Process engineering in electrochemical energy devices innovation☆
2016-05-29YingyingXieWeiminZhangShuangGuYushanYanZiFengMa
Yingying Xie ,Weimin Zhang ,Shuang Gu ,Yushan Yan ,Zi-Feng Ma ,*
1 Shanghai Electrochemical Energy Devices Research Center,Department of Chemical Engineering,Shanghai Jiao Tong University,Shanghai 200240,China
2 Department of Chemical and Biomolecular Engineering,University of Delaware,Newark,DE 19716,USA
1.Introduction
To address the growing challenges from depleting fossil fuel reserves,increasing world population,grow ing expectations for high living standards,and rising concerns over air quality and climate change,one possible solution is to develop an alternative energy system that is safe,clean,and sustainable,in place of the current major energy system that is based on the combustion of fossil fuels.Combustion has played a leading role in energy conversion throughout human history.In a combustion process,reduction and oxidation(redox)reactions are coupled together,and electrons are transferred directly from the fuel to the oxidant to produce heat.By contrast,in an electrochemical process,the redox reactions are spatially separated by an electrolyte,allowing electrons to do work as electricity which leads to intrinsically higher energy conversion efficiency yet in relatively milder conditions,compared with combustion process.Electrochemical energy engineering can be regarded as a sub-discipline of chemical engineering,and it is also closely related to process engineering.
Electrochemical energy engineering research fields typically include:(1)electro catalysis and electrode reaction kinetics;(2)electrolyte materials and ion transport;(3)device design and the corresponding electrode materials and manufacturing;(4)electrochemical energy system engineering;and(5)electrochemical energy device evaluation technologies.As a way to timely highlight the research work in the innovation for the process engineering in electrochemical energy devices,an integrated electrochemical energy system is envisioned that consists of the following electrochemical energy devices:energy storage(ES),fuel cells(FCs),electrolyzers(ELs),and solar hydrogen generators(SHs)(Fig.1).
Solar panels and w ind turbines can provide clean and renew able electricity and they are integrated into electric grid which is equipped with electrochemical energy storage devices,such as lithium-ion batteries and redox flow batteries.Fuel cells coupled with solar hydrogen generators and/or water electrolyzers can provide clean power for transportation and buildings.With minor variations,all the aforementioned electrochemical devices have the same basic three-layer structure(electrode/membrane/electrode)[1].
This article provides a perspective,rather than a comprehensive review.Hence,we use mostly our ow n recent work as examples to show the strategic switch of polymer electrolytes from PEMs to HEMs for fuel cells,and to demonstrate the innovative electrode designs and manufacturing for lithium-ion batteries.
2.From PEM Fuel Cell to HEM Fuel Cell
2.1.Key features of HEM fuel cells

Fig.1.An integrated,safe,clean,and sustainable electrochemical energy system based on fuel cells(FCs),solar hydrogen generators(SHs),electrolyzers(ELs),and energy storage(ES).With minor variations,all the aforementioned electrochemical devices share the same basic three-layer structure:electrode/membrane/electrode.Adapted from the Ref.[1].
Fuel cells are electrochemical devices which convert chemical energy stored in fuels to electricity.Fuel cells are intrinsically superior to combustion for chemical-to-electrical energy conversion in terms of conversion efficiency and environmental friendliness.Owing to the separation between the two electrodes,fuel oxidation,oxidant reduction,ion conduction through the electrolyte,and electric load can be engineered individually,and such independent engineering of each component creates excellent flexibility in device design and diagnosis.Fuel cells have been considered as promising power sources for stationary and vehicular applications.In particular,hydrogen-fueled low-temperature(typically below 100°C)fuel cells are very attractive because hydrogen has high specific energy(34 k W·h·kg-1,or 2.6 times that of gasoline),and the use of hydrogen can be free of carbon footprints if hydrogen is obtained from solar water splitting or other renew able methods.
Usually,the nature of the electrolyte used in the fuel cell dictates the selection of its electrode materials and the operational conditions.Therefore,the fuel cells are generally categorized by their electrolytes utilized.
In 1950,solid polymer electrolytes,i.e.,PEMs(based on a sulfonated polystyrene polymer),were invented[2].Similar to liquid acids,solid PEMs are also conductive for protons but are much more convenient and safer to handle for fuel cell applications.PEMs are completely free of electrolyte leakage problems.For these reasons,PEMs were quickly adopted as a new category of electrolytes into fuel cells(i.e.,PEM fuel cells,or PEMFCs)replacing the liquid acids in 1955[3].
Na fion's ability to serve as both membrane and ionomer and its outstanding chemical,thermal,and mechanical stability have enabled PEMFCs to achieve high specific power(above 1 kW·kg-1)and good device durability(over thousands of hours)[4].The success of PEMFCs has stimulated the research and commercialization interests in fuel cells over the past half-century.
PEMs provide a proton-mediated environment for electrode reactions in PEMFCs and thus precious metals(typically Pt)are demanded as electrocatalysts for PEMFCs.Largely due to their thermodynamic instability in proton-mediated environments,stable yet active nonprecious metal-based electrocatalysts have been very challenging to develop for PEMFCs[5-7].Nonprecious metals have much better stability in base than in acid,and they are also more active in base.For example,Ni and Ag were successfully demonstrated as active and chemically stable nonprecious HOR and ORR electrocatalysts,respectively,in strongly alkaline solutions[8].The idea of introducing HEMs,the counterpart of PEMs,to fuel cells can be traced back as early as PEMFCs.In part due to the great success of PEMFCs,not much work has been carried out in developing HEM fuel cells(HEMFCs).To eliminate the heavy dependence on precious metals of PEMFCs,the concept of HEMFCs was brought back to the fuel cell research community in 2001[9,10].The working principle of HEMFCs is show n in Fig.2,and electrode reactions are as follow s(based on p H=14;SHE,standard hydrogen electrode):

And the complete(four-electron)O2reduction in alkaline environment is:
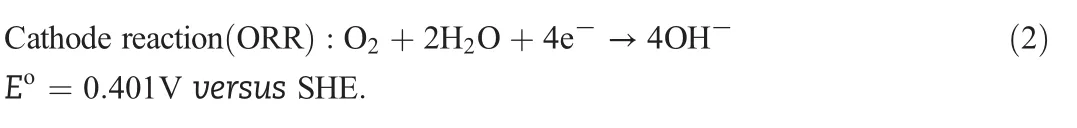
As polymer electrolytes,HEMs are convenient and safe to handle,similar to PEMs.The advantage of HEMFCs over PEMFCs is their ability to work with nonprecious metal-based electrocatalysts,enabled by the hydroxide-mediated environment in HEMs.
