Bio-inspired enantioseparation for chiral compounds☆
2016-05-29YanFuJinjinYangJinliZhangWeiLi
Yan Fu ,Jinjin Yang ,Jinli Zhang ,2,Wei Li ,*
1 Key Laboratory for Green Chemical Technology MOE,Key Laboratory of Systems Bioengineering MOE,Collaborative Innovation Center of Chemical Science and Chemical Engineering(Tianjin),School of Chemical Engineering and Technology,Tianjin University,Tianjin 300072,China
2 School of Chemistry and Chemical Engineering,Shihezi University,Shihezi 832000,China
1.Introduction
Chirality,one of the most intriguing phenomena in living organisms,has attracted much attention in the fields of chiroptical materials,helical architectures,asymmetric catalysts,and chiralbiosensors[1-4].Owing to great differences in pharmacological,toxicological and metabolic activities of enantiomers in organisms,the development of enantioseparation approaches has become a growing focus of researches,e.g.,high performance liquid chromatography,chiral extraction,ultra filtration,dynamic kinetic resolution and preferential crystallization[5-10].To achieve a continuous operation and preparative separation,it has attracted more attentions to construct chiral selectors to discriminate left-and righthanded enantiomers with high enantioselectivity in the pharmaceutical industry[11].A variety of chiral selectors including macrocyclic glycopeptide,crown ether,chiral polymer,polysaccharide,cyclodextrin,molecular imprinted polymer,protein,and aptamer,have been successfully used in the chromatographic separation[12,13].Besides,achiral zeolite,natural metal surface,dynamic polymer interface,supra molecular assembly,metal-organic cage,metal-organic framework as w ell as hydrogen-bonded organic framework,have been discovered or synthesized with highly chiral discrimination ability,which allow s for the potential development of new,sustainable and efficient enantioseparation techniques[14-22].
Supramolecular chirality is intrinsic to natural biomacromolecules involving polysaccharide,protein and nucleic acids,therefore,organisms often exhibit different biological responses to extrinsic enantiomers.Polysaccharide-based chiral stationary phases(CSPs)have been recognized as the most pow erful selectors for both analytical and preparative separations for commercial drugs.Approximately 90%of the enantiomeric excess(ee)determinations are performed using polysaccharide-based CSPs including esters of cellulose and amylose,cellulose phenylcarbamates,as well as amylose phenylcarbamates[23-26].How ever,it is difficult to study the chiral recognition mechanism at a molecular level for polysaccharide-based CSPs since a variety of interaction sites with different affinities for enantiomers exist on these chiral polymers[23-25].Owing to highly-ordered and wellde fined 3D structures,selective binding sites of protein or nucleic acids for enantiomers have been deeply investigated at molecular level by using NMRspectroscopy,X-ray crystallography,and thermodynamic and computational methods[27].These biomolecules provide a potential application of selectively separating one enantiomer from the racemic compounds through adsorption,membrane process,chromatography,or capillary electrophoresis[28-31].In this review, firstly,enantioselective recognition mechanism of proteins and nucleic acids toward different enantiomers is discussed,as w ell as their potential applications on the chiral separation of racemic compounds.Secondly,preparative enantioseparation adopting biomolecule-modi fied hybrid materials including porous microspheres,magnetic nanoparticles and affinity membranes,are summarized respectively.Finally,novel chiroptical materials constructed on the basis of chiral induction,transfer,amplification and transcription,are recognized aspromising candidates in future applications(as illustrated in Fig.1).
2.Enantioselective Resolution Based on Biomolecules
2.1.Protein
Natural proteins can discriminate a wide spectrum of charged and neutral enantiomers.The most widely used proteins nowadays in chiral separation are human serum albumin(HSA),bovine serum albumin(BSA),α1-acid glycoprotein(AGP),ovomucoid(OVM)and cellobiohydrolase I(CBHI).Amongst them AGPand OVM exhibit the broadest enantioseparation capabilities covering a wide variety of neutral,acidic and basic drug racemates.CBH preferentially resolves basic enantiomers(e.g.β-blockers)and HSA preferably acidic enantiomers[32].As show n in Fig.2,serum albumin contains w arfarinazapropazone site I(subdomain IIA)and indol-benzodiazepine site II(subdomain IIIA),which exhibits high selectivity toward chiral compounds of amino acids and their derivatives,warfarin,propranolol,ibuprofen,ofloxacin,etc.[33-37].Accordingto the X-ray crystal structures reported for HSA-warfarin,both R and S-enantiomersbound in thepocket of subdomain IIA.The main difference between two enantiomers was the conformation in the acetonyl group and the hydrogen bondings formed between Arg222 residue and the carbonyl of the coumarin ring(in R-complex)and of the acetonide(in S-complex)[34].
In order to achievechiral separation based on protein,Chung and coworkers utilized affinity ultra filtration system to separate the mixture of D,L-tryptophan using HSAw ith high reusability and recovery.The native and recovered HSA exhibited a similar separation factor of 5-7 for D,L-tryptophan.The feasibility of HSA regeneration after D,L-tryptophan separation can be demonstrated through p H adjustments[38].By using HSA in an affinity ultra filtration system,a separation factor of 12-15 and D-tryptophan yield of 95%have been successfully achieved.Owing to a fine match between HSA crystal structure and pore size,some HSA molecules were retained within membrane cross-section,offering a second-stage binding opportunity for L-tryptophan[39].Zhou and co-workers reported that the highest enantioselectivity of 9.76 toward L-tryptophan was achieved using the selective permeation enhancement-affinity dialysis unit with tw o cells in series,which selectively decreased the flux of the more weakly bound enantiomer[40].

Fig.1.Application of biomacro molecules(protein,nucleic acids,etc.)on the construction of widely used chiral selectors in the preparative enantioseparation,as well as on the development of chiral materials promising for enantioseparation.

