In-situ design and construction of lithium-ion battery electrodeson metal substrates with enhanced performances:A brief review☆
2016-05-29WeixinZhangYingmengZhangZehengYangGongdeChenGuoMaQiangWang
Weixin Zhang,Yingmeng Zhang,Zeheng Yang,Gongde Chen,Guo Ma,Qiang Wang
School of Chemistry and Chemical Engineering,Hefei University of Technology,Hefei 230009,China
Anhui Key Laboratory of Controllable Chemical Reaction and Material Chemical Engineering,Hefei 230009,China
1.Introduction
As energy crisis emerges all over the world,building better batteries has become more and more urgent.Rechargeable lithium-ion batteries(LIBs)are promising energy storage candidates for applications in portable electronics,electric vehicles and hybrid electric vehicles.Although LIBs have gained commercial success,they still face challenges in energy storagedensity,cycle life and safety,etc.Traditional commercial graphite as anode materials delivers a theoretical specific capacity of only 372 m A·h·g-1and lower rate performance due to safety concern[1-3],which are obviously far from the expectations for high energy and high power applications in future.Finding potential anode materials with high energy,long stability and good power performance to replace the conventional graphite has long been considered as a big issue.Poizot and co-workers[4]have reported that transition metal oxides can be alternative and promising anode materials for LIBs,because they can exhibit much higher capacity and higher discharge plateau in comparison with graphite,and the formation of Lidendrites can be effectively avoided to improve the safety of LIBs.Especially,nanostructured transition metal oxides such as CoO,CuO and NiO can be fully reduced through their conversion reactions with lithium(MOx+2x Li↔M+x Li2O),which are found to be electrochemically reversible[5,6].However,due to the higher surface area of nanostructured materials,more electrolytes will decompose on the surface of the electrodes,leading to the formation of thicker solid-electrolyte interface(SEI) film.This results in poor initial coulombic efficiency,one of the critical parameters for practical utilization of transition metal oxide electrode materials.
Micro-/nanostructured arrays grown directly on the current collecting substrates present attractive anode candidates for LIBs,which are different from the conventional anodes composed of active materials and additives such as electronic conducting agent(often carbon black)and polymer binder.Such kind of arrays combines the advantages of both nanosized building blocks and the microstructures.The former can improve the electrochemical reaction extent during the charge-discharge process and shorten the lithium ion diffusion pathway and the latter can accommodate the volume expansion during the charge-discharge process[7-10].Meanwhile,the micro-/nanostructured assemblies possess relatively smaller specific surface area than nanostructured materials due to some surfaces and interfaces being fused,which could con fine the excessive SEI formation,and thus reduce its initial capacity loss.On the other hand,the arrays grow ndirectly on the current collecting substrates have good contact with the substrates,which increases the electrical conductivity of the electrode and is helpful to increase their rate performance.Besides,the free space between the arrays can efficiently buffer the significant volume change associated with lithium lithiation and delithiation.
In this article,w e review and analyze design and construction of lithium-ion battery electrodes on metal substrates with enhanced performances,and reveal that self-assembling hierarchical transition metal oxide films on conducting substrates as electrodes,promise synergetic multi-functionalities to be integrated more readily into larger scale devices.An aqueous solution-based process and a microemulsion-mediated process have been respectively investigated,to control the kinetic and thermodynamic processes for the micro/nanostructured array grow th on metal substrates as current collectors.CuOnanorod arrays and micro-cog arrays have been successfully prepared on Cu foils,respectively.They have been directly used as binder-free electrodes for advanced lithium ion batteries with high energy,high safety and high stability.
2.Self-assembly of Micro-/nanostructured Cu O Arrays on Copper Substrates as Anodes
We have reported a bottom-up self-assembly approach to the in situ fabrication of micro-/nanostructured Cu(OH)2arrays on the surface of copper foils(current collectors).These Cu(OH)2arrays can be thermally transformed into a densely packed copper oxide(CuO)array,w ithout changing the architecture,and remaining integrated with the underlying copper substrate.
