Effects of Sn residue on the high temperature stability of the H2-permeable palladium membranes prepared by electroless plating on Al2O3 substrate after SnCl2–PdCl2 process:A case study☆
2016-05-30LeiWeiJianYuXiaojuanHuRongxiaWangYanHuang
LeiWei,Jian Yu,Xiaojuan Hu,Rongxia Wang,Yan Huang*
State Key Laboratory ofMaterials-oriented ChemicalEngineering,College ofChemicalEngineering,Nanjing Tech University,Nanjing 210009,China
1.Introduction
Because of their excellenthydrogen permeability and selectivity,the composite palladium membranes,which are composed of a porous substrate and a thin layer ofpalladium or palladium alloy,have been applied in many hydrogen energy systems[1–5].In particular,such a membrane concept allows the reduction of the membrane thickness even down to severalmicrons,and this not only reduces the consumption of the precious palladium but also enhances the membrane mechanical strength and H2-permeability.However,the stability of the composite palladium membranes is always a key problem for extensive membrane applications.During long-term operation at high temperatures,pinholes may gradually appear,and they willdecrease the membrane selectivity and shorten the operating life of the membranes.The causes for the membrane defects can be very complicated[1].Apart from the operation conditions such as temperature and working atmosphere[6–8],the preparation process of the composite palladium membranes plays a key role.
Porous ceramics and stainless-steelare the most common substrate materials,but the latter often stillneeds a ceramic coating layer on its surface in order to avoid intermetallic diffusion[7].As far as the membrane preparation techniques are concerned,electroless plating is obviously the most popular because it is able to create a palladium layer with high quality on various substrate surface[9,10].Prior to electroless plating,the substrate surface must be activated by seeding a layer of palladium nucleiso as to initiate the plating spontaneously and homogeneously throughout the substrate surface.The SnCl2–PdCl2process,also known as“sensitization–activation treatment”,is a traditionaland typicalmethod for substrate surface activation,which has been widely applied by most of the researchers.During such process,the nano-sized palladium nuclei,which are a highly effective catalyst for the electroless plating,are generated via reduction of Pd2+with Sn2+.Moreover,these palladium nucleican be firmly mounted onto the substrate surface because of the formation oftin colloid(e.g.,Sn(OH)1.5Cl0.5)created during hydrolysis of SnCl2[11,12].
In 1999,Paglieri et al.[13]claimed that the SnCl2–PdCl2process causes Sn residue,which leads to the formation ofpinholes in palladium membranesand consequently causespoormembrane stability(ormore precisely,the stability ofselectivity)during operation at high temperatures.This sounds reasonable because the tin oxide can be reduced by hydrogen at high temperatures,and the resulting metallic tin has a low melting point.Such a report motivated the exploration of the Sn-free activation to replace the SnCl2–PdCl2process,and indeed severalnew methods have been developed[13–15].However,none of them has been popularized so far because of their problems in effectiveness,cost,or convenience,and the SnCl2–PdCl2process remains to be the top choice for substrate activation.Consequently,the “safety”of the seemingly irreplaceable SnCl2–PdCl2process is worthy of veri fication.In this work,the Pd/Al2O3membranes were prepared via electroless plating after a SnCl2–PdCl2process,the amount of Sn residue was adjusted through SnCl2concentration,activation times and additional Sn(OH)2coating,and the correlation between Sn residue and membrane stability was investigated as a case study.
2.Experimental
2.1.Preparation
Commercialporous Al2O3tubes(outer diameter,12.5 mm;inner diameter,7.5 mm;length,75 mm;average pore size,0.2μm),which comprised a microporous ZrO2top layer,anα-Al2O3intermediate layer and a macroporousα-Al2O3base,were employed as the substrate material.Before use,the Al2O3tubes were calcined in air at600°C for 2 h to remove the organic contaminants.The outersurface of the Al2O3substrate was activated by SnCl2–PdCl2process[11,12],i.e.,the substrate was successively impregnated into the acidic SnCl2and PdCl2solutions,and a mild rinsing with deionized water was performed after each impregnation.In this experiment,the SnCl2solution contained 5 or 20 g·L-1SnCl2·2H2O and 1 ml·L-1chloride acid(37 wt%),while the PdCl2solution was composed of0.2 g·L-1PdCl2and 1 ml·L-1chloride acid(37 wt%),and the sensitization–activation cycles were repeated for 4 or 8 times.The activated substrates were denoted as Al2O3–1–4,respectively,and the detailed sensitization–activation conditions were listed in Table 1.For the sample ofAl2O3–4,the substrate was pretreated with Sn(OH)2coating as following.A Sn(OH)2suspension was synthesized by means ofadjusting the pH value of SnCl2·2H2O solution(concentration,20 g·L-1)to 8.0 with NH3·H2O(28 wt%).Afterward,the Al2O3tube was impregnated into the suspension and then dried at 120 °C over night.Palladium membranes based on the Al2O3–1–4 were prepared via electroless plating at 30°C.The plating bath was composed of PdCl22.5 g·L-1,Na2EDTA·2H2O 70 g·L-1and NH3·H2O(28 wt%)250 ml·L-1,and the reducing agent was a 0.5 mol·L-1N2H4·H2O solution.As calculated by the gravimetric method,the thicknesses of the resulting Pd/Al2O3–1–4 membranes were 5.2,5.0,5.1 and 5.3μm,respectively.