2.2.Key components of HEMFCs
2.2.1.HEMs and HEIs
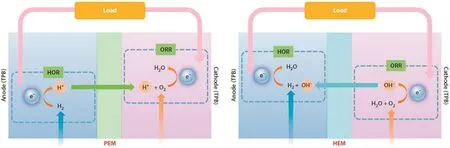
Fig.2.Working principles of PEM fuel cells(left)and HEM fuel cells(right).Abbreviations:HOR,hydrogen oxidation reaction;ORR,oxygen reduction reaction;HEM,hydroxide exchange membrane;TPB,triple phase boundary.Reprinted from the Ref.[1].
An HEM is placed between the anode and the cathode,and it plays three major roles:a)an ionic conductor offering hydroxide transport between the two electrodes to continue their electrochemical reactions;b)an electrical insulator preventing electron conduction between the two electrodes to avoid short circuit;and c)a physical separator isolating between the fuel and the oxidant to avoid direct chemical reactions.As a solubilized form of HEMs,hydroxide exchange ionomers(HEIs)are dispersed among the catalyst particles within the catalyst layer of the electrodes,creating the ionic phase of the triple phase boundary(TPB)required for high-performance HEMFC operation.The ionic phase created by HEIs provides not only the hydroxide environment for the catalysts to mediate electrochemical reactions but also the ion-conducting channels connecting between the catalytic sites to the bulk HEM,completing the hydroxide transport from one electrode to the other.
2.2.2.Nonprecious metal ORR and HOR electrocatalysts
Both ORR and HOR electrocatalysts are required to catalyze the two electrodereactions simultaneously required in fuel cell operations.Generally,the ORR kinetics is considered to be more facile in alkaline media than in acidic environments,regardless of metal catalyst used.The electrochemical reduction of an oxygen molecule can go through either complete(four-electron)pathway or incomplete(two-electron)one,demonstrating complex electrocatalytic reactions.The four-electron reaction pathway is much more preferred for ORR electrocatalysts than the two-electron reaction pathway,in part because the two-electron pathway produces hydrogen peroxide anions(HO2-)in alkaline media which can attack organic polymers causing electrolyte degradations.
In general,HOR kinetics has been widely con firmed to be much faster than that of ORR in both acid and base,albeit the HOR kinetics on Pt is around two orders of magnitude slower in base than in acid.Encouragingly,Ni-based nanoparticles have been introduced as nonprecious HOR electrocatalysts for HEMFCs[11,12],con firming the general applicability of nonprecious metal-based catalysts in HEMFCs.
2.3.Key challenges of HEMFCs
2.3.1.Development of highly durable HEMs/HEIs
Sufficient durability of HEMFCs is required for commercial applications.How ever,there is a significant gap between the demonstrated HEMFC durability and the required one.The state-of-the-art HEMFCs show a durability of less than 500 h,with ahigh cell voltagedegradation rate of over 1000 μV·h-1at a cell temperature at 50 °C[13].Such a degradation rate of HEMFCs is one order of magnitude higher than that of PEMFCs(<100 μV·h-1).The limited HEMFC durability observed may have been caused primarily by HEI degradation in electrodes,because the dispersed HEIs at the TPB are much more stressed than the bulk HEMs.The degradation mechanisms of HEIs in membrane electrode assemblies(MEAs)have been considered one of the most important research topics,and the understanding of fundamental degradation behaviors is a prerequisite for developing the next-generation highperformance and durable HEM/HEI polymer electrolytes.
Alternative polymer backbones and novel cationic groups have been explored to design new HEMs/HEIs.For example,an HEM prepared based on poly(ether ether ketone)(PEEK)backbone by blending imidazolium-functionalized PEEK and sulfonated PEEK,show ed high ion-exchange capacity(IEC)as well as fairly high hydroxide conductivity(up to 31.59 m S·cm-1at 30 °C)[14].Quaternary phosphonium-functionalized PEEK(PEEK-QPOH)HEMs were successfully prepared by a solution casting method.PEEK-QPOH membranes show ed very high hydroxide conductivity,e.g.,a PEEK-QPOH 126%membrane exhibited 61 and 89 m S·cm-1at 20 °C and 60 °C,respectively[15].
2.3.2.Development of highly active nonprecious metal-based anode electrocatalysts
In general,HOR activity of many metals is lower in alkaline media than in acidic ones,and there are tw o possible w ays to increase HOR activity:One strategy is to increase the exchange current density by tuning the metal-hydrogen binding energy,because the exchange current density on metal surfaces can be w ell correlated with their metal-hydrogen binding energies[16,17];the other strategy is to decrease the Tafel slope by tuning charge transfer coefficient.The understanding of metal-hydrogen binding energy and charge transfer coefficient is the heart of electrode kinetics.
In addition to HOR catalysts,other anode catalysts,such as alcohol oxidation catalysts,are also useful since HEMFCs can also work with alcohols or other complex compounds as fuels without much concern of fuel crossover.For example,a Pd87Cu13/Celectrocatalyst was recently developed with higher catalytic activity in alkaline media than Pd/C for the electrochemical oxidation of three types of polyalcohols:ethylene glycol,propylene glycol,and glycerol[18].