Fig.2.Crystal structure and typical ligands to site I and site II of HSA[33].
2.2.Nucleic acids
As a naturally chiral biomolecule,DNA exhibits high enantioselectivity for chiral compounds such as oligopeptide,adenosin,tyrosinamide,amino acids and their derivatives,ibuprofen,thalidomide derivative,as well as metal-organic complexes[41-47].Qu and co-workers reported that(+)-daunorubicin selectively bound to right-handed DNA,whereas its enantiomeric WP900 favored left-handed DNA.(+)-Daunorubi-cin allosterically converted the polynucleotide to a right-handed intercalated form,while WP900 converted the polynucleotide to a lefthanded form[48].Aldrich-Wright and coworkers reported that Δ-[Ru(dmphen)2dpq]2+intercalated deeply into the hexanucleotide base stack,while Λ-[Ru(dmphen)2dpq]2+only partially intercalated,contributing to the chiral discrimination of d(GTCGAC)2duplex[49,50].Qu and coworkers synthesized a series of chiral metallosupramolecular complexes since enantiomers of these cylinders can discriminate distinct DNA structures[51-53].For example,a pair of chiral helical macrocyclic lanthanide(III)complexes,(M)-Yb[LSSSSSS]3+and(P)-Yb[LRRRRRR]3+,enantioselectively bound to B-form DNA and show ed remarkably contrasting effects on GC-rich and AT-rich DNA.P-enantiomer stabilized both poly(d G-d C)2and poly(d A-d T)2.How ever,M-enantiomer stabilized poly(d A-d T)2but destabilized poly(d G-d C)2[53].
On the other hand,transition metal ion such as Cu2+,Ni2+or Pt2+,can preferably coordinate at nitrogen N7 or O6 site of guanine,consequently,it can anchor inside three-dimensional DNA structure to construct chiral microenvironment[54,55].DNA-based selector for discriminating chiral ofloxacin with high enantioselectivity and affinity was constructed through Cu(II)-coordination with DNA double helix containing successive guanine-cytosine base pairs.R-and S-ofloxacin can be directly enriched fromracemic feed solution,with the ee of 85%(R)and 78%(S)respectively by three operational stages[56,57].This discrimination was mainly attributed to the steric hindrance between the methyl group of ofloxacin and the phosphate backbone of DNA.Interestingly,distinct discrimination mechanism was involved in the enantioselective recognition of ofloxacin enantiomers between GC-and AT-rich oligonucleotides.As shown in Fig.3a,ofloxacin partially intercalated into DNA base pairs from the minor groove of GC-rich duplex,and Cu2+acted as a bridge to connect the N7 and/or O6 site of guanines with the carboxylic and the carbonyl groups of ofloxacin.In contrast,for AT-rich sequence(Fig.3b),ofloxacin interacted with Cu(II)through the groove binding model[58].
3.Biomolecule-modified Materials for Enantioseparation
3.1.Membranes
Membrane-based enantioseparation techniques facilitate industrial scale separation through channel type permeation and affinity ultra filtration,respectively.Using affinity ultra filtration,two enantiomers are separated by the adsorption of one enantiomer on the membrane with higher binding affinity than that of the opposite enantiomer.In contrast,chiral separation in the channel type permeation is achieved by the differential permeation of two enantiomers through membrane channels.Lee and co-workers developed a synthetic bio-nanotube membrane to separate racemic drugs.Silicananotubes were synthesized inside the pores of alumina films,and antibodies were attached to the inner walls of nanotubes.These membranes selectively transported the enantiomer that specifically bound to antibodies.The enantioselectivities increased as the in sidedia meter of the nanotubesdecreased[59].Separation of racemic tryptophan was performed by using BSA-immobilized polysulfone membrane and polysulfone membrane having BSA semiinterpenetrating network(IPN).BSA semi-IPN membrane exhibited higher volumetric and solute fluxes compared to BSA-immobilized membrane.Separation factor of 1.89 was achieved with BSA-immobilized membrane after 8 h-ultra filtration,while BSA semi-IPN membrane exhibited a separation factor of 1.62.BSA-immobilized membrane showed higher ee of 30.8%after 8 h than that of semi-IPN membrane(23.8%)[60].Chung and coworkers reported a one-step synthesis of a novel aldehyde-bearing graft copolymer via atom transfer radical polymerization(ATRP)to covalently capture BSA onto a polymeric membrane.This BSA modified membrane demonstrated an enantioresolution of racemictryptophan(0.184 mmol·L-1)with a time-averagedseparation factor of 2.9[61].

Fig.3.Schematic illustration of the intercalative and groovebinding of S-ofloxacin to(a)G4C4 and(b)ATin the presence of Cu2+:The purple dashed circlesdenote the methyl group at the C-3 position of the oxazine ring of S-ofloxacin and the phosphate backbone of G4C4,respectively,between which there is no apparent steric hindrance upon S-ofloxacin binding to G4C4;the green dashed linesindicate the coordination geometry of Cu IIin the CuII-DNA-ofloxacin complexes[58].(For interpretation of the reference to color in this figure legend,thereader is referred to the web version of this article.).
Salmon testes DNA was also immobilized onto the chitosan membrane via the Pt(II)-associated coordination.This DNA-immobilized chitosan membrane show ed a preferential enrichment of D-phenylalanine with a separation factor about 1.5[62,63].Chiral separation of racemic tryptophan,phenylglycine and phenylalanine was investigated on the DNA-immobilized membranes with various pore sizes by using ultra filtration technique.The ultra filtration apparatus for chiral separation is illustrated in Fig.4a.L-tryptophan preferentially permeated through the membrane with a pore size<2.0 nm while D-tryptophan preferably permeated through the membrane with a pore size>2.0 nm(Fig.4b).Interestingly,the tendency was entirely opposite in the ultra filtration of racemic phenylalanine through these DNA-based membranes[64].