2.2.Characterization
The surface morphology was characterized by scanning electron microscopy(SEM,FEIQuanta-200),and the element composition was analyzed by energy dispersive spectroscopy(EDS).Before the crosssectionalstructure analysis using metallographic microscopy(LEICADM-4000 M),allthe specimens of composite palladium membranes were firstly encapsulated with epoxy resin and then treated on a grinding-polishing system(Buehler Phoenix Beta).The permeability and stability of the prepared palladium membranes were evaluated by means of H2/N2single-gas method at 450–600 °C,and the pressure at the permeate side was always ambient.The H2permeate velocity(m3·h-1)was measured by mass flow meters,and H2flux(m3·m-2·h-1)was given by the permeate velocity per membrane area(m2),as well as the calculation of N2flux(m3·m-2·h-1).The membrane selectivity is de fined as the ratio of H2flux versus N2flux at the same pressure and temperature.Before testing,each membrane was sealed with graphite gaskets in a compact module[16],and a pretreatment with synthetic air(N2:O2=4:1)was carried out at 350°C for 2 h to remove the organic contamination on the membrane surface.
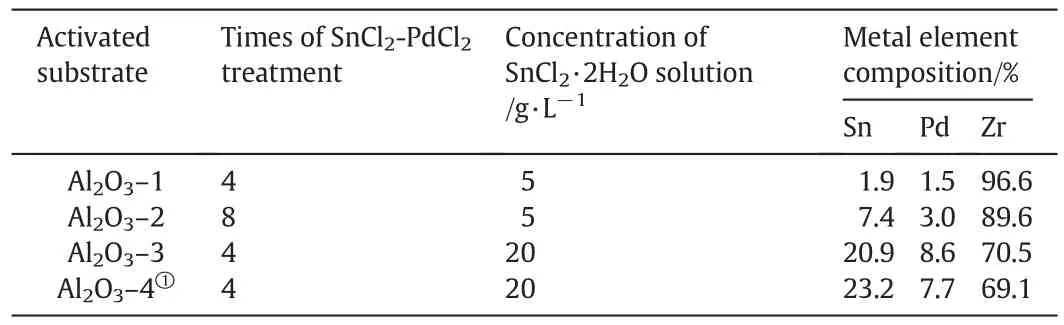
Table 1 Porous Al2O3 substrate activated by SnCl2-PdCl2 process
3.Results and Discussion
Asymmetric porous Al2O3tubes are one kind of the major substrate materials ofpalladium membranes,and the top layer is smooth and microporous,which greatly facilitates the formation ofthin and continuous palladium membranes.Additionally,the ZrO2top layer exhibits favorable chemicalstability during the preparation and application of palladium membranes.The surface and cross-sectionalmicrographs of the fresh Al2O3substrate are exhibited in Fig.1(a)and(b),the ZrO2top layer composed ofsintered particles has a smooth surface and submicron pores.Moreover,the asymmetric structure can be clearly seen in Fig.1(b).After SnCl2–PdCl2process,the color of Al2O3–1–2 was light brown,while the color ofAl2O3–3–4 was dark brown due to the increasing quantity of palladium nuclei.After drying at 120°C,the activated substrate was characterized by SEM,and their surface micrographs are shown in Fig.1(c–f).By comparing with Fig.1(a),the activated substrate surface has been coated with an activation layer thatis composed ofdried tin colloid and palladium nuclei.Furthermore,the thicknesses of the activation layers gradually increase with the increasing of SnCl2concentration and activation times.
