Solvent extraction of lanthanum and cerium ions from hydrochloric acidic aqueous solutions using partly saponified 2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester☆
2016-05-29HailongHouJianhongXuYundongWangJinnanChen
Hailong Hou ,Jianhong Xu ,Yundong Wang ,*,Jinnan Chen ,*
1 School of Chemical Engineering and Environment,Beijing Institute of Technology,Beijing 100081,China
2 The State Key Laboratory of Chemical Engineering,Department of Chemical Engineering,Tsinghua University,Beijing 100084,China
1.Introduction
Rare earth elements(REEs)have been draw ing great attention because of their extensive applications in permanent magnet[1],catalyst[2,3],metallurgy[4,5],and other high-technology domains[6].Solvent extraction method has been broadly used to separate REEs.Organo phosphorus acids,such as 2-ethylhexyl phosphoric acid-2-ethylhexyl ester(EHEHPA)[7,8],di-(2-ethylhexyl)phosphoric acid(D2EHPA)[9,10],and carboxylic acid[11,12],are w idely used.Among these,EHEHPA is more preferable due to its high selectivity and low stripping acidity[13].The mechanism with un-saponified EHEHPA is a cation exchange reaction[14],in which hydrogen ions are released.With the increase of acidity,the extraction process is inhibited.In order to enhance the extraction capability of the extractant,the saponified extractant is used in hydrometallurgy separation process.Thakur and coworkers[15]used 20%saponified alkylphosphonic acid,EHEHPA,to separate Nd(III),and some basic experimental data on the distribution of REEs against initial aqueous acidity were determined.Devi and coworkers[16]studied extraction process of Co(II)/Ni(II)from sulfate solutions with saponified extractants,D2EHPA,EHEHPA,and Cyanex 272.The optimal extraction conditions for separating the two elements were investigated.Sarangi and coworkers[17]separated Co(II)/Ni(II)from chloride solutions by using Cyanex 272 as an extractant,TBP as a phase modifier,and NaOH as a saponifier.Devi and coworkers[18]performed works on optimal conditions for the Co(II)/Mn(II)separation process with saponi fied extractant.Lee and coworkers[19]used 40%saponified EHEHPA to extract Nd(III)from chloride medium.The complex of saponified extraction was considered as Nd L3.Although the saponification extraction process is very important in industrial process,the mechanisms of the processes,including the formation of the complex,and the accompanying phenomena,have not been thoroughly clarified.
In this paper,we attempt to clarify the mechanism of the rare earth elements extraction process with partly saponified EHEHPA.Firstly,the complex was determined by the saturation extraction capacity method.The REE aqueous solution with concentration in a range of 0.0010 to 0.10 00 mol·L-1was used as the aqueous phase.The EHEHPA with 0.3(mole fraction)saponified with concentration in a range of 0.2877 to 0.8631 mol·L-1was the organic phase.Secondly,according to the relationship between the initial aqueous phase p H and equilibrium aqueous phase p H,the reaction mechanismsunder different conditions were determined.Finally,on the basis of the mechanism determined,mathematical models were developed in order to predict the REE distribution ratio.
2.Experimental Procedures
2.1.Reagents
The aqueous phase was obtained by dissolving certain amount of the LaCl3·7H2O or CeCl3·7H2O in hydrochloric acid solution with different initial p H.The diluent,sulphonated kerosene and the acidic extractant,EHEHPA were used directly without having any further purification.The organic phase was prepared from mixing the EHEHPA in sulphonated kerosene with a certain amount of strong ammonia water.The mixture was kept stirring for 12 h until a single transparent phase formed.All the reagents and the material used in the experiments are listed in Table 1.
2.2.Measurements
The un-saponified EHEHPA was quantified by electronic scale(Mettler AL204),and then dissolved in alcohol solution(75 vol%).To determine the purity of the un-saponified EHEHPA,the EHEHPA alcohol solution was titrated by 0.1001 mol·L-1standard NaOH solution with an intelligent titrator(Mettler T50).The concentration of lanthanum or cerium ions in the aqueous solution was determined by an inductively coupled plasma optical emission spectrometer(ICP-OES,IRIS Intrepid II XSP)at 295 K.The p H value in the aqueous phase was determined by Mettler Easy Five p H meter with a Mettler Toledo LE420 electrode and an ATC temperature probe.Thep Hmeter was calibrated by using standard p H buffer solutions,before each round of p H measurement.
2.3.Extraction procedure
The extraction equilibrium experiments were carried out as follow s:equal volumes(20 ml)of aqueous and organic phases were placed in conical flasks.These conical flasks were kept shaking for 1 h in water bath shaker(HZ-9212S purchased from Taicang Science and Education instrument Plant),which was sufficient to reach the thermodynamic equilibrium.The temperature of the water bath was controlled at(298 ± 1)K,and the vibration speed was at(203± 1)r·min-1.The REE concentrations in aqueous solutions before and after phase separation were measured by the ICP-OES,and the quantity of REE ions extracted into the organic phase was determined by mass balance.The distribution ratio is defined as the followings(Eq.(1)):