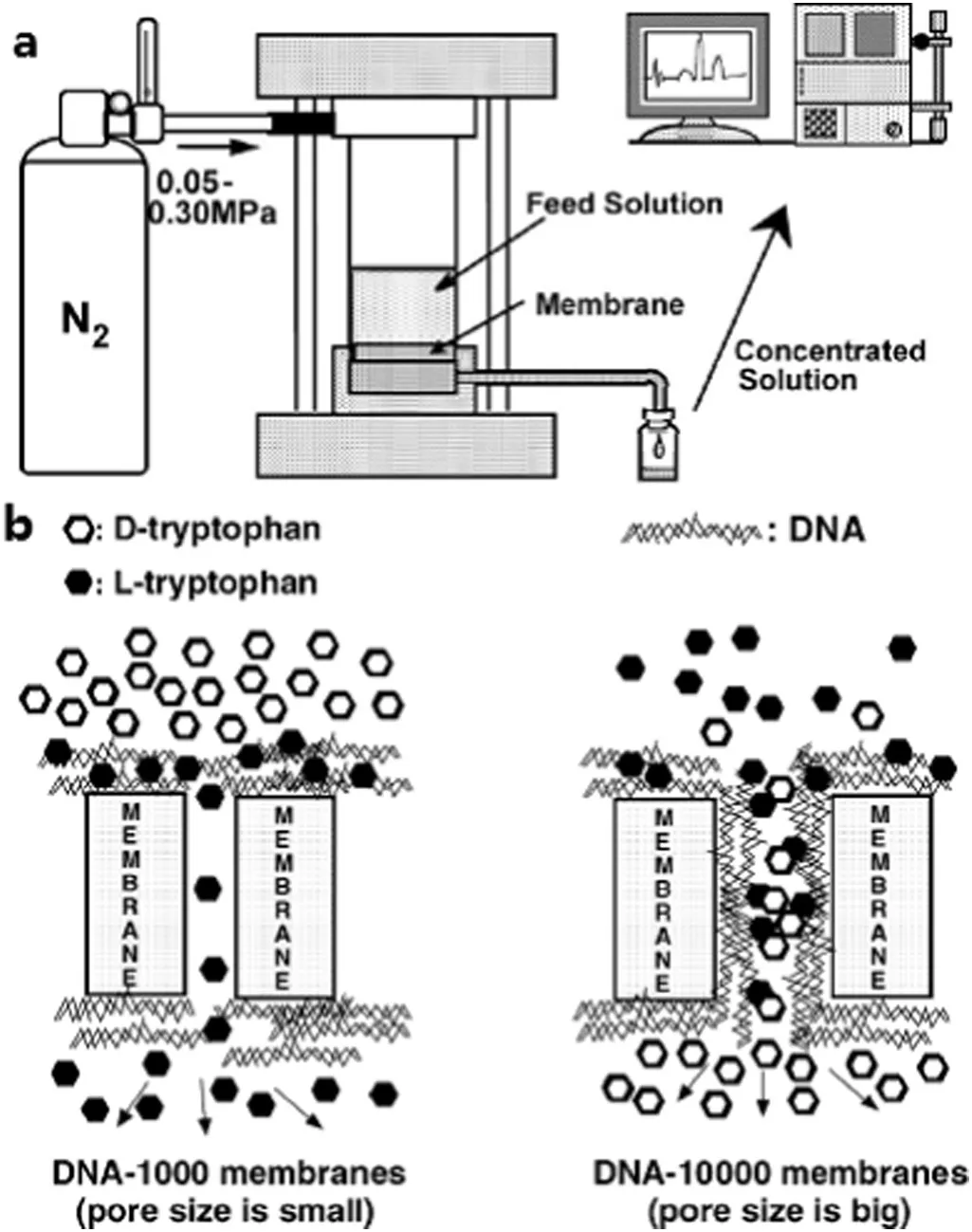
Fig.4.(a)Schematic illustration of ultra filtration apparatus for chiralseparation.(b)Chiral separation process in the ultra filtration of racemic tryptophan through DNA-based membranes[64].
3.2.Particles
Porous microspheres and nanoparticles have larger specific surface areas than membrane-based materials,which have been recognized as novel materials for continuous operation and preparative separation.Immobilized serum albumin onto other materials such as silica gels[65],carbon nanotube[66],gold nanoparticle[67],and polydopaminegraphene oxide[68],have been broadly used as chiral stationary phases(CSPs)in high performance liquid chromatography,capillary electrochromatography and chip-based capillary electrochromatography for enantioselective analysis[69,70].For preparation-scale separation,Zhai and co-workers synthesized silica microspheres with high loading of 450 mg·g-1of BSA.These micrometer-sized monodispersed spheres were packed in a column for enantioselective adsorption of racemic tryptophan and phenylalanine with the ee of 100%in the first 10 min[71].
Chiral selector-modified magnetic nanoparticles were successfully used in the chiral separation through stereoselective adsorption,with high repeatability and reproducibility[72,73].Wang and co-workers immobilized BSA on the polyamidoamine dendrimer-modified MNPs through covalent methods,as a chiral selector for direct separation of racemic tryptophan,phenylalanine and histidine.The immobilization amount of BSA was 36.7 mg·g-1,and the saturation magnetization decreased from 88.9 emu·g-1to 51.3 emu·g-1compared to bare nanospheres[74].BSA anchored on the surface of magnetic Fe3O4nanoparticles(MNPs)were prepared using electrostatic adsorption and utilized as a magnetic chiral selector for the enantioseparation of racemates of ibuprofen and o floxacin,respectively.Immobilized BSA molecules on the magnetic nanoparticles retained their stereoselective recognition for both site I-and II-binding drugs,w ith an optimal loading of 467 mg·g-1and no apparent loss of saturation magnetization.Multi-stage operation provided the ee of 54%and 39%for racemic ibuprofen and o floxacin,respectively[75].Hybrid enantioseparation process can be established by enantioselective adsorption and subsequent crystallization(illustrated in Fig.5).
