Application ofdiffusive transport modelfor better insight into retardation mechanisms involved in ion-imprinted membrane transport
2016-05-30EhsanSalehi
Ehsan Salehi*
Department ofChemicalEngineering,Faculty ofEngineering,Arak University,Arak 38156-8-8349,Iran
1.Introduction
Mathematicalmodeling is an interesting and usefulapproach to upgrade researchers'knowledge about membrane transportmechanisms.A great deal of researches has been aimed at modeling of transport phenomena in membranes.For example,the effect of concentration gradient is included in the Stephan–Maxwellmodel[1],to extend the model for describing transport of charged particles through surfacecharged porous membranes[1–3].
Membrane adsorption is a promising technology for the removalof macromolecules and heavy metals from ef fluents[4–6].Adsorptive membranes,prepared from reactive and functionalpolymers through complex physico-chemicalsynthesizing steps,carry reactive groups as adsorption sites to chelate macromolecules and heavy metals from aqueous phase[6–8].
Mathematicalmodeling ofmembrane adsorbents,due to its novelty,is ofgreat importance from both microscopic and macroscopic aspects.From macroscopic outlook,rapid prototyping and presenting ef ficient scale-up protocols are some of the major advantages of transport modeling.In microscopic view however,usefulknowledge aboutthe interactions/mechanisms involved in the process can be obtained in the light of the modeling results.Common adsorption/transport models are derived based on massbalance principles.These types ofmathematical models take advantage of convection,diffusion and adsorption mechanisms,in combination,for describing transport behavior of adsorptive membranes[9,10].Adsorption isotherms are sometimes combined with the mathematicaltransport models to characterize the equilibrium or quasi-equilibrium adsorption during transport.
Heavy metal removalfrom water resources is a great concern for environmentalists.Ion-imprinted membrane adsorbents are potential solutions for selective removal of heavy metals.Recently,the ionimprinting technique has been employed to enhance the selectivity of the adsorptive membranes[11].Moreover,speci fic recognition sites(arti ficial receptors)are contrived in an appropriate polymeric background via insertion and subsequent extraction oftemplate ions[12,13].Shape,orientation and functionality of the introduced cavities/sites are matching with the track-etched template ions.In sum,ionimprinting process results in adsorptive membranes with speci fic af finity and selectivity toward the imprinted ions.
There is a strong motivation for better understanding of the transport mechanisms involved in imprinted membrane transport.Previous researches have shown that the principle mechanisms for the removal of heavy metalions by the ion-imprinted adsorbents are adsorption(surface adsorption,complexation and chelation)and ion-exchange[14].Transport mechanisms however,are not fully understood for the ion-imprinted membranes.A novelmathematicalmodelfor describing Ni(II)ions permeation through Ni(II)-imprinted membrane was developed in our previous study[15].The proposed modelcould successfully simulate the transport of Ni(II)and Co(II)ions across the imprinted membrane for a routine dialysis permeation process.
In the current work,transportmechanism ofnickeland cobalt ions through Ni(II)-imprinted membrane was investigated with more focus on the retardation mechanisms affecting the diffusive transport.The mathematicalmodelwas developed and then applied to elucidate the controlling mechanisms/interactions involved in diffusive transport ofions through the membranes i.e.,physicalinteractions and chemical adsorption.As a result,relative retardation factor(η)was de fined to illustrate the relative importance of these retardation mechanisms during permeation.Effect ofimportant parameters such as temperature,ion concentration and time on the retardation factor was also examined.
2.Mathematical Model Extension
Unsteady state diffusive transport model(Eq.(1)),can satisfactorily describe transport ofions/molecules through an adsorptive membrane[15]:

whereεis the membrane bulk porosity,c is the ion concentration in aqueous phase,Q is the adsorption on recognition sites(solid phase),Diis the ions diffusivity across the membrane,ρsis the dry bulk density,and x indicates the transport direction perpendicular to the membrane surface.