w here D represents the distribution ratio.RE represents the REEs,La(III)or Ce(III).Square bracket refers to the concentration,mol·L-1.The subscripts eo and ea refer to the substance at the equilibrium state in the organic phase and aqueous phase respectively.All the experiments were conducted at ambient atmospheric pressure.
3.Results and Discussions
3.1.Determination of the extracted complexes
In this study,a saturation extraction capacity method was used to determine the complex formed during the extraction process by using the partly saponified EHEHPA.By computing EHEHPA to REE mole ratio in organic phase under the saturation extraction conditions,the formation of the complex was obtained.
The extraction capacities of different EHEHPA concentration solutions were evaluated by different REE concentration solutions with different initial aqueous p H.The extraction results of the lanthanum ion are show n in Fig.1.It is clear that the lanthanum ion equilibrium concentration in the organic phase,[La]eo,increased with the grow th of initial concentration of lanthanum ion,[La]ia,and became stable w hen[La]iawas larger than 0.0400 mol·L-1for 0.2877 mol·L-1[EHEHPA],and 0.0800 mol·L-1for 0.5754 mol·L-1[EHEHPA].As for the results of 0.8631 mol·L-1[EHEHPA],[La]eodid not reach any stable values.Fig.1 also shows the effect of initial aqueous p H on[La]eo.When[La]iawas less than 0.0100 mol·L-1for 0.2877 mol·L-1[EHEHPA]and 0.0400 mol·L-1for 0.5754 mol·L-1[EHEHPA],the p H effect was not obvious due to the high EHEHPA concentration.As the initial aqueous p H increased from 1.00 to 4.00,[La]eoincreased and became stable w hen p H was larger than 2.50 for 0.2877 mol·L-1[EHEHPA]and 2.00 for 0.5754 mol·L-1[EHEHPA].Fig.2 show s the extraction results of cerium ion with similar trend found.
In order to obtain the molecular formula of the complex,the EHEHPA to REE mole ratio under the stable conditions was determined.To describe easily,[RE]avwas defined as the mean value of[RE]eoof 0.080 and 0.100 mol·L-1[RE]iawith the same initial aqueous p H values,at which the[RE]eoreached the maximum and stable values.Ratio R was calculated by dividing the[EHEHPA]with[RE]av.
Table 2 show s the[La]avand R values with different[La]iaunder different extraction conditions.It indicates that,as the initial aqueous p H increased,[La]avshow ed an increase trend,and stabilized at 0.042 mol·L-1for 0.2877 mol·L-1EHEHPA,and 0.085 mol·L-1for 0.5754 mol·L-1EHEHPA.R stabilized at 6.8.Table 3 show s the[Ce]avand R with different[Ce]iaunder different extraction conditions.[Ce]avincreased as the initial aqueous phase p H increased,and also stabilized at 0.041 mol·L-1for 0.2877 mol·L-1EHEHPA,and 0.077 mol·L-1for 0.5754 mol·L-1EHEHPA.R stabilized at 7.6.The difference of stabilized R between Laand Cemay becaused by the initial aqueous p H.
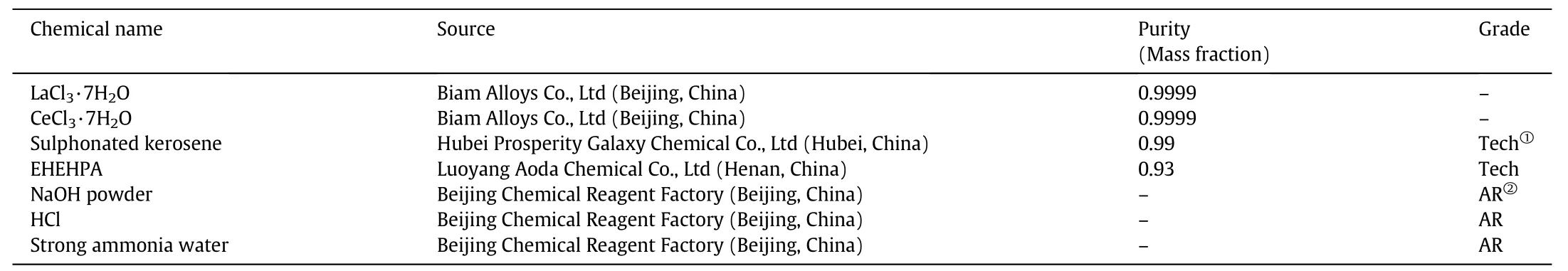
Table 1 Chemical sample details