2.1.Aqueous solution-based process to CuOnanostructured array on copper substrates
An aqueous solution-based process[11-15]has been developed to the synthesis of a series of low-dimensional Cu(OH)2nanostructures including nanorod(Fig.1a)and nanotube arrays on copper substrate surfaces.These Cu(OH)2nanostructures are formed by the direct oxidation of copper substrate in NaOH aqueous solution with(NH4)2S2O8as oxidant,at ambient temperature and pressure.Field emission scanning electron microscopy(FESEM)studies of the Cu(OH)2nanorod arrays on copper substrate show a high yield of uniform and ordered nanorods(Fig.1a).The nanorods exhibit highly smooth surfaces with average diameters of 400-600 nm and generally about 10 μm in length(Fig.1a,inset).Post-heat treatment of Cu(OH)2nanorod arrays on copper substrate in N2atmosphere at 180°C make them transformed into CuO nanorod arrays without obvious change of morphology(Fig.1e).
2.2.Microemulsion-mediated process to Cu O micro-cog hierarchical superstructure
Furthermore,a microemulsion-mediated process[16]is explored to prepare a surface film comprising dense arrays of Cu(OH)2with a unique complex morphology,cog-like hierarchical superstructure with nano filament substructure.A water-in-oil microemulsion,prepared using isooctane mixtures of sodium bis(2-ethylhexyl)sulfosuccinate(NaAOT)assurfactant and small amounts of aqueousalkalinereaction solution,has been used instead of an aqueous solution-mediated process.The aqueous alkaline solution contains NaOH and ammonia solutions with ammonium persulfate((NH4)2S2O8)as oxidant.The fabrication strategy based on con fined reaction in reverse micelle,rather than bulk reaction in aqueous solution,changes the growth habit of the micro-/nanostructured arrays grow n on the copper substrate.
FESEM studies of the Cu(OH)2film show a high yield of unusual cogshaped particles with average widths of 4-6 μm(Fig.1b).Each cogshaped particle consists of a packed radial array of biconvex plates,which are highly uniform in size and quasi-rectangular in shape(Fig.1c).These plates are spatially arranged in a spoke-like pattern to produce segmented spherical disks(Fig.1c).The top and bottom faces of the micro-cogsarehighly textured.View ed from the side,the individual blades comprise an oriented array of 5-10 nm wide nanofilamentlike domains running parallel to the vertical(radial)axis of the microcog superstructure(Fig.1d).Similarly,post-heat treatment of Cu(OH)2microcog arrays on copper substrate in N2atmosphere results in CuO micro-cog arrays with similar morphology to their precursor's(Fig.1f-1h).The structural evolution from orthorhombic Cu(OH)2to monoclinic CuO is studied by X-ray diffraction(XRD)(Fig.1i).
The obtained Cu O micro-/nanostructured arrays on copper substrates are directly used as anodes to evaluate their lithium storage properties in LIBs.Galvanostatic discharge-charge curves recorded at a current density of 0.05 C(1 C=600 m A·g-1)show three sloping potential ranges at 2-1.5,1.25-1.0 and 1.0-0 V for the first discharge cycle(Fig.1j).The data is consistent with cyclic voltammogram(CV)curves that show three reduction peaks at 1.74,0.87 and 0.70 V(Fig.1l).Multi discharge plateaus indicate that multi-step electrochemical reactions may occur during the discharge-charge process.The plateaus correspond to the formation of an intermediate composite CuII1-xCuIxO1-x/2(0≤x≤0.4)solid-solution phase,the phasetrans formation into a Cu2Ophase and the further decomposition of Cu2Ointo Cu and Li2O,respectively[17].The initial discharge capacity of the Cu O micro-cog film electrode at a rate of 0.05 C is 1052 m A·h·g-1,with a reversible capacity of 810 m A·h·g-1(Fig.1j,1m).The reversible capacity is higher than the reversible capacity(646 m A·h·g-1)for negative electrodes consisting of CuO nanorod arrays(Fig.1m),and also larger than the theoretical capacity of 674 m A·h·g-1based on a maximum uptake of 2Li/Cu O.The extra capacity is attributed to the long slope below 0.75 V,which represents reversible formation and decomposition of a polymeric gel-like film on the surface of the particles.In contrast,an electrode prepared from a commercial CuO powder blended with a polymer binder and carbon black paste,show s a lower value for the first discharge capacity(988 m A·h·g-1)and reversible capacity(330 m A·h·g-1)(Fig.1m).The corresponding initial ratios of the irreversible capacity are 23%and 67%for electrode films prepared from the Cu O micro-cog particles and commercial Cu O oxide powder,respectively.These results suggest that side reactions involving electrolyte decomposition are considerably reduced in the presence of the hierarchically assembled Cu O materials,leading to higher columbic efficiency.Significantly,the discharge capacity of the Cu O micro-cog films measured between 2 and 10 discharge-charge cycles shows only a small decrease(4%),compared with a 10%reduction for the Cu O commercial powder investigated under the same conditions.