Right-hand side of Eq.(1)demonstrates the role ofdiffusion dialysis in permeation transport.On the left-hand side of Eq.(1),the unsteadystate termindicates couple of the possible ways for the accumulation of ions in adsorptive membrane i.e.,physicalaccumulation(firstterm)and chemical adsorption(second term).Moreover,adsorption includes diffusion ofsolute from the bulk fluid to the solid surface in which the chemical surface interactions occur.At the solid surface,the solute(ions)interacts with the reactive sites through either physicalinteractions(physisorption)or chemical interactions(chemisorption).Chemical interactions with the surface result in chemical bonding,complexation and chelation.Physical step however,includes bulk diffusion,film diffusion,pore clogging,surface diffusion and sieving mechanisms.The final governing equation has been obtained by coupling Eq.(1)with the Sips isothermmodelItis worthy of mention that the Sips modelhas been veri fied among severaltested isotherms according to its higher coef ficient of determination[15].The resultant governing equation is obtained as follows:
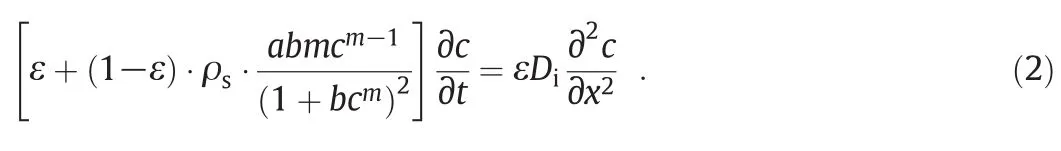
a,b and m are the equilibrium parameters of the Sips isotherm which have been obtained in another work from the current authors[15].These parameters for adsorption at different temperatures are given in Table 1.Eq.(2)includes one dependent variable(c),and couple of independent variables(x and t).The following boundary and initial conditions were applied for the mathematicalanalysis of Eq.(2):

One can ignore the chemicaladsorption term to obtain the pure effect ofphysicalinteractions on the diffusive transportof the ions.Accordingly,the reduced Partial Differential Equation(PDE)is obtained as:

On the other hand,physical interactions may be overlooked to obtain a partial differential equation indicating the pure effect of chemicaladsorption on transport mechanism:
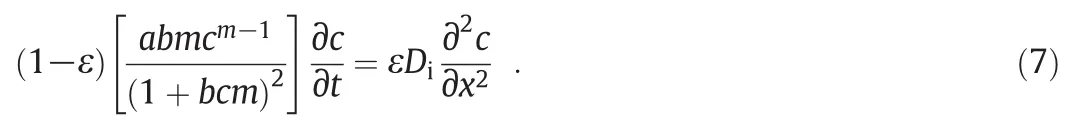
Considering Eqs.(6)and(7),relative retardation factor(η)can be de fined as ion transport-rate controlled by chemisorption to that controlled by the physical attachment mechanisms.Accordingly,a straightforward de finition forηis obtained by dividing Eq.(7)to Eq.(6),as follows:
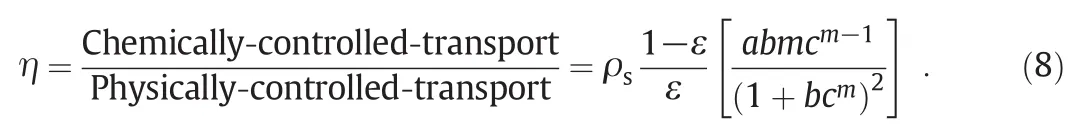
The term"retardation"(Eq.(8))is an attemptto evaluate the relative importance of the chemicalretardation mechanisms(i.e.surface chemicalinteractions)to that of the physicalones.In other words,intensity and weakness of chemicalto physicalretardation mechanisms can be monitored during ion transport with the help of Eq.(8).
3.Materials and Methods
Nickel and cobalt nitrates were obtained from Merck.All other chemicals were of analytical grade and used as received.Atomic absorption spectrometry(AA-6300 Shimadzu)was applied for analyzing the nickeland cobalt ions concentration.
Membrane fabrication method was perfectly elucidated in another work from the current authors[13].Brie fly,methacrylic acid was polymerized on the surface of PVDF micro filtration membrane in the presence of Ni-dithizone as complex agent and ethylene glycol dimethacrylate as crosslinker.Hydrochloric acid(1 mol·L-1)was employed for selective dissolution oftemplate ions from the synthesized membrane.Subsequently,the membranes were abundantly rinsed with distilled water and air-dried.