Fig.1.The effects of initial aqueous concentration of lanthanum,[La]ia,initial aqueous p H,p H i,and EHEHPA concentration with 0.3(mole fraction)of EHEHPA saponified,[EHEHPA],on lanthanum ion equilibrium concentration in the organic phase[La]eo,(a)[EHEHPA]=0.2877 mol·L-1;(b)[EHEHPA]=0.5754 mol·L-1;(c)[EHEHPA]=0.8631 mol·L-1.
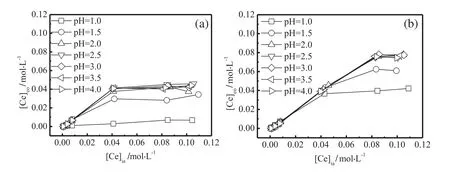
Fig.2.The effects of initial aqueous concentration of cerium,[Ce]ia,initial aqueous pH,p H i,and EHEHPA concentration with 0.3(mole fraction)of EHEHPA saponified,[EHEHPA],on cerium ion equilibrium concentration in the organic phase[Ce]eo,(a)[EHEHPA]=0.2877 mol·L-1;(b)[EHEHPA]=0.5754 mol·L-1.
Previous study[20]show ed that the extraction process of REEs with un-saponified EHEHPA can be described as:

w here H2L2is the dimmer of the EHEHPA;RE(HL)3L3is the complex,the subscripts(o)and(aq)refer to the organic and aqueous phases respectively.The R of the complex formed by the unsaponified EHEHPA is 6 which is close to the R obtained from Table 2 and 3.Compared with the complex formed by un-saponified extractants,it can be seen that the saponification may not affect the complex structure.As the EHEHPA is one kind of organic weak acid,the saponified EHEHPA can be written as NH4Lw hen the strong ammonium water is used as saponifier.Thus,the extraction process with partly saponified EHEHPA can be described as:

3.2.Equilibrium aqueous pH during extraction process
The equilibrium aqueous p H values,p Hefor lanthanum extraction process are show n in Fig.3.Fig.3(a)show s that p Heincreased with the grow th of the initial aqueous p H values,p Hi.For cases of 0.001 to0.010 mol·L-1[La]ia,the p Heabruptly ascended above 6.00 as p Hiincreased from 1.00 to 1.50.After that,the p Hebecame stable.In addition,the aqueous phase became turbid.The hydrolysis of La(III)occurred.For the case of 0.050 mol·L-1[La]ia,the pHelinearly increased as the p Hiincreased from 1.00 to 3.00,after that,p Hebe camest able.For cases of 0.100 and 0.120 mol·L-1[La]ia,p Heincreased mildly with the grow th of p Hi,and became stable as p Hiabove 2.50.Comparing Fig.3(a)and(b),increasing the EHEHPA concentration could increase the pHe.In Fig.3(b),for cases of 0.001 to 0.050 mol·L-1[La]ia,hydrolysis of La occurred.p Heincreased linearly with the p Hifor 0.100 mol·L-1[La]ia.In Fig.3(c),p Hebecame higher than the others.And the p Heincreased linearly for 0.120 mol·L-1[La]ia.Fig.4(a)show ed the extraction results of Ce(III),for cases of 0.001-0.010 mol·L-1[Ce]ia,hydrolysis occurred.p He increased linearly for case of 0.05 mol·L-1[Ce]ia.In Fig.4(b),w hen[La]iawas in the range of 0.001 to 0.050 mol·L-1,p Hewas higher than 6.20,and hydrolysis occurred.For cases of 0.100 and 0.120 mol·L-1[Ce]ia,the p He increased as p Higrew from 1.00 to 2.50,after that,became stable.

Table 2[La]av and R during extraction process with different EHEHPA concentrations,[EHEHPA],initial aqueous p H,p Hi,and initial aqueous La concentrations,[La]ia
The REE extraction process with partly saponified EHEHPA should avoid the hydrolysis process of the REEs which affects the extraction process greatly.The equilibrium aqueous p H,p He,is one key parameter indicating the hydrolysis phenomenon.When p Heis above the REE hydrolyzing p H,the REEs will precipitate.
By evaluating the equilibrium aqueous p H values in Figs.3 and 4,all of the p Hecan be divided into following three zones:Zone I,p He>6.00(La),or 6.20(Ce);Zone II,p Hi<p He<6.00(La)or 6.20(Ce);and Zone III,p He<p Hi.
In Zone I of Figs.3 and 4,the equilibrium aqueous phase became turbid.The partly saponified extractant in the organic phase was aptto hydrolyze with the hydrogen ions in the aqueous phase and turned to be an un-saponified extractant,which led to the increase of p Hegreatly:

Table 3[Ce]av and R during extraction process with different EHEHPA concentrations,[EHEHPA],initial aqueous p H,p Hi,and initial aqueous Ce concentrations,[Ce]ia

When the p Hereached the REE hydrolysis p H,6.00 for lanthanum ion and 6.20 for cerium ion,a hydrolysis process occurred,responsible for the formation of the aqueous turbid phase(Eq.(5)).Another reason for the appearance of turbid equilibrium aqueous phase is that the partly saponified extractant,a kind of surfactant,could easily form micelles in the aqueous phase.

In Zone II,the p Hewas larger than p Hiwhich means hydrogen ion decreasing reaction dominated.How ever,the aqueous phase was clear.Similar to Zone I,the p Heincreased due to a hydrolysis of the saponified extractant(Eq.(4)).The REE reaction with saponified EHEHPA(Eq.(3))was the dominating one.Meanwhile,the unsaponified extraction process existed(Eq.(2)).However,the released hydrogen ions reacted with the saponified extractant(Eq.(4)),which turned the un-saponified reaction to be a saponified reaction.
In Zone III,the p Hewas smaller than thep Hi,and the overall effect of the reactions increased the hydrogen ion concentration,which means that all of the saponified EHEHPA reacted completely(Eq.(3)).The un-saponified EHEHPA began to react with REE(Eq.(2)),and the cation exchange reaction released hydrogen ion which led to the increase of the acidity in the aqueous phase.