2.3.3.Development of highly active nonprecious metal-based cathode electrocatalysts
Development of nonpreciousmetal-based ORRcatalysts has been an activeresearch focus for HEMFCs.It should benoted however that many non-precious metal ORR catalysts are available that show similar or slightly better ORR activity than Pt/C.Therefore the goal of the development of non-precious metal ORR catalysts should be ones with much better activity than Pt/C.Some recent examples of ORR catalysts are as follow s.Guo and co-workers[19]developed anew method of preparing Fe/N/C electrocatalysts with soybean biomass as the nitrogen source,denoted as Fe/C-SOYB.The number of electron transfer was observed to be~3.1 under the electrode potential of-0.5 to-0.7 V vs.RHE in 0.1 mol·L-1KOH solution,which suggests that the ORR catalyzed by the prepared Fe/C-SOYB catalyst occurs via a mixed process of both two-and four-electron transfer pathways.A novel FeTEPA/C catalyst was first reported by Zhang and co-w orkers[20]who employed a simple N5 structure tetraethylenepentamine ligand as the nitrogen source.In 0.1 mol·L-1KOH solution,the ORR peak potential(Ep)and the peak current(Ip)observed were 0.978 V vs.RHE and-0.08 m A,respectively,for the prepared catalyst.The co-incorporation of S and N into multi walled carbon nanotubes(CNTs)and its effects on the ORR catalytic performance in alkaline electrolytes has been studied by Dominguez and co-workers[21].Chao and co-workers[22]developed a strategy that utilizes an octahedral Co(II)complex with 2,6-bis(benzimidazol-2-yl)pyridine(BBP)as the precursor to prepare Co/N-co-doped carbon nonprecious metal catalyst.The catalysts have a unique hollow ed-out octahedral structure(Co/N-HCOs),and comparable ORR activity to Pt/C in alkaline media.
Wang and co-workers[23]also prepared a carbon-supported cobalteiron(II,III)oxide(Co-Fe3O4)hybrid nanoparticles(Co-Fe3O4/C)as efficient ORR catalyst in alkaline media,with the number of electron transfer of 3.99 over a very wide range of electrode potentials of 0.1-0.8 V vs.RHE.The Co-Fe3O4/C was also tested in HEMFCs showing good performance and excellent durability.This is one of the best results for the nonprecious ORR catalysts reported up to date.
3.Innovative Manufacturing of Materials and Device for Low Cost Lithium-ion Batteries
3.1.Key materials synthesis route and process engineering
Rechargeable lithium batteries are attractive power sources because of their high energy density and long operation life.Lithium-ion batteries have been widely used in portable electronic devices,such as cameras,mobile phones,and laptop computers.The large-scale lithium-ion batteries have great potential for electric vehicles,distributed energy storage systems.For the large-scale lithium-ion batteries,high performance key materials,such as electrode materials,electrolytes and separators,with low cost and improved safety need to be developed.The industrial synthesis routes of the key materials greatly depend on the process design and optimization.
3.1.1.Cathode materials—LiFePO4
Olivine LiFePO4(LFP), first reported by Padhi and co-workers[24],has attracted much attention as one of the lithium-ion cathode materials.The electrochemical performance of LiFePO4/C cathode is closely related to its surface area,particle morphology,size and size distribution,carbon content,carbon morphology,phase purity,and so on,which are dependent on its synthesis route[25].Various preparation methods have been reported for LiFePO4and LiFePO4/C,such as hydrothermal synthesis,sol-gel synthesis process,carbothermal reduction technology[26],supercritical hydrothermal method[27]and mechanochemical activation[28].How ever,the selection of the starting materials for the iron source has proved most important in the synthesis route.It not only affects the conditions for the preparation process but also its commercial viability.In our previous w ork,a novel synthesis route based on ball milling process for high-performance LiFePO4/C cathode materials has been developed,and the mixtures of metal iron pow der,FePO4and Li3PO4·2H2O were used as the starting materials with sucrose added as a conductive phase precursor.The resulting product is only LiFePO4/C,and it show s excellent cycle performance with a high capacity of 164 m A·h·g-1at 0.1C discharge rate and 137 m A·h·g-1at 1C discharge rate,respectively,with 1C being the theoretical current density of full discharge in 1 h,namely 172 m A·g-1[29].The effect of ball milling time and the type of liquid medium on the electrochemical performance of the LiFePO4/C cathode material were investigated in depth.Different ways of ball milling such as dry ball milling and w et ball milling in benzene or acetone,various ball milling durations from 0 to 8 h,have been tested with Li2CO3,NH4H2PO4,and FeC2O4as raw materials and citric acid as organic carbon source.The optimized parameters to the cathode materials with initial discharge capacities of 153 and 120 mA·h·g-1at 0.1 and 10C rates at room temperature were obtained under the conditions of w et milling in acetone liquid medium for 4 h follow ed by thermal treatment at 700°C[30].In many studies and manufacturing processes of LiFePO4/C production,FePO4has been often adopted as a precursor.Moreover,FePO4contains iron and phosphate in a single precursor,therefore reducing the number of phases in the solid state reaction which may accelerate the calcination process.Recently,nano-FePO4was generated by a novel method using a con fined area impinging jet reactor,w here rapid mixing creates a uniform supersaturation distribution for nucleation,follow ed by crystal grow th and other particle processes to take place in an environment with an uniform temperature and pressure distribution[31].
Rate performance of lithium-ion cathode materials is one of the important factors for electric vehicle application.Many efforts have been devoted to enhancing the rate capability.The effects of fluorine substitution on the electrochemical properties of LiFePO4/Ccathode materials were investigated,and the results indicated that F-substitution can improve the rate capability[32].Graphene wrapped LiFePO4/C composites also show good rate capability because of the conductivity improvement[33].Nevertheless,low-temperature performance is still a challenging requirement for LiFePO4cathode material,and new electrolytes are in demand to improve the low-temperature performance of lithium-ion cells.A number of ternary and quaternary,all carbonate-based electrolytes,such as LiPF6/EC+DEC+DMC+EMC,have been demonstrated useful to improve the low-temperature performance[34].Besides the electrolyte,nano-LFP has show n much better low-temperature performance than micro-LFP.Thus,a simple process using low cost precursors to mass produce high quality nano-LFP/C is likely to trigger a revolution.Based on the above analysis,the manufacturing process of nano-LiFePO4/C cathode material was designed,and the flow chart is show n in Fig.3.The full cell tests were conducted with a 1 A·h pouch cell using carbonaceous mesophase spherule(CMS)as anode,and the electrolyte solution was 1 mol·L-1LiPF6in ethylene carbonate_diethyl carbonate(1:1 in volume).The electrochemical performances of the full cell,using nano-LiFePO4/C cathode of 7 g active material,are show n in Fig.4.The nano-LiFePO4/C material show s excellent cycle performance at 1C discharge rate,and the discharge capacity kept over 90%after 900 cycles.