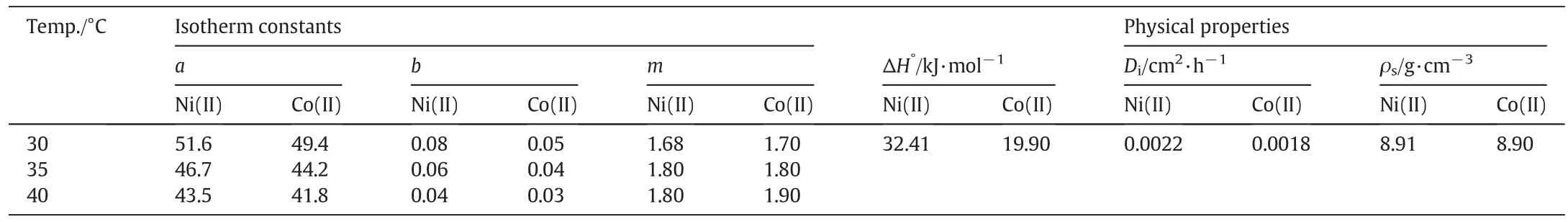
Table 1 Physicaland thermodynamic parameters for Ni(II)-imprinted membrane adsorption[15]
Static(batch)adsorption tests were performed to obtain the equilibrium parameters of the adsorption isotherms.The procedure was fully described in our prior paper[15].Diffusive transport of ions through the imprinted membrane was investigated according to straightforward dialysis permeation procedure.The experimentalsetup and applied procedure were explained in detailelsewhere[13,15].In brief,a two-section dialysis cellwas employed fordialysis permeation tests.Membrane samples were placed and sealed between the two half-cells of the setup.Feed-and receive-side half-cells were filled with 100 mlofnickel(orcobalt)nitrate solution(with concentration range of 1 to 25 mg·L-1)and distilled water,respectively.Three hours once,a few milliliter sample solution was taken outfrom the receive solution and then,immediately compensated with the same amount ofdistilled water.
4.Results and Discussion
The governing equation(Eq.(2))has been numerically solved to obtain the concentration of the ions as a function of permeation time and location throughout the imprinted membrane.Fig.1,indicates the results for T=35 °C,pH=8 and c0=25 mg·L-1.The results were comparable for different initial concentrations(not shown).Model predictions were compared with the experimentaldata in Fig.2.Results indicate thatthe mathematicalmodelcan satisfactorily simulate the permeation ofNi(II)ions through the Ni(II)-imprinted membrane.Itis obvious from Fig.1,that the dispersion has negligible effect on the permeation of the ions through the membrane.Therefore,onedimensionalanalysis approach could be employed without any meaningfulerror.As inferred from Fig.1,the concentration of the ions inside the membrane matrix increases with time.This fact demonstrates the potentialof the imprinted membrane for the ion uptake.Moreover,the ions can easily access the vacantrecognition sites via facilitated transport mechanism[13–15].
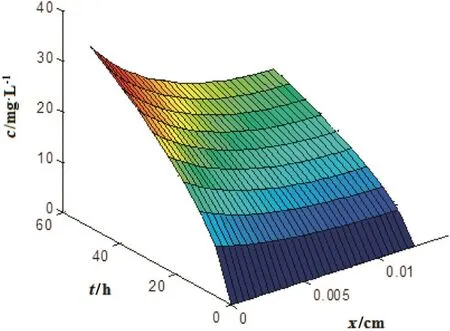
Fig.1.Concentration distribution of Ni(II)ions versus time and position in the Ni(II)-imprinted membrane at T=35 °C,pH=8 and c0=25 mg·L-1.

Fig.2.Permeate concentration versus time:model prediction(dash-line)versus experimentaldata(triangles).
Gradual extension of the concentration gradient inside the membrane matrix can facilitate the adsorption/transportmechanism.Itis obvious from Fig.1,that the concentration of the ions is higher in regions closer to the membrane inlet presumably due to superior ion uptake rate as well as minimum possible mass-transfer resistance against mass transfer in these regions.