Fig.3.The effects of initial aqueous p H,p Hi,initial aqueous concentration of lanthanum,[La]ia,and EHEHPA kerosene solution with 0.3(mole fraction)EHEHPA saponified,[EHEHPA],on the equilibrium aqueous p H,p He,(a)[EHEHPA]=0.2877 mol·L-1;(b)[EHEHPA]=0.5754 mol·L-1;(c)[EHEHPA]=0.8631 mol·L-1.
3.3.Modeling
In this part,the extraction processes of Zone II and III are modeled by the controlling mechanisms.In order to realize the modeling of extraction process,the following assumptions are made:
(1)When the extractant is partly saponified,the REE ion is preferably reacting with the saponified extractant;w hen the saponified extractant is used up,the un-saponified extractant begins to react.
(2)The mechanism of Zone II can be illustrated by Eqs.(3)and(4),and other relative reactions can be neglected.
(3)The mechanism of Zone III can be illustrated by Eqs.(2)and(3),and other relative reactions can be neglected.
According to the law of mass action,the extraction equilibrium constants of Eqs.(2),(3)and(4)are set as K1,K2,and K3:

Fig.4.The effects of initial aqueous p H,p Hi,initial aqueous concentration of cerium,[Ce]ia,and EHEHPA kerosene solution with 0.3(mole fraction)EHEHPA saponified,[EHEHPA],on the equilibrium aqueous p H,p He,(a)[EHEHPA]=0.2877 mol·L-1;(b)[EHEHPA]=0.5754 mol·L-1.

The square bracket represents the concentration of the matter.
3.3.1.Modeling of cases in Zone II
From Eqs.(7)and(8),relationship between distribution ratio D of the REE and the equilibrium aqueous p Hecan be derived as:

with K=1/2lg K2-3/2lg K3.
According to assumption(1),the equilibrium concentration of the H2L2can be represented as:

where[H2L2]iis the initial concentration of the un-saponi fied EHEHPA;[RE3+]iis the initial aqueous REE concentration;[H+]iis the initial aqueous acidity and[H+]eis the equilibrium aqueous acidity.Thus,K can be calculated as:

K values can be calculated from Eq.(11).The initial concentration of theun-saponified dimmer of EHEHPA,the initial REE concentration,and the initial aqueous p H values can be obtained before the experiments.The equilibrium aqueous acidity can be obtained by a p H meter.The K values used for calculation are listed in Table 4.The nonlinear equations Eqs.(9)and(10)can be solved by using MATLAB software to calculate the lg Dcal.Fig.5 show s the comparison between the experimental results and the calculated ones.It can be seen that w hen lg Dexpis lower than 2,the experimental results agree well with the semi-empirical model for both lanthanum and cerium,and most relative error is within±30%.
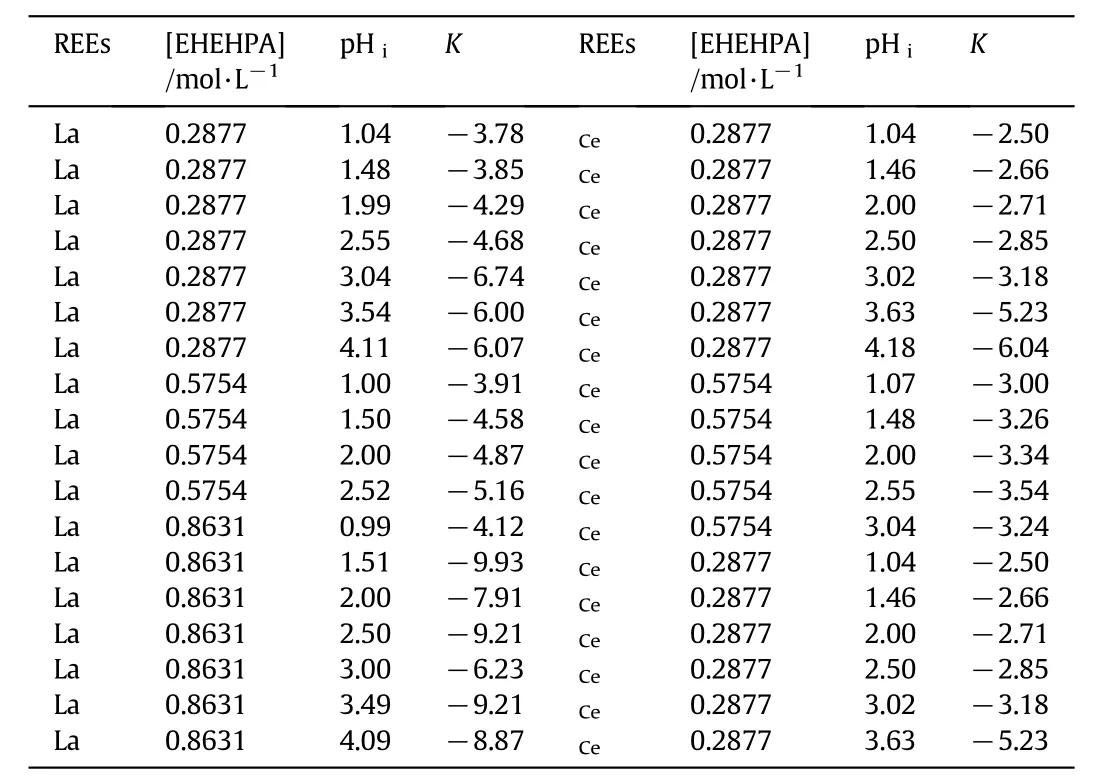
Table 4 K①used in calculation models for REEs extraction process