A variety of oligonucleotides have been selected using SELEX procedure to enantioselectively discriminate chiral compounds.Peyrin and coworkers developed a series of aptamer-based high performance liquid chromatography and aptamer-modified micellar electrokinetic chromatography for enantioselective analysis[76,77].For the aptamerbased preparative separation,Huang and co-workers employed a method for the enantioseparation of racemic tryptophan using aptamermodified AuNPs as the chiral selector.The maximum separation efficiency was achieved with 50 nm-Au NPs at a centrifugation speed of 5000 r·min-1.The aptamer-AuNPs can be reused for at least eight times without a signi ficant decrease of their binding affinity.Complete enantioseparation can be achieved after five repeated additions of aptamer-Au NPs into racemic feed solution[78].
4.New Hybrid Materials for Enantioseparation
4.1.Chiral self-assembled materials
Chiral assembled materialshaveattracted grow inginterestsin many fields,especially focusing on their potential applications in the enantiomeric resolution[79,80].For the principles on the construction of chiral materials,the formation of metal-organic frameworks and nanocages is based on the mechanisms of chiral induction and transfer,whereas supramolecular gels are formed on the basis of chiral amplification mechanism.In the fields of chiral recognition,a kind of metallogels based on the copper(II)complex of quinolinol-substituted L-glutamine,show ed new enantioselective recognition toward chiral aromatic amino acids.A blue emission band at 393 nm appeared after addition of L-enantiomer,whereas no new emission band was observed for D-enantiomer.Such enantioselectivity only occurred in the gel state not in the solution[81].
Cuiand coworkersdeveloped ahomochiraloctanuclear cage through the self-assembly of enantiopure pyridyl functionalized metallosalan units.Upon coordination the optical rotation of each ligand was increased by a factor of 10.The crystalline cages existed in two different porous structures,in which 1a was built of helicatecages interconnected by channels and 1b was built of helicate cages interconnected by pentahedral cages.By immersing the crystalline cages 1a in the racemic solution of 1-(methylsul finyl)benzene,1-phenylethanol,1-phenylpropanol and 1-phenylethylamine,enantioselectivity was up to 20.1%,4.7%,13.0%and 14.6%(S-enantiomer excess),respectively.Adopting the crystalline cages 1b,enhanced enantioselectivity was detected as 37.5%,13.8%,18.6%and 10.3%,respectively[82].A homochiral MOF separation membrane was synthesized via the solvothermal reaction of metal ions,chiral ligands and organic connectors.The resulting MOF membrane with the pore size of 5 Å(0.5 nm)exhibited good enantioseparation performance for sulfoxide enantiomers,with a preferential adsorption ability to(S)-methyl phenyl sulfoxide(SMPS)ver R-MPS[83].
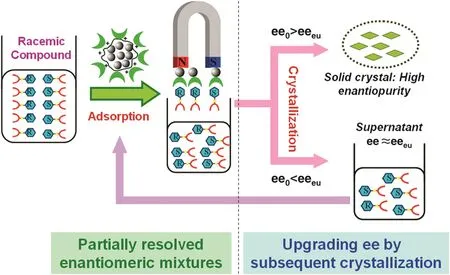
Fig.5.Hybrid enantioseparation approach involving enantiomeric adsorption and subsequent crystallization[75].If the ee0 of the partially resolved mixtures generated by using magnetically chiral selectors higher than that of the eutectic point(ee0>eeeu),enantiopure enantiomer is directly obtained from solid crystal through subsequent crystallization.If the ee0<eeeu,another cycle of enantioselective adsorption of the supernatant after crystallization(ee≈eeeu)is proposed to enhance the ee higher than the eeeu.
4.2.Transcription of chirality from enantiopuremoleculestoachiral materials
Chiral transcription mechanism is mainly adopted to explore chiral porous inorganic materials,in which the intrinsic chirality of enantiopure molecules can be imprinted into organic or inorganic materials[84,85].Phenylated silica sol-gel(PSG)matrices were doped with the chiral cationic surfactant,(-)-N-dodecyl-N-methylephedrinium bromide,and then the surfactants can be extracted by methanol.Good enantioselectivity was observed toward propranolol,naproxen and binaphthyl-2,2-diyl hydrogen phosphate(BINAP),with discrimination ratios in the range of 1.22-1.34[86].Liu and co-workers successfully synthesized gold nanorod@chiral mesoporous silica core-shell nanoparticles(GNR@CMS NPs)through the coating of chiral mesoporous silica(CMS)shells on the surface of gold nanorod(GNR)by using amino acid-derived surfactants as the templates for shell grow th.Surface enhanced Raman scattering spectroscopy demonstrated a distinct chiral recognition toward cysteine loaded in the porous shells[87].Jung and co-workers reported a controllable synthesis of helical silica-graphene-guest hybrids,with the enantioselective resolution ability for racemic phenylalanine,tryptophan and alanine derivatives(Fig.6).Helical silica nanotube was firstly obtained through the transcription of chiral diaminocyclohexane-based organogels.Then the encapsulation of graphene sheets on the(3-aminopropyl)triethoxysilane(APTES)-modi fied silica nanotubes was driven by electrostatic interactions.Finally,the negative charged hybrid facilitated the supramolecular chiral assembly of a positively charged guest molecule N1,N3,N5-tri(4-pyridinyl)cyclohexane-1,3,5-tricarboxamide(TPHC)[88].