Other experiments investigate the rate performance of the Cu O micro-cog film electrode in comparison with the Cu O nanorod film electrode.At a rate of 4 C(1C=600 m A·g-1),the micro-cog films still retain a discharge capacity of 583 m A·h·g-1(Fig.1k,1n).By contrast,the electrodes comprising CuO nanorod films have a reduced discharge capacity of 419 m A·h·g-1at a rate of 3 C,and show hardly any discharge capacity w hen the rate is increased to 4 C.Comparing the discharge capacities at various rates indicates that the Cu O microcog films show much slow er capacity decay than the Cu O nanorod films as the current density increased.
It can be seen that the speci fic capacities keep increasing with the rate of 0.1 C,0.25 C,0.5 C until having a sudden decrease at rate of 1 C.This increase might be attributed to a reversible formation of the gellike polymer layer resulting from kinetically activated electrolyte degradation,which may not be common but do happen on many anode materials[18-20].Tarascon's group[19,20]found that the nano particle-driven electrolyte reduction leads to the formation of polymeric gel-like film when they investigated the cycling of CoO/Li cells,which is similar to that of Cu O/Li cells in our work.The capacity increases together with the appearance of a maximum in the capacity pro file with increasing cycle numbers.
The results demonstrate that formation of the hierarchical superstructure considerably improves electrochemical performance compared with the simple structured materials.
2.3.Cosur fact ant-mediated micro emulsion process to free-standing hierarchical CuO arrays on copper substrate

Fig.1.(a)FESEM image of the Cu(OH)2 nanorod array grown on acopper substrate.Inset:a higher magnification FESEM image of asingle Cu(OH)2 nanorod.(b)Low magnification FESEM image of Cu(OH)2 micro-cog particles grown on a copper substrate.(c)High magnification FESEM image of microcog-like Cu(OH)2 particles.(d)A higher magnification FESEM image of the side-view of Cu(OH)2 blade-like elements.(e)FESEM image of CuO nanorod array obtained by thermal dehydration of Cu(OH)2 nanorod array.(f-h)FESEM images of CuO films of micro-cog particles obtained by thermal dehydration of Cu(OH)2 films.(f)Low magnification image showing densely packed CuO micro-cogs.(g)A single particle showing retention of the micro-cog morphology.(h)Side-view showing striated CuO blade-like elements within an individual micro-cog superstructure.(i)XRD patterns of Cu(OH)2 and Cu O microcogs on copper substrates.(j-n)Electrochemical properties of Cu O micro-cog films in the voltage range of 0.005-3 V(1 C=600 m A·g-1).(j)Discharge-charge curves cycling at a rate of 0.05 C.(k)Voltage pro files for the first galvanostatic discharge and charge curves at various rates of CuO micro-cog films.(l)CV curves at a scan rate of 0.1 m V·s-1.(m)Specific capacity as a function of cycle number and charging( filled symbols)or discharging(open symbols)for electrodes produced from CuO micro-cog filmsCuO nanorod filmsand CuO commercial powder.(n)Rate performance of CuO micro-cog films.(The average mass of per CuO micro-cog film is 0.8 mg.)Reproduced from Refs.[13]and[16].Copyright 2015 Elsevier and Wiley.