Fig.5.Comparison between the experimental and the calculation results for zone II.
3.3.2.Modeling of cases in Zone III
According to the assumption(1)and(3),the cases in Zone III can be illustrated by Eqs.(2)and(3).The relationship between the distribution ratio and equilibrium p H values can be represented as:

w here K′=lg K2;[H2L2](o)is obtained by subtracting the components of extractant reacted with the saponified extractant and the portion reacted individually with REEs.Thus,[H2L2](O)can be expressed as:

w here[EHEHPA]is the total mass of the EHEHPA before extraction process(including the saponified fraction);[RE3+]irepresenting the initial RE3+concentration.Thus K′can be calculated as:

K′values can be obtained from Eq.(14).The initial concentration of the total EHEHPA and the initial concentration of REEs can be obtained before extraction.The equilibrium aqueous p H can be measured.The K′values used for calculation are listed in Table 5.lg Dcalcan be calculated according to Eqs.(12)and(13)by MATLAB.Fig.6 show s the comparison between the experimental and calculated results.It can be seen that theexperimental results agree w ell with the model prediction for lanthanum and cerium,since the largest relative error is smaller than 20%.

Table 5 K′① used in calculation models for REEs extraction process
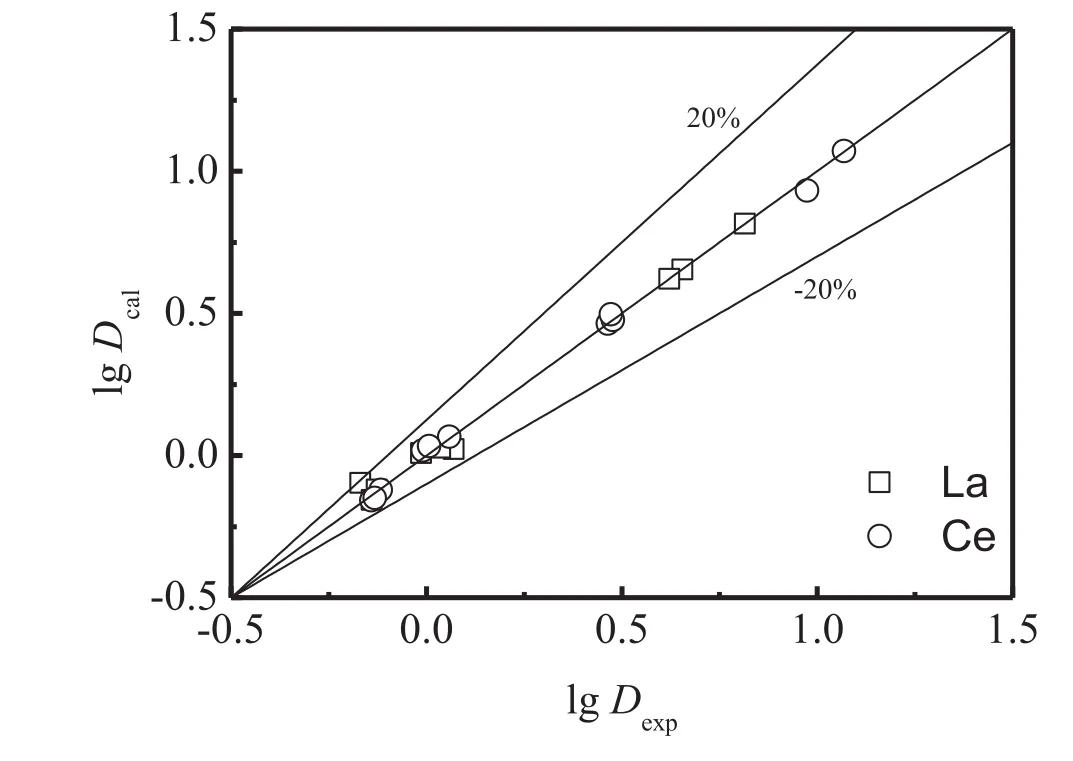
Fig.6.Comparison between the experimental and the calculation results for zone III.
4.Conclusions
In this paper,lanthanum and cerium extraction experiments using partly saponified EHEHPA were conducted.The REE aqueous solutions with aconcentration in a range of 0.0010 to 0.1000 mol·L-1and the initial aqueousp Hin a range of 1.00-4.00 were used as the aqueous phase.The EHEHPA with 0.3(mole fraction)saponified in kerosene solutions in a range of 0.2877 to 0.8631 mol·L-1were used as the organic phase.The mechanism of extraction process using partly saponified EHEHPA was clarified.The results show ed that the complex formed with saponified EHEHPA was the same as the one that formed with the un-saponified EHEHPA.According to the equilibrium aqueous p H,the extraction by using partly saponified EHEHPA can be divided into three processes:extraction with saponified EHEHPA,extraction with un-saponified EHEHPA,and hydrolysis process.On the basis of mechanism,semi-empirical models were developed to predict the REE distribution ratio between two phases.The calculation data agreed w ell with the experimental results.