4.3.Prospects of biomacromolecules on the development of chiral materials
Biomacromolecules especially deoxyribonucleic acids(DNA)so far have been widely used to develop new biosensors and chiral nanomaterials[89-91].Cooperative amplification of chiral bias of each nucleotide on the DNA chain leads to structural polymorphism through different base pairings including Watson-Crick,reverse Watson-Crick,Hoogsteen,reverse Hoogsteen pairings,with the resulting helical structures of Watson-Crick duplex,parallel duplex,triplex,G-quadruplex,imotif,A-motif,etc.[92,93].Intriguing features of speci fic recognition(DNA-DNA,DNA-ligand,DNA-protein,DNA-metal),structural interconversion triggered by chemical stimulus,and the feasibility to chemical modification,providefunda mental principleson theconstruction of chirally supramolecular assembly,chiroptical materials and helical architectures on the basis of chiral transfer,amplification and transcription.For example,sulfonated nickel(II)porphyrin molecules can assemble into chirally supermolecular structures templated by left-handed Z-DNA in the presence of spermine[94].DNA origami enabled the high-yield production of plasmonic structures that contain nanoparticles arranged in nanometer-scale helices(Fig.7a)[1].Chiral liquid-crystal-phase of calf thymus DNA directed the silica mineralization,resulting in the formation of enantiomeric helical architectures(Fig.7b)[2].
In the fields of chiral separation,although DNAmoleculesalonehave the ability to discriminate drug enantiomers such as tyrosine,tryptophan,ibuprofen and ofloxacin,there striction of drug specieshaslimited the development of DNA-based chiral selectors.Owing to the advantages of DNA structures on the exploration of chiroptical materials,it is promising to develop chiral DNA-based hybridshaving distinct chemical properties to broaden the enantioselective separation,on the basis of chiral induction,transfer,amplification and transcription.Although biomacromolecules possess high enantioselectivity in the molecular recognition of chiral compounds,in future more efforts should be devoted to enhance their stability against p H changes,temperature and solvents,as w ell as to reduce the cost involved in the chiral separation process.
5.Conclusions
Polymorphous structures of proteins and nucleic acids with inherent chirality provide apotential platform to chiral resolution for enantiomers.Preparative enantioseparation can be achieved through biomolecule modified hybrid materials including porous microspheres,magnetic nanoparticles and affinity ultra filtration membranes.On the basis of chiral induction,transfer,amplification and transcription,biomacromolecules are recognized as promising candidates to construct chiral hybrid materialshaving multiplefunctionsto broaden the enantioselectiveseparation of more drug enantiomers.

Fig.6.(a)Preparation of modified helical SNTs and GO,(b)wrapping of GO onto modified SNTs,(c)reduced GO hybrids with SNTs,(d)chiral transcription of TPHC[88].

Fig.7.(a)Left-and right-handed nanohelices(diameter 34 nm,helical pitch 57 nm)are formed by ninegold nanoparticles each of diameter 10 nm that are attached to the surface of DNA origami 24-helix bundles(each of diameter 16 nm)[1];(b)macroscopic enantiomeric helical morphologies and corresponding opposite DNA chiral packing of the impeller-like helical DNA-silica complexes(IHDSCs)[2].
[1]A.Kuzyk,R.Schreiber,Z.Fan,G.Pardatscher,E.M.Roller,A.Högele,F.C.Simmel,A.O.Govorov,T.Liedl,DNA-based self-assembly of chiral plasmonic nanostructures with tailored optical response,Nature 483(2012)311-314.
[2]B.Liu,L.Han,S.Che,Formation of enantiomeric impeller-like helical architectures by DNA self-assembly and silica mineralization,Angew.Chem.Int.Ed.51(2012)923-927.
[3]C.Wang,G.Jia,J.Zhou,Y.Li,Y.Liu,S.Lu,C.Li,Enantioselective Diels-Alder reactions with G-quad rup lex DNA-based catalysts,Angew.Chem.Int.Ed.51(2012)9352-9355.
[4]L.Feng,C.Zhao,Y.Xiao,L.Wu,J.Ren,X.Qu,Electrochemical DNA three-way junction based sensor for distinguishing chiral metallo-supramolecular complexes,Chem.Commun.48(2012)6900-6902.
[5]T.J.Ward,K.D.Ward,Chiral separations:a review of current topics and trends,Anal.Chem.84(2011)626-635.
[6]C.Ma,X.L.Xu,P.Ai,S.M.Xie,Y.C.Lv,H.Q.Shan,L.M.Yuan,Chiral separation of D,L-mandelic acid through cellulose membranes,Chirality 23(2011)379-382.
[7]K.Petruševska-Seebach,A.Seidel-Morgenstern,M.P.Elsner,Preferential crystallization of L-asparagine in water,Cryst.Growth Des.11(2011)2149-2163.
[8]K.Tang,P.Zhang,C.Pan,H.Li,Equilibrium studies on enantioselective extraction of oxybutynin enantiomers by hydrophilic β-cyclodextrin derivatives,AIChE J.57(2011)3027-3036.
[9]P.Tufvesson,J.Lima-Ramos,J.S.Jensen,N.Al-Haque,W.Neto,J.M.Woodley,Process considerations for the asymmetric synthesis of chiral amines using transaminases,Biotechnol.Bioeng.108(2011)1479-1493.
[10]K.Würges,K.Petruševska-Seebach,M.P.Elsner,S.Lütz,Enzyme-assisted physicochemical enantioseparation processes—part III:overcoming yield limitations by dynamic kinetic resolution of asp aragine via preferential crystallization and enzymatic racemization,Biotechnol.Bioeng.104(2009)1235-1239.
[11]N.M.Maier,P.Franco,W.Lindner,Separation of enantiomers:needs,challenges,perspectives,J.Chromatogr.A 906(2001)3-33.
[12]A.Berthod,Chiral recognition mechanisms,Anal.Chem.78(2006)2093-2099.