The EDS analyzing results on Al2O3–1–4 were listed in Table 1.Note that the aluminum element was not detected because the outmost surface of the Al2O3tube is actually a layer of ZrO2.The percentages of tin in the metallic elements on Al2O3–1–3 surfaces are 1.9,7.4 and 20.9%respectively,indicating that the amount of Sn residue increases with the increasing of SnCl2concentration and the activation times.The largest Sn residue is found to be 23.2%on the substrate Al2O3–4 that has been coated with a Sn(OH)2suspension in advance.In the literature[7,11]on the substrate activation before electroless plating of palladium membranes,the reported concentration of SnCl2·2H2O for substrate activation is just 1–5 g·L-1,and the sensitization–activation process are often repeated for 3–6 times.Therefore,the Sn residue after a normalactivation treatmentwillde finitely be much lower than those on the Al2O3–3 and Al2O3–4 in this work,but these two samples,as an extreme case,can help to verify the effect of the Sn residue.
After electroless plating on the Al2O3–1–4 substrates,fourpalladium membranes were obtained,and allof them looked highly lustrous and smooth.Fig.2 displays the surface micrographs of the resulting palladium membranes,which is extremely typicaland similar to those reported in the literature[7,12,16,19,20].Principally,the kinetics of hydrogen permeation through palladium membranes follows the solution–diffusion mechanism:

where JH2is the hydrogen flux,F is the permeance,PRetand PPermare the pressures atthe retentate and permeate sides,and n is the pressure exponent[1,9,10].When the palladium membrane is not completely defect-free,the hydrogen can also permeate through the membrane defects,but this partis negligible against JH2.In general,the defects of the membranes can be re flected by nitrogen flux.
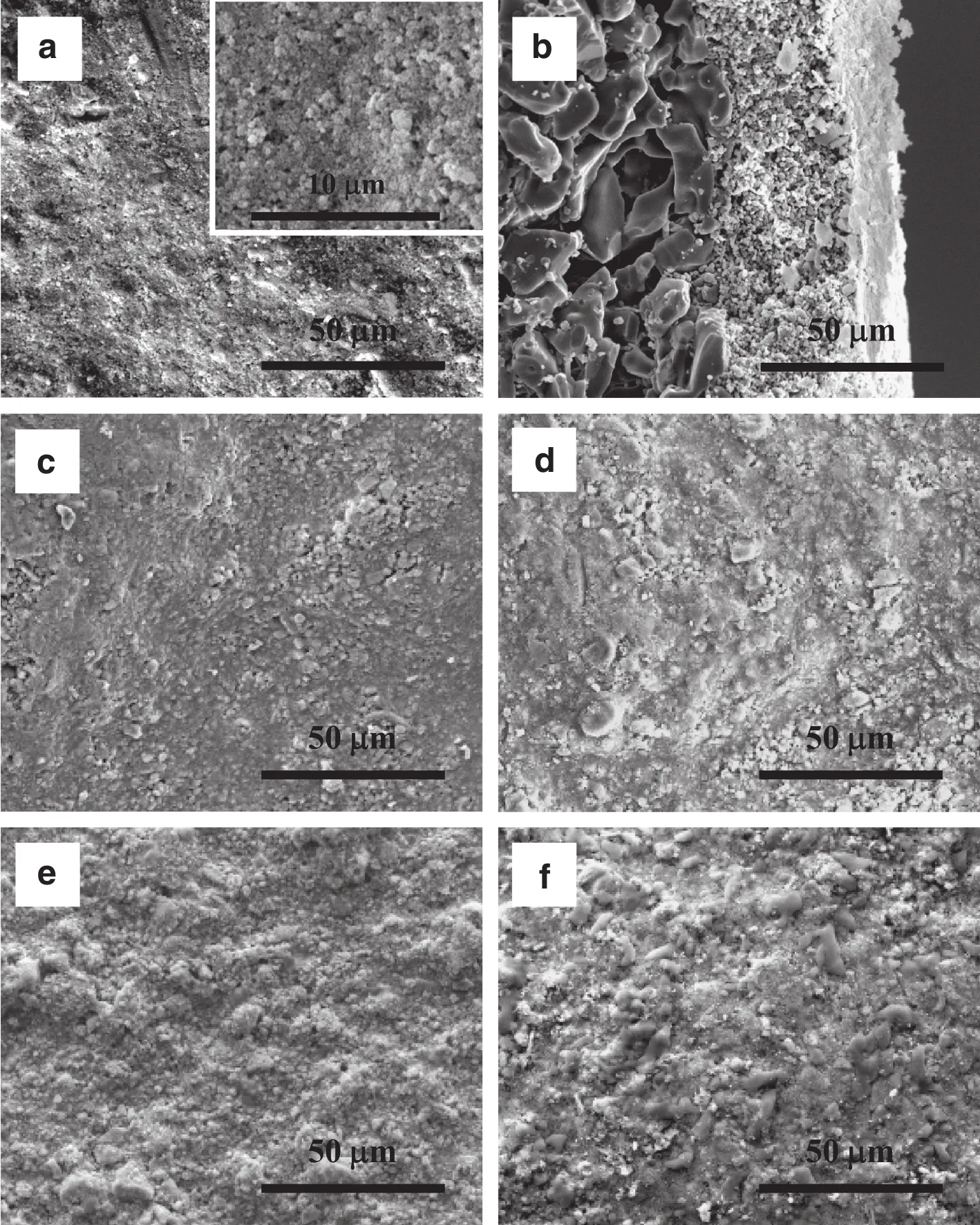
Fig.1.(a)and(b):Surface and cross-sectionalSEMmicrographs of the fresh Al2O3 substrate;(c)–(f):Surface SEMmicrographs of Al2O3–1,Al2O3–2,Al2O3–3 and Al2O3–4,respectively.