[1]J.S.Preston,The recovery of rare earth oxides from a phosphoric acid byproduct.Part 4.The preparation of magnet-grade neodymium oxide from the light rare earth fraction,Hydro metallurgy 42(2)(1996)151-167.
[2]J.A.Z.Pieterse,H.Top,F.Vollink,K.Hoving,R.W.van den Brink,Selective catalytic reduction of NO x in real exhaust gas of gas engines using unburned gas:Catalyst deactivation and advances toward long-term stability,Chem.Eng.J.120(1-2)(2006)17-23.
[3]X.H.Cao,J.Ren,C.L.Xu,K.H.Zhang,C.C.Zhan,J.Lan,Preparation,characterization of Dawson-type heteropoly acid cerium(III)salt and its catalytic performance on the synthesis of n-butyl acetate,Chin.J.Chem.Eng.21(5)(2013)500-506.
[4]H.H.Cheng,H.G.Yang,S.L.Li,X.X.Deng,D.M.Chen,K.Yang,Hydrogen storage properties of melt-spun LaNi4.25Al0.75,J.Alloys Compd.458(1-2)(2008)330-334.
[5]T.Xu,H.Q.Peng,Formation cause,composition analysis and comprehensive utilization of rare earth solid wastes,J.Rare Earths 27(6)(2009)1096-1102.
[6]H.C.Kao,P.S.Yen,R.S.Juang,Solvent extraction of La(III)and Nd(III)from nitrate solutions with 2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester,Chem.Eng.J.119(2-3)(2006)167-174.
[7]G.S.Lee,M.Uchikoshi,K.Mimura,M.Isshiki,Distribution coefficients of La,Ce,Pr,Nd,and Sm on Cyanex 923-,D2EHPA-,and PC88A-impregnated resins,Sep.Purif.Technol.67(1)(2009)79-85.
[8]L.Pei,L.M.Wang,X.L.Fu,Separation of Eu3+using a novel dispersion combined liquid membrane with P507 in kerosene as the carrier,Chin.J.Chem.Eng.19(1)(2011)33-39.
[9]W.D.Zhang,C.H.Cui,Y.Q.Yang,Mass transfer of copper(II)in hollow fiber renewal liquid membrane with different carriers,Chin.J.Chem.Eng.18(2)(2010)346-350.
[10]W.W.Wang,Y.Pranolo,C.Y.Cheng,Recovery of scandium from synthetic red mud leach solutions by solvent extraction with D2EHPA,Sep.Purif.Technol.108(2013)96-102.