[13]M.Michaud,E.Jourdan,A.Villet,A.Ravel,C.Grosset,E.Peyrin,A DNA aptamer as a new target-specific chiral selector for HPLC,J.Am.Chem.Soc.125(2003)8672-8679.
[14]M.M.Wanderley,C.Wang,C.-D.Wu,W.Lin,A chiral porous metal-organic framework for highly sensitive and enantioselective fluorescence sensing of amino alcohols,J.Am.Chem.Soc.134(2012)9050-9053.
[15]T.Liu,Y.Liu,W.Xuan,Y.Cui,Chiral nanoscale metal-organic tetrahedral cages:Diastereoselective self-assembly and enantioselective separation,Angew.Chem.122(2010)4215-4218.
[16]P.Li,Y.He,J.Guang,L.Weng,J.C.-G.Zhao,S.Xiang,B.Chen,A homochiral microporous hydrogen-bonded organic framework for highly enantioselective separation of secondary alcohols,J.Am.Chem.Soc.136(2014)547-549.
[17]A.Shundo,K.Hori,T.Ikeda,N.Kimizuka,K.Tanaka,Design of a dynamic polymer interface for chiral discrimination,J.Am.Chem.Soc.135(2013)10282-10285.
[18]K.Huang,X.Dong,R.Ren,W.Jin,Fabrication of homochiral metal-organic framework membrane for enantioseparation of racemic diols,AIChE J.59(2013)4364-4372.
[19]T.Eralp,A.Ievins,A.Shavorskiy,S.J.Jenkins,G.Held,The importance of attractive three-point interaction in enantioselective surface chemistry:Stereospecific adsorption of serine on theintrinsically chiral Cu{531}surface,J.Am.Chem.Soc.134(2012)9615-9621.
[20]Y.Yun,A.J.Gellman,Enantioselective separation on naturally chiral metal surfaces:D,L-aspartic acid on Cu(3,1,17)R&S surfaces,Angew.Chem.Int.Ed.52(2013)3394-3397.
[21]T.S.van Erp,T.P.Caremans,D.Dubbeldam,A.Martin-Calvo,S.Calero,J.A.Martens,Enantioselective adsorption in achiral zeolites,Angew.Chem.122(2010)3074-3077.
[22]A.Martin-Calvo,S.Calero,J.A.Martens,T.S.van Erp,Adsorption of polar enantiomers in achiral zeolites,J.Phys.Chem.C 117(2013)1524-1530.
[23]C.Yamamoto,E.Yashima,Y.Okamoto,Structural analysis of amylose tris(3,5-dimethylphenylcarbamate)by NMR relevant to its chiral recognition mechanism in HPLC,J.Am.Chem.Soc.124(2002)12583-12589.
[24]S.Ma,S.Shen,H.Lee,M.Eriksson,X.Zeng,J.Xu,K.Fandrick,N.Yee,C.Senanayake,N.Grinberg,Mechanistic studies on the chiral recognition of polysaccharide-based chiral stationary phases using liquid chromatography and vibrational circular dichroism:reversal of elution order of N-substituted alpha-methyl phenylalanine esters,J.Chromatogr.A 1216(2009)3784-3793.
[25]R.B.Kasat,E.I.Franses,N.H.L.Wang,Experimental and computational studies of enantioseparation of structurally similar chiral compounds on amylose tris(3,5-dimethylphenylcarbamate),Chirality 22(2010)565-579.
[26]T.Ikai,Y.Okamoto,Structure control of polysaccharide derivatives for efficient separation of enantiomers by chromatography,Chem.Rev.109(2009)6077-6101.
[27]J.Ståhlberg,H.Henriksson,C.Divne,R.Isaksson,G.Pettersson,G.Johansson,T.A.Jones,Structural basis for enantiomer binding and separation of a common βblocker:crystal structure of cellobiohydrolase Cel7A with bound(S)-propranolol at 1.9 Å resolution,J.Mol.Biol.305(2001)79-93.
[28]L.Zhang,M.Song,Q.Tian,S.Min,Chiral separation of L,D-tyrosine and L,D-tryptophan by ct DNA,Sep.Purif.Technol.55(2007)388-391.
[29]W.Li,Y.Li,Y.Fu,J.Zhang,Enantioseparation of chiral ofloxacin using biomacromolecules,Korean J.Chem.Eng.30(2013)1448-1453.
[30]J.Haginaka,Enantiomer separation of drugs by capillary electrophoresis using proteins as chiral selectors,J.Chromatogr.A 875(2000)235-254.
[31]J.Haginaka,Protein-based chiral stationary phases for high-performance liquid chromatography enantioseparations,J.Chromatogr.A 906(2001)253-273.
[32]M.Lämmerhofer,Chiral recognition by enantioselectiveliquid chromatography:Mechanisms and modern chiral stationary phases,J.Chromatogr.A 1217(2010)814-856.
[33]V.T.G.Chuang,M.Otagiri,Stereoselective binding of human serum albumin,Chirality 18(2006)159-166.
[34]C.Bertucci,A.Canepa,G.A.Ascoli,L.F.L.Guimaraes,G.Felix,Site I on human albumin:Differences in the binding of(R)-and(S)-warfarin,Chirality 11(1999)675-679.
[35]T.Itoh,Y.Saura,Y.Tsuda,H.Yamada,Stereoselectivity and enantiomer-enantiomer interactions in the binding of ibuprofen to human serum albumin,Chirality 9(1997)643-649.
[36]H.Hödl,J.Koidl,M.G.Schmid,G.Gübitz,Chiral resolution of tryptophan derivatives by CE using canine serum albumin and bovine serum albumin as chiral selectors,Electrophoresis 27(2006)4755-4762.
[37]I.Petitpas,A.A.Bhattacharya,S.Twine,M.East,S.Curry,Crystal structure analysis of w arfarin binding to human serum albumin anatomy of drug site I,J.Biol.Chem.276(2001)22804-22809.