The hydrogen permeation kinetics was analyzed at 450°C,and the results are demonstrated in Fig.3.A good linear relationship between the JH2and the difference of square root of the pressures can be established at n=0.5,indicating that the hydrogen diffusion obeys the Sievert's Law,and the rate-determining step should be the diffusion ofhydrogen atoms in palladium bulk[17,18].The calculated hydrogen permeance F of the Pd/Al2O3–1–4 membranes are 7.02,6.76,7.96 and 8.23 m3·m-2·h-1·kPa-0.5,respectively.
Fig.4 shows the results of the membrane stability tested at 450–600°C under a pressure of 300 kPa during a time-on-stream of 400 h.The hydrogen flux always increases with the increasing ofoperation temperature because the high temperature speeds up the velocity of the hydrogen diffusion in palladium[19].The nitrogen flux is always far from the corresponding hydrogen flux under the same conditions for each membrane despite of the difference among the membranes.At 450°C,H2/N2selectivities of the prepared palladium membranes are allstable and in the following order:Pd/Al2O3–1 ≫ Pd/Al2O3–2 > Pd/Al2O3–3,indicating that the increase in Sn residue on the substrate surface tends to decrease the membrane selectivity.As con firmed by Fig.1 and Table 1,the excessive activation of the substrate surface willdeposit a thicker layer that is composed of Sn colloid and palladium nuclei,and a too thick layer may lead to more membrane defects and poor membrane adhesion because such a colloidallayer is metastable against the plating bath.H2/N2selectivity of the Pd/Al2O3–4 at 450 °C is higher than that of Pd/Al2O3–3,and this should be attributed to the pretreatment on the Al2O3tube with Sn(OH)2suspension,which improves the surface properties of the Al2O3tube.
With the increasing of temperature from 450 to 500°C,Fig.4(a)shows that the selectivity of Pd/Al2O3–1 slightly increases.This is reasonable because the increase in temperature tends to cause an increase in hydrogen flux and a decrease in nitrogen flux.At the temperature of550 and 600°C,however,the H2/N2selectivity of Pd/Al2O3–1 continues to decrease during the testing,indicating that either new defects are created or the original defects are enlarged or even both.Similar tendency of the H2/N2selectivity can be observed from Fig.4(b),which reveals that the stabilities of the Pd/Al2O3–1 and Pd/Al2O3–2 membranes are similar.For the membrane of Pd/Al2O3–3,Fig.4(c)shows that the H2/N2selectivity is stable at all the testing temperatures,and it normally increases with the increasing of the temperature.It can be concluded that the Pd/Al2O3–3 is almost stable throughout the whole testing period.In Fig.4(d),an interesting tendency of the nitrogen flux and the H2/N2selectivity for Pd/Al2O3–4 is exhibited.The nitrogen flux continues to decrease during the testing at500 and 550°C,and similar phenomenon has been also reported as a“self-repairing effect”in our previous work[20].In addition,Fig.4(d)shows a sharp increase in selectivity and a sharp decrease in nitrogen flux when the temperature is increased from 550 to 600°C.