4.1.Retardation mechanism
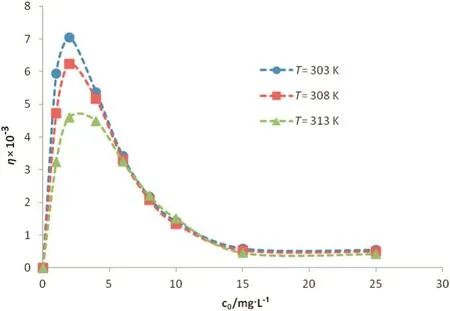
Fig.3.Retarding factor versus initialconcentration atdifferenttemperatures for Ni(II)ions.
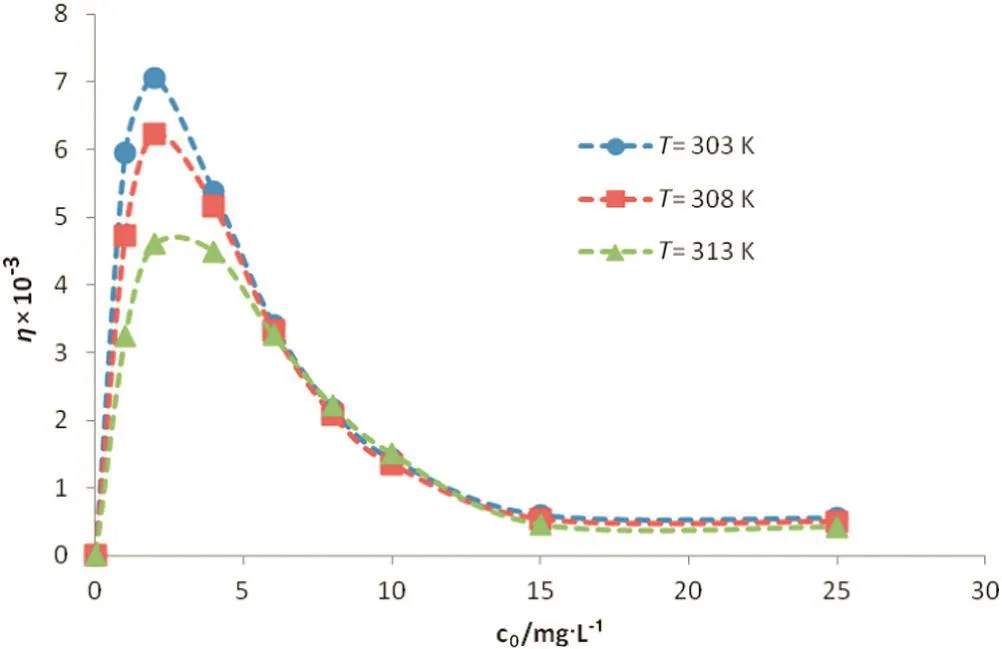
Fig.4.Retarding factor versus initialconcentration at different temperatures for Co(II)ions.
Based on Eq.(8),retardation factor(η)is the function ofequilibrium concentration and temperature.Moreover,isotherm parameters(a,b and m)were employed to calculateηbased on Eq.(8).These constants are clearly temperature dependent.In addition,equilibrium concentration is another independent variable of retardation factor.Results for Ni(II)and Co(II)ions are shown in Figs.3 and 4,respectively.Initialconcentration of the ions in the feed phase is in the range of1 to 25 mg·L-1.According to the modeling results,the chemisorption-induced transport rate is around one thousand order ofmagnitude greater than the physical-interaction-controlled rate.This factreveals thatthe imprinted membrane is originally an ‘adsorptive’rather than a ‘size-exclusive’membrane[11,15].Generation ofarti ficialreceptor-like adsorption sites as a result of the imprinting process provides the condition for the imprinted membrane to act as a selective-adsorption carrier.In an ion-imprinted membrane,reactive reception sites play an important role in transport of the template ions.These sites can memorize,recognize and interactwith the imprinted ions through differentmechanisms such as chemicalbonding,chelation and complexation.In other words,accumulation ofions in aqueous solution filled in the membrane pores is in lesser importance compared to the selective-chemisorption mechanism in ion-imprinted membrane transport.It is obvious from Figs.3 and 4 thatthe retardation factor is higher for the nickelcompared to the cobalt ions.This fact is originated from the imprinting process.Moreover,the arti ficialreception sites are better matching with Ni(II)(the imprinted ion)than with Co(II)ions[14].Accordingly,the Ni(II)-imprinted membrane offers higher af finity toward Ni(II)ions.