3.1.2.Anode materials
Various carbon based lithium insertion materials,such as hard carbon,purified flake natural graphite,synthetic graphite,graphitic carbon,have been studied as anode active materials for rechargeable lithium batteries.Most graphitic carbonaceous anode materials include mesocarbon microbeads(MCMB)or carbonaceous mesophase spherule(CMS)are prepared by liquid-or gas-phase pyrolysis from various organic resins or hydrocarbon precursors at high temperatures[35].Recently,we made use of commercial Si powder and prepared a novel bath lily-like graphene sheet w rapped nano-Si(GS-Si)by a simple spray drying method.In this process,no surfactant, filtration or washing processes were required,not to mention high vacuum conditions.This process is safe and environmentally friendly.The GS-Si composite was tested as an anode material for lithium ion battery.The as-synthesized composite exhibits a high reversible capacity of 1525 m A·h·g-1and superior cycling stability.These encouraging results make spray drying one of the most suitable methods for large-scale preparation of graphene-based materials[36].
The GS-Si prepared by spray drying can be thermally treated at a mild temperature of 700°C,and such strategy can also be used to fabricate 3D crumpled GS-w rapped Fe2O3composites,where,a certain amount of nano-Fe2O3pow der mixed with the graphene oxide(GO)suspension and then were spray-dried to form a Fe2O3@GO composite.It was found that the crumpled GS around Fe2O3particlescould not only provide a 3D conductive matrix but also buffer the volume change of Fe2O3during cycling[37].The spray drying process was further optimized to produce more anode materials.For example,spherical GS-encapsulated Li4Ti5O12nanoparticle composites were prepared by a one-pot spray-drying assisted solid-phase reaction process[38].As an anode material for a nonaqueous,hybrid battery-capacitor(BatCap)system,the Li4Ti5O12@GS composite exhibits good high-rate capability and long cycle life.The specific capacitance still retains90%of the initial value after 20000 cycles,while the Coulombic efficiencies maintained at 100%.This can reasonably be attributed to the interesting crumpled graphene-encapsulated structure and extraordinary synergistic effects between the tw o components.

Fig.3.Manufacturing process of nano-LiFePO4/C cathode materials.

Fig.4.Charge-discharge curves and cycling performance of as-prepared nano-LiFePO4/C.The data are provided by Jiangsu Lenengy Battery Co.Ltd.
3.2.Manufacturing process of lithium-ion batteries
In general,conventional lithium-ion batteries are fabricated by casting anode and cathode materials on various current collector(Al foil for cathode and Cu foil for anode,respectively),with an intermediate layer of a separator or polymer electrolyte.Electrode manufacturing is the key process,followed by the assembly and formation processes which may affect the quality of lithium-ion batteries.The modified manufacturing process of lithium-ion batteries improved by LG Chem is illustrated in Fig.5,where the separator is replaced by the nano-scaleceramic particle coated separator in order to improve the mechanical and thermal stability,and to combine stacking and folding process together to make cell so as to obtain safety and performance excellence[39].
In the electrode manufacturing process,unit operations such as paste mixing,coating and drying for electrode significantly influence the product quality and the process energy consumption.These operations can be improved and optimized based on the principles of chemical engineering.Conventional coating and solvent drying process consume large amounts of energy with a long tunnel dryer,resulting in significantly increased manufacturing costs.
Coating and drying process design is closely related to the battery chemistries.The components of electrode,such as active materials,binders,solvents and conductive agent,and the structure of the dryer play important roles on the electrode manufacturing quality and the batteries cost.Conventional slurry casting processes dominate the cost of lithium ion battery electrodes.Based on different binders and solvents or drying method,a series of electrode manufacturing processes are under development.For the lithium iron phosphate batteries,the processes include optimizing blend of phosphate and oxide cathode powders with respect to energy,safety and compatibility with dry electrode fabrication,modifying low-or zero-solvent binders for anodic compatibility,and optimizing the cathode and anode formulations.
Son[40]developed an integrated cell with non-N-methyl-2-pyrrolidone(NMP)electrodes with direct coated separators,and also planning to develop an additive and new formulation for process improvement to achieve electrode target with 50%manufacturing cost reduction.Wood and co-workers[41]replaced NMP processing with water-based chemistry for all active materials.The cost of electrode processing could be cut,primarily due to fast drying of water(i.e.higher solids loading,lower drying T,lower air flow rate&higher volatility than for NMP)and elimination of solvent recovery process.They also developed roll-to-roll electrode processing non-destructive evaluation and in-line electrode quality control method to reduce lithium secondary batteries system cost[42].An entire two-sided electrode coating&curing process was designed by Miltec Inc[43].They started with liquid UV curable mixture to demonstrate utilization of UV curable binder to produce lithium-ion batteries with performance equal to or greater than PVDF baseline(Fig.6).The results show that the Miltec's UV electrode coating process is simpler and can reduce the electrode manufacturing expense by 80%.
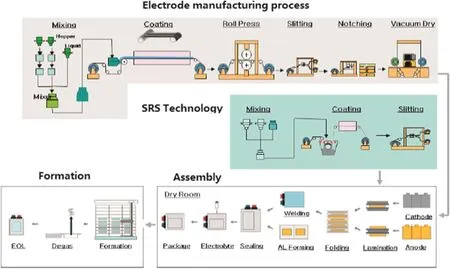
Fig.5.Modified industrial manufacturing processes of lithium-ion batteries[39].
Bae and co-workers[44]developed a scalable high density binder-free low-tortuosity electrode.The freeze casting process was successfully adopted to cathode materials in order to increase cell-level energy density compared to conventional lithium-ion technology.
4.Designs and Thermal Characterization of a High-power Lithium-ion Battery
4.1.Modeling and optimization of lithium-ion battery design
In order to realize their potential application,the lithium-ion batteries must be advanced through a disruptive technological development or a series of incremental improvements in design and chemistry.The most critical design parameters include energy density,power density,cost,lifetime,and safety.Each of the parameters depends on both system chemistry and system design.Traditional lithium-ion battery designs comprise individual cells based on an anode,an electrolyte,and a cathode,and the components of electrode containing active materials,additive,binder,and current collectors.A schematic of traditional composite electrode and their average mass breakdown is depicted in Fig.7.