Moreover,a cosurfact ant-mediated microemulsion process[21]is successfully established to the synthesis of free-standing Cu O arrays with hierarchical micro-cog architectures on copper substrates.We carry the previous study forward and n-butanol is introduced as a cosurfactant into an AOT-isooctane-water microemulsion.This significant change results in the grow th of free-standing Cu(OH)2cog-arrays on Cu substrates in a well-aligned manner.And the derived CuO arrays show good contact between the cog-arrays and the current collecting substrates,thus exhibiting better rate performance and enhanced cycling life as anodes in LIBs.
A large scale coverage of free-standing Cu(OH)2cog-arrays can be seen on the substrate(Fig.2a)and the cog-like structures are densely packed on the Cu foil(Fig.2b).The well-defined assemblies consist of about 10-20 pieces of blade-like elements assembling from the center of the cog(Fig.2c).The assemblies have average heights of over 6 μm and diameters of 1-2 μm,and the thickness of the blade-like elements typically varies from 100-200 nm(Fig.2c).After thermal dehydration at 180°C for 5 h,although a few cracks appear on the blade-like elements,the cog-like hierarchical structure is mostly intact and the Cu O cog-arrays remain over 6 μm in length and 1-2 μm in diameter(Fig.2d-2f).The structural evolution from orthorhombic Cu(OH)2to monoclinic CuO is studied by X-ray diffraction(XRD)(Fig.2g).
The free-standing CuOcog-arrays on copper substrates are also used as anodes directly in LIBs.The discharge-charge curves at a current density of 1 C(1 C=670 m A·g-1)show three obvious potential plateaus appear at about 2.2-1.8 V,1.2 V and 0.8 V during the first discharge process(Fig.2h).The data is consistent with CV curves that show three reduction peaks centered at 1.86,0.98 and 0.74 V in the first CV curve(Fig.2j).The stable capacity retention at such a high current density over 300 cycles(91.6%at 1 C and 86.9%at 2 C)indicates its excellent cycling stability and good capacity retention(Fig.2k).As expected,the electrode containing hierarchical arrays show s a good rate performance and still exhibits relatively high capacities even at high current rates(Fig.2i,2l).The average capacity decreases from 812 to 754 mA·h·g-1to 720,679 and 635 mA·h·g-1,when the current density increases from 0.1 to 0.5 C to 1,2 and 4 C.When the current density increases further to 5,6,10 and 12 C,the average capacity decreases to 615,589,501 and 466 m A·h·g-1,respectively.Even at a high rate of 15 C,it is remarkable to note that the Cu O micro-cog arrays still retain a very high discharge capacity of about 418 m A·h·g-1(Fig.2l).When reducing the rate from 15 C down to 0.5 C,the reversible capacity increases to 674 m A·h·g-1,suggesting that the CuO cog-array electrode exhibits superior rate capability to the Cu O micro-cog particles described in Fig.1.

Fig.2.(a)Low magnification FESEM image of hierarchical Cu(OH)2 array film grow n on a copper substrate,(b)High magnification FESEM image of cog-like arrays,(c)Top view of asingle Cu(OH)2 cog.(d-f)FESEM images of CuO free-standing cog-arrays obtained from thermal dehydration of Cu(OH)2 precursors.(g)XRD patterns of Cu(OH)2 and CuO free-standing cogarrays grown on copper substrates.(h-l)Electrochemical performance of CuO cog-array films in the voltage range of 0.01-3 V(1 C=670 m A·g-1).(h)Discharge-charge curves cycling at a rate of 1 C.(i)Voltage pro files for the first galvanostatic discharge and charge curves at various rates.(j)CV curves at a scan rate of 0.1 m V·s-1.(k)Cycling performance at a current density of 1 C(open symbols)and 2 C( filled symbols).(l)Rate cyclability at various rates.(The average mass of per CuO free-standing cog-arrays is 0.7 mg.)