4.2.Effectofequilibrium concentration
Retardation factor increases sharply with increasing concentration,meets a maximum and then,decreases and switches to a plateau at higher concentrations.Increasingηindicates the impact of chemisorption as controlling mechanism at initial concentration less than 3 mg·L-1(low concentrations).This may be attributed to the large volume of vacant reactive sites at lower concentrations due to lower ion uptake rate.In this condition,the imprinted membrane is a better candidate for adsorption compared to physicalretardation.Physicalinteractions are strengthened with increasing ion concentration.Severe uptake ofions at higher concentrations impels the ions to accumulate in the pores due to limited number of the recognition sites.Therefore,the relative retardation factor decreases.This means gradualweakness of chemical adsorption mechanism.At higher concentrations(more than 15 mg·L-1),saturation condition brings about as a result of the concentration-gradient development.Unchanged value of retardation factor con firms this conclusion.At saturation condition;whereas,all the adsorption sites are occupied,there is a balance in the ion exchange rate between the recognition sites(solid phase)and the fluid filled the pores(liquid phase).Accordingly,the relative retardation factor remains constant.This reveals thatthe relative impactofchemisorption to physical interactions in controlof mass transport does not further change.In this condition,imprinted membrane acts as a facilitatedtransport carrier.Fig.5 schematically shows the change in the imprinted-membrane transport behavior from chemically adsorptive to facilitated-exchange transport.This conclusion is in tune with the results obtained by other researchers[11–14].
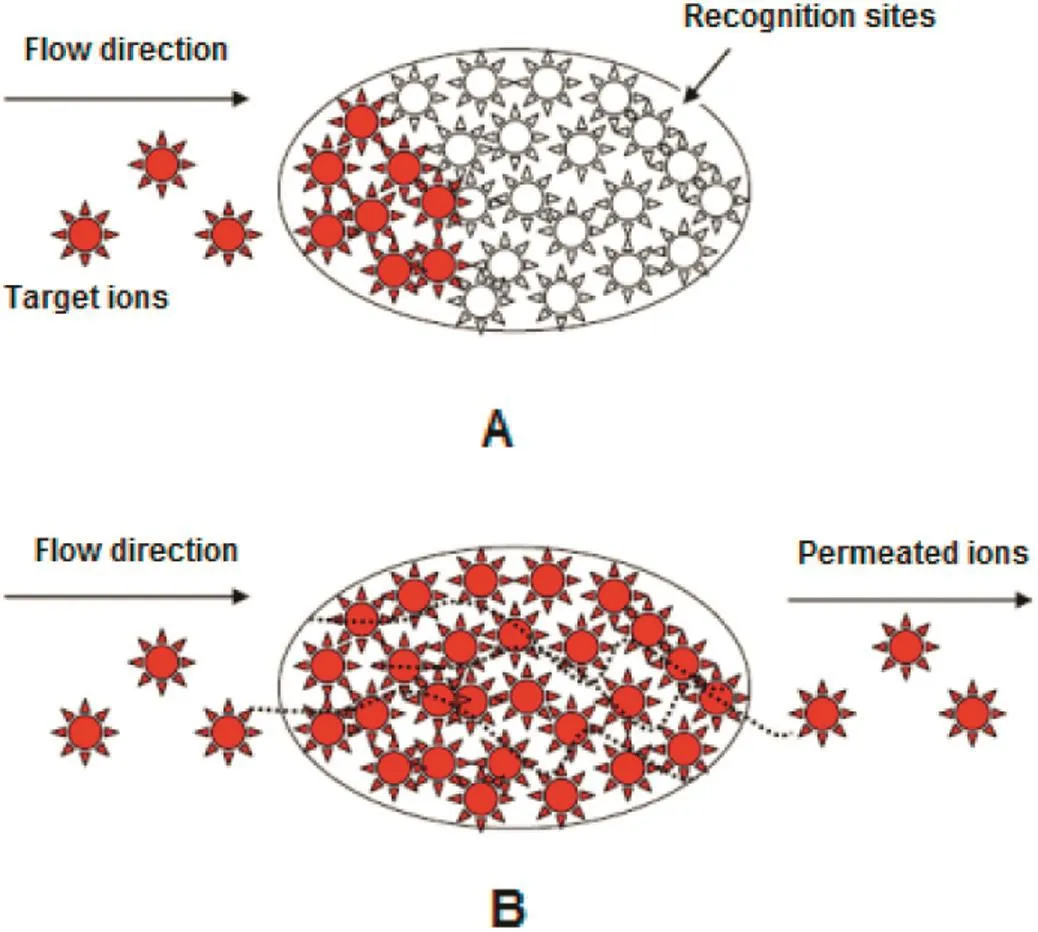
Fig.5.Ion-imprinted membrane transportmechanism;before(A)and after(B)saturation ofrecognition sites.(Dash lines:ion-exchange paths).