One of the important factors determining the performance of lithium-ion battery is the cell internal resistance.This factor,including the resistances of most hardware w here current passes through,such as steel can,foil substrates,tabs,top header,etc.,is a w ell-know n issue.The influence of tab position and quantity as w ell as multi-segment electrodes in cell design for the performance of an 18650 power-oriented lithium-ion cell was investigated by Chen and coworkers[46].From the industrial view point,they established a simplified model.The resistances of cells with traditional and center-tab designs are simulated for rapidly estimating the influences of a change in the tab position on the extreme high-rate performance of a real 18650-type power-oriented lithium-ion battery.In addition to cylindrical cell(18650-type),the large-capacity pouch and prismatic cells have been designed for electric vehicle and energy storage application[47].For the large capacity lithium-ion battery design,the performance of a battery electrode is influenced by the ratio of components and the size as w ell as placement of the current collecting tabs.For developing low-cost battery case of lightweight,aluminum or plastic was used in the battery case design.

Fig.6.Comparison of conventional coating and drying process(a)and UV curing process(b)[43].

Fig.7.Schematic of components of a lithium-ion battery electrode(a)and Pie charts showing an average mass breakdown for a commercial lithium-ion battery(b)[45].
A two-dimensional modeling was developed to calculate the potential and current density distribution on the electrodes of a lithium-polymer battery[48].Based on the two-dimensional modeling,Kim and co-workers[49]reported a modified modeling to predict the potential,current density and temperature distributions of the electrodes of a large-scale battery.In their work,a lithium-ion polymer battery(LIPB)consisting of a Li[NiCoMn]O2positive electrode,a graphite negative electrode and a plasticized electrolyte was modeled.The two-dimensional temperature distributions from the experiment and model were in good agreement.By comparing the experimental discharge curves of 10 and 26 A·h LIPBs with the modeling results,it was con firmed that the parameters tuned for the electrodes of a small-scale battery can be applied for the electrodes of a large-scale battery provided that the materials and compositions of the electrodes as w ell as the manufacturing processes are the same.Therefore,an optimum design of the electrode is pertinent for the production of large-scale lithium-polymer batteries.They also studied the effect of local current density on electrode design.Wang and co-workers[50]used a simplified model based on the Doyle-New man general model to estimate the performance of LiFePO4electrode,and simulated the local current density,surface concentration and local state of discharge distribution across the cathode area at 1C discharge.A“critical thickness”was proposed as a parameter for battery design.Simultaneous multi-parameter optimization of battery design parameters using a physics-based porous electrode theory model has been implemented for the efficient design of porous electrodes[51].The simultaneous optimization of electrode design parameters can achieve a significant improvement in energy drawn from a battery,and the optimal design of state-of-the-art batteries for minimizing the temperature gradient across a cell for safe operation and prevention of thermal runaway.
4.2.Thermal modeling of lithium-ion batteries
Thermal modeling is an effective way to understand how the design and operating variables affect the thermal behavior of the lithium-ion battery during charging and discharging.Several two-dimensional and three-dimensional thermal models have been developed to predict and examine the thermal behavior of a lithium-ion battery[52,53].In the field of thermal modeling,two types of techniques have been considered:analytical techniques and numerical techniques.The former gives continuous solutions and can show explicitly how the parameters affect the solutions.Numerical techniques are employed for complex models depending on the design and the multidirectional heat transfer of the battery.Numerical methods are usually implemented with commercial software packages such as ANSYS Fluent,COMSOL Multiphysics.
The heat generation in a battery cell is determined by the battery chemistry and kinetics.The battery operation conditions and design parameters have a strong influence on the kinetics.Consequently,the heat generation rate for a battery system may vary greatly with its actual design.A coupled thermal-electrochemical model has been developed,and the model was implemented for a Lix C6|LiPF6(EC/DMC)|LiyMn2O4cell[54].Samba and co-w orker[55]developed a tw o dimensional thermal model to predict the cell temperature distribution over the surface of the battery,and a LiFePO4/graphite lithium-ion pouch cell with a rated capacity of 45 A·h used.The higher the charge-discharge current rate is,the more the battery temperature rises due to the increase in heat generation from the electrodes domain and tabs.In Fig.8,during 4C discharge,i.e.the discharge current of 45 A·h,the area near the positive tab become hotter than the rest area of the battery because the generated heat at the positive tab is higher than the negative tab due to the higher resistance of the aluminum.
Recently,an analytical model is proposed to describe the two dimensional distribution of potential and current in planar electrodes of pouch-type lithium-ion batteries,and a simple concentration-independent polarization expression is employed to describe the collective behavior of complex processes in the electrolyte solution between the electrodes.A pouch type lithium-ion battery(20 A·h)is characterized experimentally to obtain its polarization expressions during constant current discharge processes[56].In order to develop a rapid,non-destructive,quality control method for lithium battery production,a theoretical equivalent circuit model(ECM)of 40 A·h commercial batteries was formulated to interpret electrochemical impedance spectra as a function of temperature at zero state-of charge(SOC)[57].This work provided primary benchmark data on which a battery quality control system may be based.Based on ECM,Jung and Kang[58]developed a noble multi-D mathematical model of large-scale lithium-ion batteries which predicts current,voltage,temperature,SOC,and state of health(SOH)distribution.In order to consider the effect of temperature and SOC on the cell performance,model parameters were bilinearly interpolated.While,ECM sub-model inserted into the present multi-D model needs to be modified if the electrode is blended with phase-changing materials such as LiFePO4and lithium titanate oxide in order to reflect phase-transition phenomena.
Typically,the commercial batteries require optimal design battery management systems(BMS)for reliable,safe and efficient operation.Two key functions of BMS are SOC estimation and SOH monitoring.It is w ell know n that the determination of open-circuit-voltage(OCV)and SOC relationship is the basic principle of almost all methods for model-based SOC estimation approach.The OCV polynomial model and its corresponding nonlinear semi-in finite programming model are firstly described by He and co-workers[59].They provide a systematic framework for automatically constructing monotonicity guaranteed OCV model and it is helpful to obtain accurate SOC estimation and SOH monitoring.More recently,they propose a systematic wavelet analysis-based multi-scale Gaussian process regression modeling method.The proposed model has been applied to SOH estimation of lithium-ion batteries[60].Three datasets of lithium-ion batteries obtained from the data repository of the NASAAmes Prognostics Center of Excellence(PCoE)were used to illustrate the effectiveness of the proposed method.For the one-step-ahead prediction,the predicted SOH is quite close to the actual SOH,and the smallest mean absolute percentage error(MAPE)on battery Nos.5,6,and 7 is 0.60%,0.98%,and 0.49%,respectively.