[11]W.W.He,W.P.Liao,C.J.Niu,D.Q.Li,Synergistic extraction of rare earthsusing acid-base coupling extractants of Calix[4]arene carboxyl derivative and primary amine N1923,Sep.Purif.Technol.62(3)(2008)674-680.
[12]W.Wang,Y.Liu,A.M.Xu,H.L.Yang,H.M.Cui,Solvent extraction of yttrium by taskspecific ionic liquidsbearing carboxylic group,Chin.J.Chem.Eng.20(1)(2012)40-46.
[13]G.X.Xu,C.Y.Yuan,Solvent Extraction of Rare Earths,Science press,Beijing,1987.142.
[14]M.Ochsenkuhnpetropulu,T.Lyberopulu,G.Parissakis,Selective separation and determination of scandium from yttrium and lanthanides in red mud by a combined ion exchange/solvent extraction method,Anal.Chim.Acta 315(1-2)(1995)231-237.
[15]N.V.Thakur,D.V.Jayaw ant,N.S.Iyer,K.S.Koppiker,Separation of neodymium from lighter rare earths using alkyl phosphonic acid,PC-88A,Hydrometallurgy 34(1)(1993)99-108.
[16]N.B.Devi,K.C.Nathsarma,V.Chakravortty,Separation and recovery of cobalt(II)and nickel(II)from sulphate solutions using sodium salts of D2EHPA,PC 88A and Cyanex 272,Hydrometallurgy 49(1-2)(1998)47-61.
[17]K.Sarangi,B.R.Reddy,R.P.Das,Extraction studies of cobalt(II)and nickel(II)from chloride solutions using Na-Cyanex 272.Separation of Co(II)/Ni(II)by the sodium salts of D2EHPA,PC88A and Cyanex 272 and their mixtures,Hydrometallurgy 52(3)(1999)253-265.
[18]N.B.Devi,K.C.Nathsarma,V.Chakravortty,Separation of divalent manganese and cobalt ions from sulphate solutions using sodium salts of D2EHPA,PC 88A and Cyanex 272,Hydrometallurgy 54(2-3)(2000)117-131.
[19]M.S.Lee,J.Y.Lee,J.S.Kim,G.S.Lee,Solvent extraction of neodymium ions from hydrochloric acid solution using PC88A and saponified PC88A,Sep.Purif.Technol.46(1-2)(2005)72-78.
[20]G.X.Xu,C.Y.Yuan,Solvent Extraction of Rare Earths,Science press,Beijing,1987.143.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Scoping biology-inspired chemical engineering☆
- Review on the nanoparticle fluidization science and technology☆
- Multi-functional forward osmosis draw solutes for seawater desalination☆
- Bio-inspired enantioseparation for chiral compounds☆
- Process engineering in electrochemical energy devices innovation☆
- In-situ design and construction of lithium-ion battery electrodeson metal substrates with enhanced performances:A brief review☆