[38]F.Edwie,Y.Li,T.-S.Chung,Exploration of regeneration and reusability of human serum albumin as a stereoselective ligand for chiral separation in affinity ultra filtration,J.Membr.Sci.362(2010)501-508.
[39]H.Wang,Y.Li,T.S.Chung,A fine match between the stereoselective ligands and membrane pore size for enhanced chiral separation,AIChE J.55(2009)2284-2291.
[40]Z.Zhou,Y.Xiao,T.A.Hatton,T.S.Chung,Novel membrane processes for the enantiomeric resolution of tryptophan by selective permeation enhancements,AIChE J.57(2011)1154-1162.
[41]M.Ravikumar,S.Prabhakar,M.Vairamani,Chiral discrimination of α-amino acids by the DNA triplet GCA,Chem.Commun.(2007)392-394.
[42]J.Malina,O.Novakova,M.Vojtiskova,G.Natile,V.Brabec,Conformation of DNA GG intrastrand cross-link of antitumor oxaliplatin and its enantiomeric analog,Biophys.J.93(2007)3950-3962.
[43]M.Michaud,E.Jourdan,C.Ravelet,A.Villet,A.Ravel,C.Grosset,E.Peyrin,Immobilized DNA aptamers as target-specific chiral stationary phases for resolution of nucleoside and amino acid derivative enantiomers,Anal.Chem.76(2004)1015-1020.
[44]C.Ravelet,R.Boulkedid,A.Ravel,C.Grosset,A.Villet,J.Fize,E.Peyrin,A L-RNA aptamer chiral stationary phase for the resolution of target and related compounds,J.Chromatogr.A 1076(2005)62-70.
[45]P.-H.Lin,S.-J.Tong,S.R.Louis,Y.Chang,W.-Y.Chen,Thermodynamic basis of chiral recognition in a DNA aptamer,Phys.Chem.Chem.Phys.11(2009)9744-9750.
[46]Y.S.Kim,C.J.Hyun,I.Kim,M.B.Gu,Isolation and characterization of enantioselective DNA aptamers for ibuprofen,Bioorg.Med.Chem.18(2010)3467-3473.
[47]A.Shoji,M.Kuwahara,H.Ozaki,H.Sawai,Modified DNA aptamer that binds the(R)-isomer of a thalidomide derivative with high enantioselectivity,J.Am.Chem.Soc.129(2007)1456-1464.
[48]X.Qu,J.O.Trent,I.Fokt,W.Priebe,J.B.Chaires,Allosteric,chiral-selective drug binding to DNA,Proc.Natl.Acad.Sci.97(2000)12032-12037.
[49]P.P.Pellegrini,J.R.Aldrich-Wright,Evidence for chiral discrimination of ruthenium(II)polypyridyl complexes by DNA,Dalton Trans.(2003)176-183.
[50]J.G.Collins,J.R.Aldrich-Wright,I.D.Greguric,P.P.Pellegrini,Binding of the delta-and lambda-enantiomers of[Ru(dmphen)2dpq]2+to the hexanucleotide d(GTCGAC)2,Inorg.Chem.38(1999)5502-5509.
[51]C.Zhao,J.Geng,L.Feng,J.Ren,X.Qu,Chiral metallo-supramolecular complexes selectively induce human telomeric G-quadruplex formation under salt-deficient conditions,Chem.Eur.J.17(2011)8209-8215.
[52]H.Yu,X.Wang,M.Fu,J.Ren,X.Qu,Chiral metallo-supramolecular complexes selectively recognize human telomeric G-quadruplex DNA,Nucleic Acids Res.36(2008)5695-5703.
[53]C.Zhao,J.Ren,J.Gregoliński,J.Lisow ski,X.Qu,Contrasting enantioselective DNA preference:Chiral helical macrocyclic lanthanide complex binding to DNA,Nucleic Acids Res.40(2012)8186-8196.
[54]E.-J.Lee,J.-A.Yeo,K.Jung,H.J.Hwangbo,G.-J.Lee,S.K.Kim,Enantioselective binding of o floxacin to B form DNA,Arch.Biochem.Biophys.395(2001)21-24.
[55]H.J.Hw angbo,B.H.Yun,J.S.Cha,D.Y.Kwon,S.K.Kim,Enantioselective binding of S-and R-o floxacin to various synthetic polynucleotides,Eur.J.Pharm.Sci.18(2003)197-203.
[56]Y.Fu,X.Duan,X.Chen,J.Zhang,W.Li,Enantioselective separation of chiral ofloxacin using functional Cu(II)-coordinated G-rich oligonucleotides,RSC Adv.4(2014)1329-1333.
[57]Y.Fu,X.Duan,X.Chen,H.Zhang,J.Zhang,W.Li,Chiral discrimination of ofloxacin enantiomers using DNA double helix regulated by metal ions,Chirality 26(2014)249.
[58]W.Li,X.Chen,Y.Fu,J.Zhang,Enantioselective recognition mechanism of ofloxacin via Cu(II)-modulated DNA,J.Phys.Chem.B 118(2014)5300-5309.
[59]S.B.Lee,D.T.Mitchell,L.Tro fin,T.K.Nevanen,H.Soderlund,C.R.Martin,Antibodybased bio-nanotube membranes for enantiomeric drug separations,Science 296(2002)2198-2200.
[60]K.Singh,H.Bajaj,P.Ingole,A.Bhattacharya,Comparative stud y of enantioseparation of racemic tryptophan by ultra filtration using BSA-immobilized and BSA-interpenetrating network polysulfone membranes,Sep.Sci.Technol.45(2010)346-354.
[61]J.K.Yong,Y.Xiao,T.-S.Chung,The facile synthesis of an aldehyde-containing graft copolymer membrane for covalent protein capture with retention of protein functionality,J.Chromatogr.A 1217(2010)1904-1911.