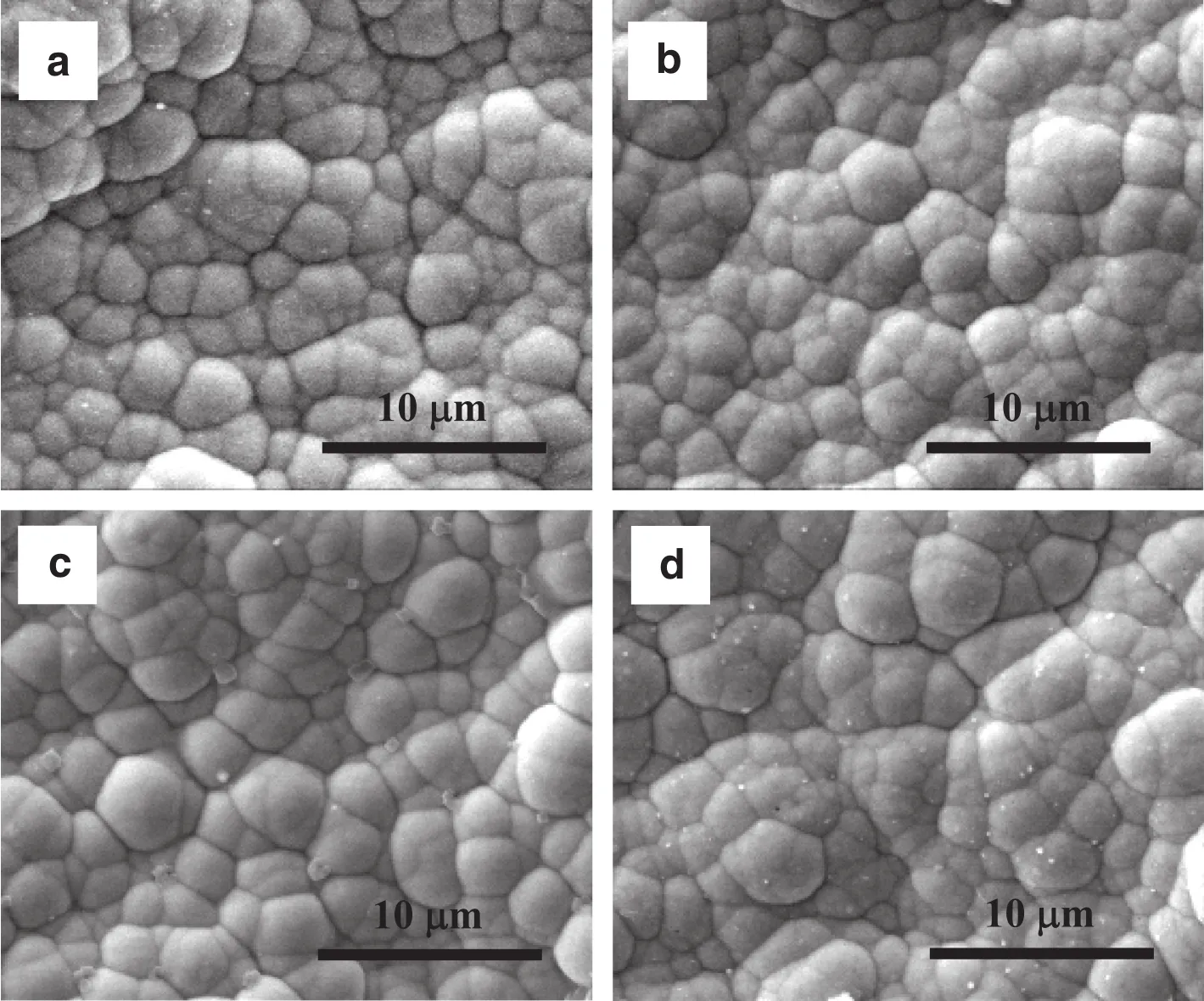
Fig.2.SEMmicrographs of the resulting palladium membranes:(a)Pd/Al2O3–1;(b)Pd/Al2O3–2;(c)Pd/Al2O3–3;(d)Pd/Al2O3–4.

Fig.3.Kinetic analysis on hydrogen permeation through the prepared palladium membranes.