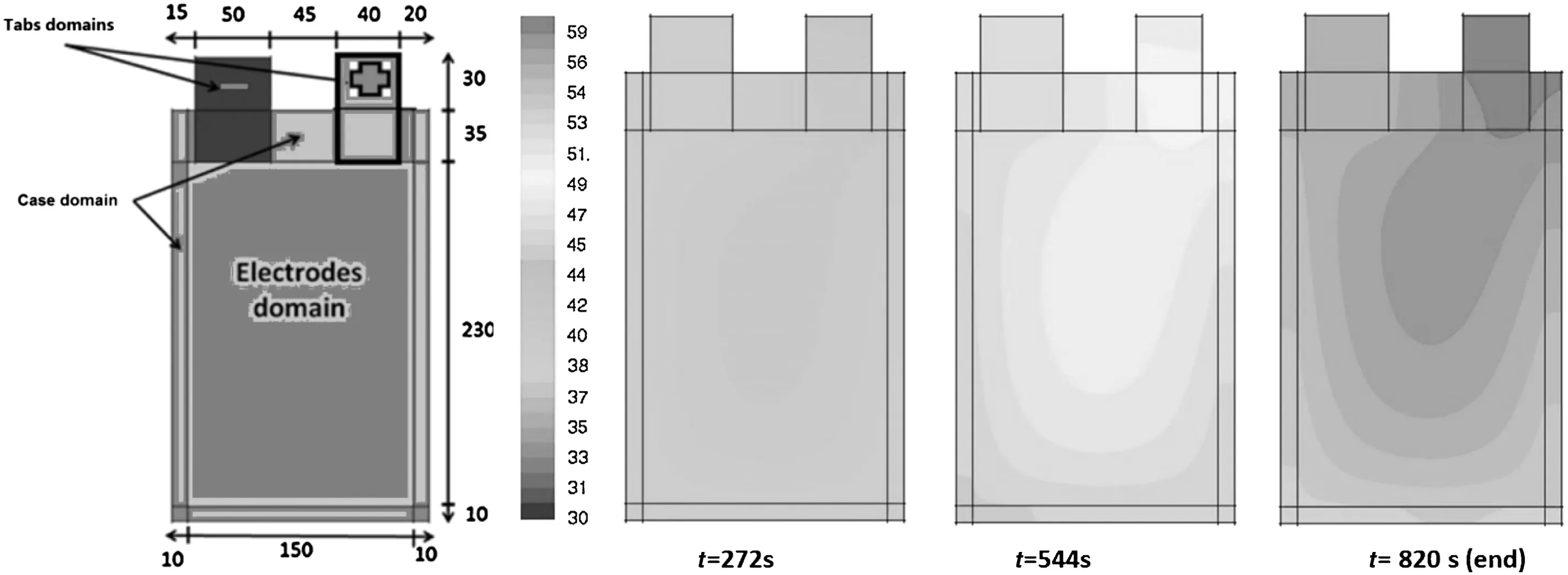
Fig.8.Schematic diagram and dimension(mm)of pouch Li-ion battery(left),and thermal distributions based on modeling at 4C discharge rate(right)[55].
5.Conclusions
In summary,electrochemical energy engineering will likely become a vibrant sub-discipline of chemical engineering and a fertile ground for chemical engineering innovation.This trend has been illustrated by several examples.The simple switch from PEMs to HEMs has led to innovative cell designs and more importantly enabled the use of low-cost catalytic materials.The process optimization and scale-up of the LiFePO4/C material preparation and the design and thermal characterization of large scale lithium-ion battery manufacturing show that process engineering principles are important for innovative manufacturing of low cost lithium-ion batteries.Electrochemical energy materials and device innovations are closely related to the fundamentals involved in transport phenomena and unit operations.Process system engineering methodology can be used to establish a series of models for SOC and SOH estimation,which is based on the electrochemical reaction,occurred within lithium ion battery.
[1]S.Gu,B.J.Xu,Y.S.Yan,Electrochemical energy engineering:A new frontier of chemical engineering innovation,Annu.Rev.Chem.Biomol.Eng.5(2014)429-454.
[2]W.Juda,W.A.Mc Rae,Coherent ion-exchange gels and membranes,J.Am.Chem.Soc.72(1950)1043-1044.
[3]W.T.Grubb,Fuel cell,US Patent No.2913511(1959).
[4]M.S.Wilson,S.Gottesfeld,Thin- film catalyst layers for polymer electrolyte fuel-cell electrodes,J.Appl.Electrochem.22(1992)1-7.
[5]R.Bashyam,P.Zelenay,A class of non-precious metal composite catalysts for fuel cells,Nature 443(2006)63-66.
[6]G.Wu,K.L.More,C.M.Johnston,P.Zelenay,High-performance electrocatalysts for oxygen reduction derived from polyaniline,iron,and cobalt,Science 332(2011)443-447.
[7]X.Yuan,X.L.Ding,C.Y.Wang,Z.F.Ma,Use of polypyrrole in low temperature fuel cells,Energy Environ.Sci.6(4)(2013)1105-1124.
[8]E.W.Justi,A.W.Winsel,The DSK system of fuel cell electrodes,J.Electrochem.Soc.108(1961)1073-1079.
[9]E.Agel,J.Bouet,J.F.Fauvarque,Characterization and use of anionic membranes for alkaline fuel cells,J.Power Sources 101(2001)267-274.
[10]J.R.Varcoe,R.C.T.Slade,Prospects for alkaline anion-exchange membranes in low temperature fuel cells,Fuel Cells 5(2005)187-200.
[11]S.F.Lu,J.Pan,A.B.Huang,L.Zhuang,J.T.Lu,Alkaline polymer electrolyte fuel cells completely free from noble metal catalysts,Proc.Natl.Acad.Sci.U.S.A.105(2008)20611-20614.
[12]S.Gu,W.C.Sheng,R.Cai,S.M.Alia,S.Q.Song,Y.S.Yan,An efficient Ag-ionomer interface for hydroxide exchange membrane fuel cells,Chem.Commun.49(2013)131-133.
[13]M.Piana,M.Boccia,A.Filpi,E.Flammia,H.A.Miller,H2/air alkaline membrane fuel cell performance and durability,using novel ionomer and non-platinum group metal cathode catalyst,J.Power Sources 195(2010)5875-5881.