Such excellent rate performance can probably be attributed to well aligned arrays directly constructed on the copper surface as w ell as the particularly assembled feature of the hierarchical micro-cog architecture.Introduction of n-butanol improves the contact between the active Cu O film of free-standing arrays and the copper collector in comparison with cog particles randomly lying on the copper substrate(Fig.1).This improvement provides efficient transport for electrons among the Cu/Cu O/electrolyte interfaces.Furthermore,the hierarchical and porous CuO arrays can not only provide the efficient transport of lithium ions,but also facilitate the diffusion of electrolyte into the inner region of the material.And the array structure can accelerate the electrochemical reaction kinetics and decrease the polarization of the electrode,by accommodating sufficient space to sustain the volume change associated with lithium insertion and extraction.As a result,fully displayed discharge capacity,excellent cycling stability and enhanced rate performance can be obtained at high rates.
To investigate more closely the lithium-driven structural and morphological changes,w e studied the Cu O electrode collected from the disassembled cell after discharge-charge testing.Fig.3 presents the FESEM images of the Cu O electrode after 100 discharge-charge cycles at the rate of 1 C[21].We can still identify the largely intact cog-arrays(Fig.3b and c),which are still attached on the current collector(Fig.3a)after repeated phase conversion reactions.The results reveal that the hierarchical structures are stable and can be sustained during cycling.Each free-standing micro-cog is assembled by blade like elements that interconnect and support each other.This construction can more efficiently buffer the significant volume change than the simple-structured arrays during the discharge-charge processes upon lithium insertion and removal.The results discussed above indicate that hierarchical CuO free-standing cog-arrays could possibly tolerate those volume changes,thus leading to the excellent rate capability and capacity retention of the electrode even at high current rates.
3.Full Cell Construction Based on Cu O Nanorod Array Film Anode and Spinel LiNi0.5Mn1.5O4 Cathode Materials
Lithium-ion batteries with advanced performance are required to meet the needs for next generation power batteries.A new full cell has been successfully assembled based on CuO nanorod array anode and spinel LiNi0.5Mn1.5O4cathode materials(acquired from BASF SE),as show n in Fig.4[22].Different from the conventional prelithiation of transition metal oxides anodes[23],a CuO-limited full cell has been assembled directly by adjusting the positive/negative capacity ratio of 1.2:1,which could deliver a discharge capacity of 660 mA·h·g-1with estimated energy density of about 217 W·h·kg-1at 0.1 C rate.The Cu O/LiNi0.5Mn1.5O4full cell exhibits good cycle stability(w ith capacity retention of 84%at 0.5 C over 100 cycles)and superior rate capability(about 240 m A·h·g-1at a high rate of 10 C),which mainly resulted from Cu O arrays directly constructed on copper substrate and the hierarchical structure of LiNi0.5Mn1.5O4materials.The innovation in electrode engineering and full cell matching may shed new light upon constructing high energy,high rate capability and high safety full cells.
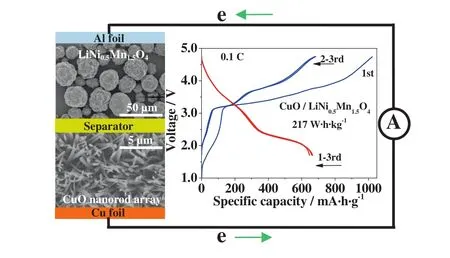
Fig.4.A lithium-ion full cell assembled based on CuO nanorod array anode and spinel LiNi0.5Mn1.5O4 cathode materials.