After the stability test at high temperatures,the surface and crosssectional morphologies of the Pd/Al2O3–1–4 were investigated and exhibited in Fig.5.According to the cross-sectional images,the membrane thicknesses of Pd/Al2O3–1–4 were 4.8,5.2,5.3 and 3.8 μm,respectively.The thickness of the Pd/Al2O3–4 is signi ficantly thinner than that calculated,whereas the measured and calculated thicknesses of the other membranes are quite consistent.The boundaries between the palladium layers and the substrates of the four membranes can be observed from the cross-sectional images in Fig.5.Such boundaries for Pd/Al2O3–1–3 are very clear,while that for the Pd/Al2O3–4 looks relatively blurry(see Fig.5(g)).It seems that the palladium diffused into the substrate during the stability test,and this may be associated with the “self-repairing effect”that is observed in Fig.4(d).Hence,this needs further investigation.Comparison between Fig.5 and Fig.2 indicates that the palladium clusters become flat after stability test,which is quite normalbecause the palladium clusters tend to sinter at high temperatures[6,7,21].
Up to now,the stability of the composite palladium membranes is stilla bottle-neck for their extensive applications.Most of the reported palladium membranes are prepared by electroless plating thatis often associated with the SnCl2–PdCl2process,and therefore it is a serious concern whether the SnCl2–PdCl2process worsens the membrane stability as has been already reported.In Ref.[13],eight porous Al2O3tubes were activated through SnCl2–PdCl2treatmentwith differentconcentration of the sensitizers,three tubes were activated by dip-coating with chloroform solution of palladium acetate,and palladium membranes with thicknesses of3–27 μm were prepared by electroless plating.The initialselectivities of the corresponding 8 membranes at 450 °C are 5,12,39,∞,34,200,73 and 51,and the selectivities of the other 3 membranes are 49,50 and 40,respectively.The authors claimed thatthe decrease in the SnCl2concentration helps to stabilize the membrane stability,and the membranes with Sn-free activation showed the beststability in selectivity.To summarize the results in Fig.4,the selectivities of Pd/Al2O3–1 and Pd/Al2O3–2 decrease from 950 and 160 at 450 °C down to 180 and 150 at 600 °C after testing for 400 h,while the selectivities of Pd/Al2O3–3 and Pd/Al2O3–4 increase from 60 and 120 up to 90 and 550,respectively.Although the initialselectivities of the Pd/Al2O3–1–4 are greatly different,their finalselectivities of the Pd/Al2O3–1–3 are quite close and much lower than that of the Pd/Al2O3–4.If this result is associated with the results in Table 1,no clear correlation between the Sn residue and the membrane stability can be observed,and therefore the worry about Sn residue on membrane stability is not necessary at all.