4.3.Effectoftemperature
Retardation factor was calculated at different temperatures.As previously mentioned,isotherm constants which play a pivotalrole in determination ofηare temperature dependent.It is clear from Figs.3 and 4 that adsorption is superior controlling mechanism at lower temperatures.It is in agreement with the exothermic nature of the adsorption according to the positive amount of enthalpy(Table 1)[15].In other word,reducing temperature provides more favorable condition for adsorption ofions from thermodynamic viewpoint.The effect of temperature is not signi ficant at higher concentrations as obvious from Figs.3 and 4.This may be attributed to the dominating effectofexchange rather than adsorption mechanism.In this condition,the kinetic of the adsorption/desorption cycle is not highly affected by raising the temperature.
4.4.Time dependency
Variation ofretardation factor versus time for Ni(II)permeation at two differentinitialconcentrations is shown in Fig.6.At first,ηincreases with time and then switches to a constant.Concentration gradient(or more exactly mass transfer zone)is extended inside the membrane matrix by gradual occupation of adsorption sites located on or near the surface.Adsorption capacity is ful filled after fulldevelopment of the concentration gradient.In this condition,the ion adsorption rate is in tune with the desorption rate.So,the concentration of the ions in the aqueous phase and that on the solid phase remains unchanged i.e.,ηswitches to a constant.
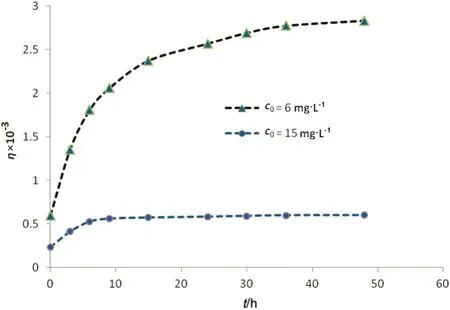
Fig.6.Retarding factor versus time at different initialconcentrations for Ni(II)ions.
As inferred from Fig.6,adsorption is dominating retardation mechanism at lower concentrations.This is attributed to the limited adsorption capacity of the imprinted membrane.Athigher concentrations,larger number of the ions can accumulate in the fluid filled in the membrane pores.Accordingly,saturation occurs sooner and transport mechanism rapidly changes from adsorption-conducted to physicalion-exchange.
5.Conclusions
Diffusive transportmodelwas utilized to derive a key-factor indicating relative importance of chemicaladsorption to physicalinteractions during ion-imprinted membrane transport.Some bulletresults inferred from the current study:
1.Chemisorption is much important controlling mechanism for ionimprinted membrane permeation compared to physicalretardation.
2.Retardation factor increases with reducing temperature due to exothermalnature ofadsorption.
3.Retardation factor is also greater for the permeation of Ni(II)ions compared to Co(II)ions as a result of the imprinting effect.
4.Separation mechanism of the imprinted membrane changes from adsorption to facilitated transport.
5.Adsorption to physical-attachment ratio meets a constant after saturation of the membrane.
Nomenclature

Acknowledgements
The author acknowledges Arak University for supporting during this study.