[14]Z.Li,Z.Y.Jiang,H.M.Tian,S.Wang,B.Zhang,H.Wu,Preparing alkaline anion exchange membrane with enhanced hydroxide conductivity via blending imidazolium-functionalized and sulfonated poly(ether ether ketone),J.Power Sources 288(2015)384-392.
[15]X.Yan,S.Gu,G.He,X.Wu,W.Zheng,X.Ruan,Quaternary phosphoniumfunctionalized poly(ether ether ketone)as highly conductive and alkali-stable hydroxide exchange membrane for fuel cells,J.Membr.Sci.466(2014)220-228.
[16]B.E.Conway,G.Jerkiewicz,Relation of energies and coverages of underpotential and overpotential deposited H at Pt and other metals to the ‘volcano curve’for cathodic H-2 evolution kinetics,Electrochim.Acta 45(2000)4075-4083.
[17]W.C.Sheng,M.Myint,J.G.Chen,Y.S.Yan,Correlating the hydrogen evolution reaction activity in alkaline electrolytes with the hydrogen binding energy on monometallic surfaces,Energy Environ.Sci.6(2013)1509-1512.
[18]F.Munoz,C.Hua,T.Kw ong,L.Tran,T.Q.Nguyen,J.L.Haan,Palladium-copper electrocatalyst for the promotion of the electrochemical oxidation of polyalcohol fuels in the alkaline direct alcohol fuel cell,Appl.Catal.B Environ.174-175(2015)323-328.
[19]C.Z.Guo,W.L.Liao,C.G.Chen,Design of a non-precious metal electrocatalyst for alkaline electrolyte oxygen reduction by using soybean biomass as the nitrogen source of electrocatalytically active center structures,J.Power Sources 269(2014)841-847.
[20]H.J.Zhang,H.L.Li,X.T.Li,S.Y.Zheng,B.Zhao,J.H.Yang,Highly active electrocatalyst for oxygen reduction reaction from pyrolyzing carbon-supported iron tetraethylenepentamine complex,Appl.Catal.B Environ.160(2014)676-683.
[21]C.Dominguez,F.J.Perez-Alonso,S.A.Al-Thabaiti,S.N.Basahel,A.Y.Obaid,A.O.Alyoubi,J.L.G.de la Fuente,S.Rojas,Effect of N and S co-doping of multi walled carbon nanotubes for the oxygen reduction,Electrochim.Acta 157(2015)158-165.
[22]S.J.Chao,Z.Y.Bai,Q.Cui,H.Y.Yan,K.Wang,L.Yang,Hollowed-out octahedral Co/N-codoped carbon as a highly ef ficient non-precious metal catalyst for oxygen reduction reaction,Carbon 82(2015)77-86.
[23]C.H.Wang,C.W.Yang,Y.C.Lin,S.T.Chang,S.L.Y.Chang,Cobalt-iron(II,III)oxide hybrid catalysis with enhanced catalytic activities for oxygen reduction in anion exchange membrane fuel cell,J.Power Sources 277(2015)147-154.
[24]A.K.Padhi,K.S.Nanjundasw amy,J.B.Goodenough,Phosp hoolivines as positiveelectrode materials for rechargeable lithium batteries,J.Electrochem.Soc.144(4)(1997)1188-1194.
[25]X.Z.Liao,Z.F.Ma,Y.S.He,X.M.Zhang,L.Wang,Y.Jiang,Electrochemical behavior of LiFePO4/C cathode material for rechargeable lithium batteries,J.Electrochem.Soc.152(10)(2005)A1969-A1972.
[26]K.D.Yang,F.X.Tan,F.Wang,Y.F.Long,Y.X.Wen,Response surface optimization for processparameters of LiFePO4/C preparation by carbothermal reduction technology,Chin.J.Chem.Eng.20(4)(2012)793-802.
[27]Y.J.Zhang,Y.F.Yang,X.Y.Wang,S.S.Li,Synthesis of sub-micrometer lithium iron phosphate particles for lithium ion battery by using supercritical hydrothermal method,Chin.J.Chem.Eng.22(2)(2014)234-237.
[28]Z.W.Xiao,G.R.Hu,K.Du,Z.D.Peng,A facile route for synthesis of LiFePO4/C cathode material with nano-sized primary particles,Chin.J.Chem.Eng.22(5)(2014)590-595.
[29]X.Z.Liao,Z.F.Ma,L.Wang,X.M.Zhang,Y.Jiang,Y.S.He,A new synthesis route for LiFePO4/C cathode materials for lithium ion batteries,Electrochem.Solid-State Lett.7(12)(2004)A522-A525.
[30]D.Zhang,R.Cai,Y.K.Zhou,Z.P.Shao,X.Z.Liao,Z.F.Ma,Effect of milling method and time on the properties and electrochemical performance of LiFePO4/C composites prepared by ball milling and thermal treatment,Electrochim.Acta 55(2010)2653-2661.
[31]X.M.Liu,P.Yan,Y.Y.Xie,H.Yang,X.D.Shen,Z.F.Ma,Synthesis of superior fast charging-d ischarging nano-LiFePO4/C from nano-FePO4generated using a con fined area impinging jet reactor approach,Chem.Commun.49(47)(2013)5396-5398.
[32]X.Z.Liao,Y.S.He,Z.F.Ma,X.M.Zhang,L.Wang,Effects of fluorine-substitution on the electrochemical behavior of LiFePO4/C cathode materials,J.Power Sources 174(2)(2007)720-725.
[33]Y.Shi,S.L.Chou,J.Z.Wang,D.Wexler,H.J.Li,H.K.Liu,Y.P.Wu,Graphene wrapped LiFePO4/C composites as cathode materials for Li-ion batteries with enhanced rate capacity,J.Mater.Chem.22(32)(2012)16465-16470.
[34]X.Z.Liao,Z.F.Ma,Q.Gong,Y.S.He,L.Pei,L.J.Zeng,Low-temperature performance of LiFePO4/C cathode in a quaternary carbonate-based electrolyte,Electrochem.Commun.10(2008)691-694.
[35]Z.F.Ma,X.Z.Yuan,D.Dan Li,X.Z.Liao,Structrual and electrochemical characterization of carbonaceous mesophase spherule anode material for rechargeable lithium batteries,Electrochem.Commun.4(2)(2002)188-192.