Fig.4.Stability testing of the palladium membranes under a pressure of300 kPa:(a)Pd/Al2O3–1;(b)Pd/Al2O3–2;(c)Pd/Al2O3–3;(d)Pd/Al2O3–4.
The difference between the conclusions in this work and Ref.[13]is due to the fact that many other factors,such as the uniformity in membrane thickness distribution,the membrane physical strength,and the boundary between the palladium layer and the substrate,are more important than the Sn residue.For example,Paglieri and coworkers[13]prepared 5 membranes on the substrates activated under same conditions,and the membrane thicknesses are 6.8,9.3,10.3,14.1 and 19.6μm.The initial membrane selectivities are 5,12,39,∞and 34,respectively,and great differences were also found among the membrane stabilities and cannot be explained just by the difference in membrane thickness.
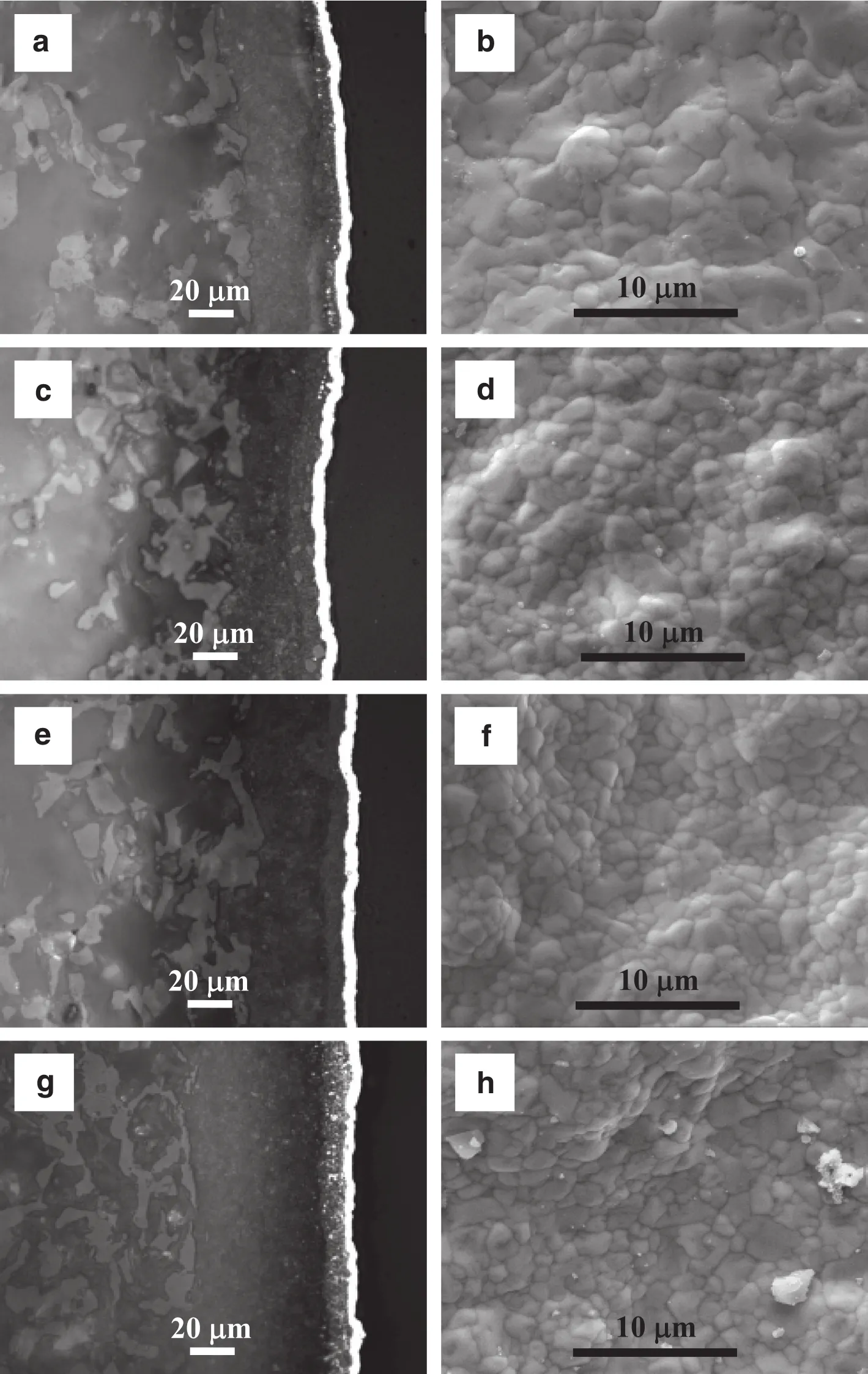
Fig.5.Cross-sectionalmetallographic micrographs(Left)and surface SEMmicrographs(Right)of the palladium membranes after stability test:(a,b)Pd/Al2O3–1;(c,d)Pd/Al2O3–2;(e,f)Pd/Al2O3–3;(g,h)Pd/Al2O3–4.
Even if the residual tin can alloy with palladium,the permeation properties of the resulting Pd–Sn alloy cannotbe signi ficantly different from the pure palladium.On one hand,the amount of residualtin on the substrate surface is so low in a normal SnCl2–PdCl2treatment;on the other hand,the low melting point of Sn facilitates the formation of homogeneous Pd–Sn alloy.Itwas wellknown thatmany homogeneous palladium alloys such as Pd–Cu and Pd–Ag are not only permeable to hydrogen but also resistant to hydrogen embrittlement[1,10].Eventually,the SnCl2–PdCl2process has been the mostpopular method for substrate activation in electroless plating ofpalladium membranes,and it might remain to be so in future.
4.Conclusions
During surface activation of the porous Al2O3substrate through the SnCl2–PdCl2process for electroless plating of palladium membranes,the increase in the SnCl2concentration and the activation times led to the increasing amount of Sn residue on the substrate surface,which resulted in a lower initialselectivity but a better membrane stability of the prepared palladium membranes during a time-on-stream of400 h at 450–600 °C.As an extreme case,the additional Sn(OH)2coating further increased the Sn residue on the Al2O3substrate,but the membrane selectivity was even greatly improved.As a consequence,it can be concluded that the Sn residue on the substrate surface from SnCl2–PdCl2process is not the main factor in fluencing the stability(or more precisely,the stability ofselectivity)of the palladium membranes at high temperatures.
[1]S.N.Paglieri,J.D.Way,Innovations in palladium membrane research,Sep.Purif.Methods 31(2002)1–169.
[2]J.Han,I.S.Kim,K.S.Choi,High purity hydrogen generator for on-site hydrogen production,Int.J.Hydrog.Energy 27(2002)1043–1047.