[1]S.S.Madaeni,H.R.Godini,Transfer characterization of charged particles through charged conductive membrane via dialysis process,J.Porous.Mater.15(4)(2008)467–473.
[2]M.S.Hall,V.M.Starov,D.R.Loyd,Reverse osmosis of multicomponent electrolyte solution:Part I.Theoreticaldevelopment,J.Membr.Sci.128(1)(1997)23–27.
[3]R.J.Hill,Electric-field-enhanced transport in polyacrylamide hydrogelnanocomposites,J.Colloid Interface Sci.316(2)(2007)635–644.
[4]R.S.Vieira,E.Guibal,E.A.Silva,Beppu MM.Adsorption and desorption of binary mixtures of copper and mercury ions on natural and crosslinked chitosan membranes,Adsorption 13(5)(2007)603–611.
[5]M.M.Beppu,E.J.Arrud,R.S.Vieira,N.N.Santos,Adsorption of Cu(II)on porous chitosan membranes functionalized with histidine,J.Membr.Sci.240(1–2)(2004)227–235.
[6]A.G.Boricha,Z.V.P.Murthy,Acrylonitrile butadiene styrene/chitosan blend membranes:Preparation,characterization and performance for the separation of heavy metals,J.Membr.Sci.339(1–2)(2009)239–249.
[7]C.Liu,R.Bai,Adsorptive removalofcopper ions with highly porous chitosan/cellulose acetate blend hollow fiber membranes,J.Membr.Sci.284(1–2)(2006)313–322.
[8]Z.Cheng,X.Liu,M.Han,W.Ma,Adsorption kinetic character of copper ions onto a modi fied chitosan transparent thin membrane from aqueous solution,J.Hazard.Mater.182(1–3)(2010)408–415.
[9]F.Cattoli,C.Boi,M.Sorci,G.C.Sarti,Adsorption of pure recombinant MBP-fusion proteins on amylose af finity membranes,J.Membr.Sci.273(1–2)(2006)2–11.
[10]C.Boi,S.Dimartino,G.C.Sarti,Modeling and simulation of af finity membrane adsorption,J.Chromatogr.A 1162(1)(2007)24–33.
[11]X.Wang,L.Zhang,C.Ma,R.Song,H.Hou,D.Li,Enrichment and separation ofsilver from waste solutions by metalion imprinted membrane,Hydrometallurgy 100(1–2)(2009)82–86.
[12]J.H.Chen,G.P.Li,Q.L.Lio,J.C.Ni,W.B.Wo,J.M.Lin,Cr(III)ionic imprinted polyvinyl alcohol/sodium alginate(PVA/SA)porous composite membranes for selective adsorption of Cr(III)ions,Chem.Eng.J.165(2)(2010)465–473.
[13]V.Vatanpour,S.S.Madaeni,S.Zinadini,H.R.Rajabi,Development of ion imprinted technique for designing nickel ion selective membrane,J.Membr.Sci.373(1–2)(2011)36–42.
[14]H.Su,J.Li,T.Tan,Adsorption mechanism for imprinted ion(Ni2+)of the surface molecular imprinting adsorbent(SMIA),Biochem.Eng.J.39(3)(2008)503–509.
[15]E.Salehi,S.S.Madaeni,V.Vatanpour,Thermodynamic investigation and mathematical modeling of ion-imprinted membrane adsorption,J.Membr.Sci.389(1–2)(2012)334–342.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Heat transfer ofnano fluidics in hydrophilic pores:Insights from molecular dynamics simulations☆
- Numericalsimulation ofstirred tanks using a hybrid immersed-boundary method☆
- Numericalsimulation ofmicromixing effect on the reactive flow in a co-rotating twin screw extruder☆
- Coalescence behaviour ofwater droplets in water-oilinterface under pulsatile electric fields
- Effects of Sn residue on the high temperature stability of the H2-permeable palladium membranes prepared by electroless plating on Al2O3 substrate after SnCl2–PdCl2 process:A case study☆
- Highly selective synthesis for 4,4′-bisphenol F from phenoland formaldehyde catalyzed with[C4mim][HSO4]ionic liquid☆