4.Conclusions
We review ed our efforts on developing a bottom-up selfassembly strategy to the preparation of micro/nanostructured Cu O arrays on copper substrates as efficient lithium-ion battery electrodes.This solution-based chemical approach features mildness,scalability,and process simplicity,and holds function-oriented potential for lithium-ion batteries through controlling the nucleation/grow th,and thus the composition,crystal structure,size,and morphology of the arrays.This facile and mild chemical method can be extended to prepare other transition metal oxide arrays on corresponding metal substrates,for example,NiO arrays on nickel foil or nickel foam substrates.These ordered arrays on metal substrates can be used directly as binder-free anodes without the conventional electrode fabrication process related with powder materials.The innovation in electrode engineering facilitates better electric contact between the current collector and active materials,and provides enhanced accommodation of strains which resulted from lithium ion lithiation/delithiation,thus reducing the contact resistance between the current collector and active materials,and enhancing the power density and rate performance in LIBs.
The growth of metal oxide arrays on conductive substrates(that can be employed directly ascurrent collectors)has show n great potential in improving the transport pathway of electrons and enhancing the stability of the nanostructure,but further effort is still highly required.There is still a huge space to exploit more scalable strategies for growing other potential micro-/nanostructured metal oxide array electrodes for LIBs.It is know n that the current LIB system is not limited only by the performance of the anode.The idea using ordered arrays directly grow n on current collectors to improve the transport pathways of electrons as well as to enhance the electrochemical reaction kinetics can be possibly applied to cathode materials.It is hopeful to expect a great progress of the cycling life and rate performance of LIBs,if electrode materials can be systematically investigated in combination with the improvement in electrolyte and membrane.
[1]J.R.Dahn,T.Zheng,Y.H.Liu,J.S.Xue,Mechanisms for lithium insertion in carbonaceous materials,Science 270(1995)590-593.
[2]H.L.Wang,Y.Yang,Y.Y.Liang,J.T.Robinson,Y.G.Li,A.Jackson,Y.Cui,H.J.Dai,Graphene-wrapped sulfur particles as a rechargeable lithium-sulfur battery cathode material with high capacity and cycling stability,Nano Lett.11(2011)2644-2647.
[3]Y.H.Xu,Q.Liu,Y.J.Zhu,Y.H.Liu,A.Langrock,M.R.Zachariah,C.S.Wang,Uniform nano-Sn/C composite anod es for lithium ion batteries,Nano Lett.13(2013)470-474.
[4]P.Poizot,S.Laruelle,S.Grugeon,L.Dupont,J.M.Tarascon,Nano-sized transition metal oxides as negative-electrode materials for lithium-ion batteries,Nature 407(2000)496-499.
[5]H.Li,P.Balaya,J.Maier,Li-storage via heterogeneous reaction in selected binary metal fluorides and oxides,J.Electrochem.Soc.151(2004)A1878-A1885.
[6]F.Badway,I.Plitz,S.Grugeon,S.Laruelle,M.Dolle,A.S.Gozdz,J.M.Tarascon,Metal oxides as negative electrode materials in Li-ion cells,Electrochem.Solid-State Lett.5(2002)A115-A118.
[7]P.L.Taberna,S.Mitra,P.Poizot,P.Simon,J.M.Tarascon,High rate capabilities Fe3O4-based Cu nano-architectured electrodes for lithium-ion battery applications,Nat.Mater.5(2006)567-573.
[8]C.K.Chan,H.Peng,G.Liu,K.Mcilwrath,X.F.Zhang,R.A.Huggins,Y.Cui,Highperformance lithium battery anodes using silicon nanow ires,Nat.Nanotechnol.3(2008)31-35.
[9]F.S.Ke,L.Huang,B.Zhang,G.Z.Wei,L.J.Xue,J.T.Li,S.G.Sun,Nanoarchitectured Fe3O4array electrode and its excellent lithium storage performance,Electrochim.Acta 78(2012)585-591.