[3]A.S.Augustine,Y.H.Ma,N.K.Kazantzis,High pressure palladium membranes reactor for the high temperature water–gas shift reaction,Int.J.Hydrog.Energy 36(2011)5350–5360.
[4]Y.M.Lin,M.H.Rei,Study on the hydrogen production from methanol steam reforming in supported palladium membrane reactor,Catal.Today 67(2001)77–84.
[5]Y.Swesi,D.Ronze,I.Pitault,R.Dittmeyer,F.Heurtaux,Puri fication process for chemicalstorage ofhydrogen for fuelcellvehicles applications,Int.J.Hydrog.Energy 32(2007)5059–5066.
[6]F.Guazzone,Y.H.Ma,Leak growth mechanism in composite Pd membranes prepared by the electroless deposition method,AIChE J.54(2008)487–494.
[7]Y.Huang,R.Dittmeyer,Preparation and characterization of composite palladium membranes on sinter-metal supports with a ceramic barrier against intermetallic diffusion,J.Membr.Sci.282(2006)296–310.
[8]H.Y.Gao,Y.S.Lin,Y.D.Li,B.Q.Zhang,Chemical stability and its improvement of palladium-based metallic membranes,Ind.Eng.Chem.Res.43(2004)6920–6930.
[9]S.Yun,S.T.Oyama,Correlations in palladium membranes for hydrogen separation:a review,J.Membr.Sci.375(2011)28–45.
[10]Y.Huang,X.Li,Y.Fan,N.Xu,Palladium-based composite membranes:principle,preparation and characterization,Prog.Chem.18(2006)230–238.
[11]P.P.Mardilovich,Y.She,Y.H.Ma,Defect-free palladium membranes on porous stainless-steelsupport,AIChE J.44(1998)310–322.
[12]Y.Huang,R.Dittmeyer,Preparation of thin palladium membranes on a porous support with rough surface,J.Membr.Sci.302(2007)160–170.
[13]S.N.Paglieri,K.Y.Foo,J.D.Way,J.P.Collins,D.L.Harper-Nixon,A new preparation technique for Pd/alumina membranes with enhanced high-temperature stability,Ind.Eng.Chem.Res.38(1999)1925–1936.
[14]L.Wu,N.Xu,J.Shi,Preparation ofa palladium composite membrane by an improved electroless plating technique,Ind.Eng.Chem.Res.39(2000)342–348.
[15]S.Yun,J.H.Ko,S.T.Oyama,Ultrathin palladium membranes prepared by a novel electric field assisted activation,J.Membr.Sci.369(2011)482–489.
[16]W.Chen,X.Hu,R.Wang,Y.Huang,On the assembling of Pd/ceramic composite membranes for hydrogen separation,Sep.Purif.Technol.72(2010)92–97.
[17]T.L.Ward,T.Dao,Model of hydrogen permeation behavior in palladium membranes,J.Membr.Sci.153(1999)211–231.
[18]A.Caravella,F.Scura,G.Barbieri,E.Drioli,Sieverts law empirical exponent for Pdbased membranes:critical analysis in pure H2permeation,J.Phys.Chem.B 114(2010)6033–6047.
[19]X.Hu,Y.Huang,S.Shu,Y.Fan,N.Xu,Toward effective membranes for hydrogen separation:multichannel composite palladium membranes,J.Power Sources 18(2008)135–139.
[20]X.Hu,W.Chen,Y.Huang,Fabrication of Pd/ceramic membranes for hydrogen separation based on low-cost macroporous ceramics with pencil coating,Int.J.Hydrog.Energy 35(2010)7803–7808.
[21]F.Guazzone,E.A.Payzant,S.A.Speakman,Y.H.Ma,Microstrains and stress analysis in electroless deposited thin Pd films,Ind.Eng.Chem.Res.45(2006)8145–8153.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Heat transfer ofnano fluidics in hydrophilic pores:Insights from molecular dynamics simulations☆
- Numericalsimulation ofstirred tanks using a hybrid immersed-boundary method☆
- Numericalsimulation ofmicromixing effect on the reactive flow in a co-rotating twin screw extruder☆
- Coalescence behaviour ofwater droplets in water-oilinterface under pulsatile electric fields
- Application ofdiffusive transport modelfor better insight into retardation mechanisms involved in ion-imprinted membrane transport
- Highly selective synthesis for 4,4′-bisphenol F from phenoland formaldehyde catalyzed with[C4mim][HSO4]ionic liquid☆