[36]Y.S.He,P.F.Gao,J.Chen,X.W.Yang,X.Z.Liao,J.Yang,Z.F.Ma,A novel bath lily-like graphene sheet-w rapped nano-Si composite as a high performance anode material for Li-ion batteries,RSC Adv.1(2011)958-960.
[37]G.W.Zhou,J.L.Wang,P.F.Gao,X.W.Yang,Y.S.He,X.Z.Liao,J.Chen,Z.F.Ma,A facile spray drying route for the 3D graphene-encapsulated Fe2O3nanoparticles for lithium ion battery anodes,Ind.Eng.Chem.Res.52(3)(2013)1197-1204.
[38]T.Yuan,W.T.Li,W.M.Zhang,Y.S.He,C.M.Zhang,X.Z.Liao,Z.F.Ma,One-pot spraydried graphene sheets-encapsulated nano-Li4Ti5O12microspheres for a hybrid batCap system,Ind.Eng.Chem.Res.53(27)(2014)10849-10857.
[39]R.Koo,Advanced Li-ion polymer battery cell manufacturing plant in USA,2012 DOE AMR Meeting,May 16-20,Washington DC,arravt001,2012.
[40]Y.K.Son,Significant cost improvement of Li-ion cells through non-NMP electrode coating,direct separator coating,and fast formation technologies,2014 DOE AMR Meeting,June 17-20,Washington DC,ES133,2014.
[41]D.L.Wood,J.L.Li,C.Daniel,D.Mohanty,S.Nagpure,Overcoming processing cost barriers of high performance lithium-ion battery electrodes,2014 DOE AMR Meeting,June 17-20,Washington DC,ES164,2014.
[42]D.Mohanty,J.L.Li,R.Born,L.C.Maxey,R.B.Dinwiddie,C.Daniel,D.L.Wood,Nondestructive evaluation of slot-die-coated lithium secondary battery electrodes by in-line laser caliper and IR thermography methods,Anal.Methods 6(3)(2014)674.
[43]J.Arnold,G.Voelker,Utilization of UV or EB curing technology to significantly reduce costs and VOCs in the manufacture of lithium-ion battery electrode,2014 DOE AMR Meeting,June 17-20,Washington DC,ES132,2014.
[44]C.J.Bae,C.K.Erdonmez,J.W.Halloran,Y.M.Chiang,Design of battery electrodes with dual-scale porosity to minimize tortuosity and maximize performance,Adv.Mater.25(2013)1254-1258.
[45]S.J.Dillon,K.Sun,Microstructural design considerations for Li-ion battery systems,Curr.Opin.Solid State Mater.Sci.16(2012)153-162.
[46]Y.S.Chen,K.H.Chang,C.C.Hu,T.T.Cheng,Performance comparisons and resistance modeling for multi-segment,electrode designs of pow er-oriented lithium-ion batteries,Electrochim.Acta 55(2010)6433-6439.
[47]M.Majima,T.Tada,S.Ujiie,E.Yagasaki,S.Inazaw a,K.Miyazaki,Design and characteristics of large-scale lithium ion battery,J.Power Sources 81-82(1999)877-881.
[48]K.H.Kw on,C.B.Shin,T.H.Kang,C.S.Kim,A two-dimensional modeling of a lithiumpolymer battery,J.Power Sources 3(2006)151-157.
[49]U.S.Kim,C.B.Shin,C.S.Kim,Modeling for the scale-up of a lithium-ion polymer battery,J.Power Sources 189(2009)841-846.
[50]M.Wang,J.J.Li,X.M.He,H.Wu,C.R.Wan,The effect of local current density on electrode design for lithium-ion batteries,J.Power Sources 207(2012)127-133.
[51]S.De,P.W.C.Northrop,V.Ramadesigan,V.R.Subramanian,Model-based simultaneous optimization of multiple design parameters for lithium-ion batteries for maximization of energy density,J.Power Sources 227(2013)161-170.
[52]S.C.Chen,C.C.Wan,Y.Y.Wang,Thermal analysis of lithium-ion batteries,J.Power Sources 140(2005)111-124.
[53]U.S.Kim,J.Yi,C.B.Shin,T.Han,S.Park,Modelling the thermal behaviour of a lithium-ion battery during charge,J.Power Sources 196(2011)5115-5121.
[54]W.Wu,X.R.Xiao,X.S.Huang,The effect of battery design parameters on heat generation and utilization in a Li-ion cell,Electrochim.Acta 83(2012)227-240.
[55]A.Samba,N.Omar,H.Gualous,Y.Firouz,P.V.Bossche,J.V.Mierlo,T.I.Boubekeur,Development of an advanced tw o-dimensional thermal mod el for large size lithium-ion pouch cells,Electrochim.Acta 117(2014)246-254.
[56]P.Taheri,A.Mansouri,M.Yazdanpour,M.Bahrami,Theoretical analysis of potential and current distributions in planar electrodes of lithium-ion batteries,Electrochim.Acta 133(2014)197-208.
[57]P.S.Attidekou,S.Lambert,M.Armstrong,J.Widmer,K.Scott,P.A.Christensen,A study of 40 A h lithium ion batteries at zero percent state of charge as a function of temperature,J.Power Sources 269(2014)694-703.
[58]S.Jung,D.Kang,Multi-dimensional modeling of large-scale lithium-ion batteries,J.Power Sources 248(2014)498-509.
[59]Y.J.He,J.N.Shen,J.F.Shen,Z.F.Ma,Embedding monotonicity in the construction of polynomial open-circuit voltage model for lithium-ion batteries:A semi-in finite programming formulation approach,Ind.Eng.Chem.Res.54(12)(2015)3167-3174.
[60]Y.J.He,J.N.Shen,J.F.Shen,Z.F.Ma,State of health estimation of lithium-ion batteries:A multiscale Gaussian process regression modeling approach,AIChE J.61(5)(2015)1589-1600.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Scoping biology-inspired chemical engineering☆
- Review on the nanoparticle fluidization science and technology☆
- Multi-functional forward osmosis draw solutes for seawater desalination☆
- Bio-inspired enantioseparation for chiral compounds☆
- In-situ design and construction of lithium-ion battery electrodeson metal substrates with enhanced performances:A brief review☆
- Developments in the understanding of gas-solid contact efficiency in the circulating fluidized bed riser reactor:A review☆