[10]Q.Q.Xiong,X.H.Xia,J.P.Tu,J.Chen,Y.Q.Zhang,D.Zhou,C.D.Gu,X.L.Wang,Hierarchical Fe2O3@Co3O4nanowire array anode for high-performance lithium-ion batteries,J.Power Sources 240(2013)344-350.
[11]W.X.Zhang,S.H.Yang,In situ fabrication of inorganic nanow ire arrays grown from and aligned on metal substrates,Acc.Chem.Res.42(2009)1617-1627.
[12]J.Xu,W.X.Zhang,Z.H.Yang,S.H.Yang,Lithography inside Cu(OH)2nanorods:a general route to controllable synthesis of the arrays of copper chalcogenide nanotubes with double walls,Inorg.Chem.47(2008)699-704.
[13]W.X.Zhang,J.Xu,Z.H.Yang,S.X.Ding,Mesoscale organization of Cu7S4nanow ires:formation of novel sheath-like nanotube array,Chem.Phys.Lett.434(2007)256-259.
[14]W.X.Zhang,X.G.Wen,S.H.Yang,Y.Berta,Z.L.Wang,Single crystalline scroll-type nanotube arrays of copper hydroxide synthesized at room temperature,Adv.Mater.15(2003)822-825.
[15]W.X.Zhang,X.G.Wen,S.H.Yang,Controlled reactionson a copper surface:Synthesis and characterization of nanostructured copper compound films,Inorg.Chem.42(2003)5005-5014.
[16]W.X.Zhang,M.Li,Q.Wang,G.D.Chen,M.Kong,Z.H.Yang,S.Mann,Hierarchical self-assembly of microscale cog-like superstructures for enhanced performance in lithium-ion batteries,Adv.Funct.Mater.21(2011)3516-3523.
[17]A.Débart,L.Dupont,P.Poizot,J.B.Leriche,J.M.Tarascon,A transmission electron microscopy study of the reactivity mechanism of tailor-made Cu O particles tow ard lithium,J.Electrochem.Soc.148(2001)A1266-A1274.
[18]Y.M.Zhang,W.X.Zhang,Z.H.Yang,H.Y.Gu,Q.Zhu,S.H.Yang,M.Li,Self-sustained cycle of hydrolysis and etching at solution/solid interfaces:A general strategy to prepare metal oxide micro-/nanostructured arrays for high-performance electrodes,Angew.Chem.Int.Ed.54(2015)3932-3936.
[19]S.Grugeon,S.Laruelle,L.Dupont,J.M.Tarascon,An update on the reactivity of nanoparticles Co-based compounds towards Li,Solid State Sci.5(2003)895-904.
[20]S.Laruelle,S.Grugeon,P.Poizot,M.Dolle,L.Dupont,J.M.Tarascon,On the origin of the extra electrochemical capacity displayed by MO/Li cells at low potential,J.Electrochem.Soc.149(2002)A627-A634.
[21]Y.M.Zhang,W.X.Zhang,M.Li,Z.H.Yang,G.D.Chen,Q.Wang,Cosurfactant mediated microemulsion to free-standing hierarchical CuO arrays on copper substrates as anodes for lithium-ion batteries,J.Mater.Chem.A 1(2013)14368-14374.
[22]W.X.Zhang,G.Ma,H.Y.Gu,Z.H.Yang,H.Cheng,A new lithium-ion battery:Cu O nanorod array anode versus spinel LiNi0.5Mn1.5O4cathode,J.Power Sources 273(2015)561-565.
[23]J.Hassoun,F.Croce,I.Hong,B.Scrosati,Lithium-ion battery:Fe2O3anode versus LiFePO4cathode,Electrochem.Commun.13(2011)228-231.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Scoping biology-inspired chemical engineering☆
- Review on the nanoparticle fluidization science and technology☆
- Multi-functional forward osmosis draw solutes for seawater desalination☆
- Bio-inspired enantioseparation for chiral compounds☆
- Process engineering in electrochemical energy devices innovation☆
- Developments in the understanding of gas-solid contact efficiency in the circulating fluidized bed riser reactor:A review☆