[62]A.Higuchi,Y.Higuchi,K.Furuta,B.O.Yoon,M.Hara,S.Maniw a,M.Saitoh,K.Sanui,Chiral separation of p henylalanine by ultra filtration through immobilized DNA membranes,J.Membr.Sci.221(2003)207-218.
[63]Y.Matsuoka,N.Kanda,Y.M.Lee,A.Higuchi,Chiral separation of phenylalanine in ultra filtration through DNA-immobilized chitosan membranes,J.Membr.Sci.280(2006)116-123.
[64]A.Higuchi,A.Hayashi,N.Kanda,K.Sanui,H.Kitamura,Chiral separation of amino acids in ultra filtration through DNA-immobilized cellulose membranes,J.Mol.Struct.739(2005)145-152.
[65]K.Sakai-Kato,M.Kato,H.Nakakuki,T.Toyo'oka,Investigation of structure and enantioselectivity of BSA-encap sulated sol-gel columns prepared for capillary electrochromatography,J.Pharm.Biomed.Anal.31(2003)299-309.
[66]X.Weng,H.Bi,B.Liu,J.Kong,On-chip chiral separation based on bovine serum albumin-conjugated carbon nanotubes as stationary phase in a microchannel,Electrophoresis 27(2006)3129-3135.
[67]H.F.Li,H.Zeng,Z.Chen,J.M.Lin,Chip-based enantioselective open-tubular capillary electrochromatography using bovine serum albumin-gold nanoparticle conjugates as the stationary phase,Electrophoresis 30(2009)1022-1029.
[68]R.-P.Liang,X.-N.Wang,C.-M.Liu,X.-Y.Meng,J.-D.Qiu,Facile preparation of protein stationary phase based on polydopamine/graphene oxide platform for chip-based open tubular capillary electrochromatography enantioseparation,J.Chromatogr.A 1323(2014)135-142.
[69]M.Martínez-Gómez,J.Martínez-Pla,S.Sagrado,R.Villanueva-Camañas,M.Medina-Hernández,Chiral separation of oxprenolol by af finity electrokinetic chromatography-partial filling technique using human serum albumin as chiral selector,J.Pharm.Biomed.Anal.39(2005)76-81.
[70]C.-M.Liu,R.-P.Liang,X.-N.Wang,J.-W.Wang,J.-D.Qiu,A versatile polydopamine platform for facile preparation of protein stationary phase for chip-based open tubular capillary electrochromatography enantioseparation,J.Chromatogr.A 1294(2013)145-151.
[71]Z.Zhai,Y.Chen,Y.J.Wang,G.S.Luo,Chiral separation performance of micrometersized monodispersed silica spheres with high protein loading,Chirality 21(2009)760-768.
[72]H.J.Choi,M.H.Hyun,Separation of enantiomers with magnetic silica nanoparticles modi fied by a chiral selector:Enantioselective fishing,Chem.Commun.(2009)6454-6456.
[73]X.Chen,J.Rao,J.Wang,J.J.Gooding,G.Zou,Q.Zhang,A facile enantioseparation for amino acids enantiomers using β-cyclodextrins functionalized Fe3O4nanospheres,Chem.Commun.47(2011)10317-10319.
[74]Y.Wang,P.Su,S.Wang,J.Wu,J.Huang,Y.Yang,Dendrimer modified magnetic nanoparticles for immobilized BSA:a novel chiral magnetic nano-selector for direct separation of racemates,J.Mater.Chem.B 1(2013)5028-5035.
[75]Y.Fu,T.Huang,B.Chen,J.Shen,X.Duan,J.Zhang,W.Li,Enantioselective resolution of chiral drugs using BSA functionalized magnetic nanoparticles,Sep.Purif.Technol.107(2013)11-18.
[76]J.Ruta,S.Perrier,C.Ravelet,B.Roy,C.Perigaud,E.Peyrin,Aptamer-modified micellar electrokinetic chromatography for the enantioseparation of nucleotides,Anal.Chem.81(2009)1169-1176.
[77]R.Huang,W.Xiong,D.Wang,L.Guo,Z.Lin,L.Yu,K.Chu,B.Qiu,G.Chen,Label-free aptamer-based partial filling technique for enantioseparation and determination of DL-tryptophan with micellar electrokinetic chromatography,Electrophoresis 34(2013)254-259.
[78]R.Huang,D.Wang,S.Liu,L.Guo,F.Wang,Z.Lin,B.Qiu,G.Chen,Preparative separation of enantiomers based on functional nucleic acids modi fied gold nanoparticles,Chirality 25(2013)751-756.
[79]Q.Jin,L.Zhang,X.Zhu,P.Duan,M.Liu,Amphiphilic Schiff base organogels:Metal-ionmediated chiral twists and chiral recognition,Chem.Eur.J.18(2012)4916-4922.
[80]H.Jintoku,M.Takafuji,R.Oda,H.Ihara,Enantioselective recognition by a highly ordered porphyrin-assembly on a chiral molecular gel,Chem.Commun.48(2012)4881-4883.
[81]W.Miao,L.Zhang,X.Wang,H.Cao,Q.Jin,M.Liu,A dual-functional metallogel of amphiphilic copper(II)quinolinol:Redox responsiveness and enantioselectivity,Chem.Eur.J.19(2013)3029-3036.
[82]W.Xuan,M.Zhang,Y.Liu,Z.Chen,Y.Cui,A chiral quadruple-stranded helicate cage for enantioselective recognition and separation,J.Am.Chem.Soc.134(2012)6904-6907.
[83]W.Wang,X.Dong,J.Nan,W.Jin,Z.Hu,Y.Chen,J.Jiang,A homochiral metal-organic framework membrane for enantioselective separation,Chem.Commun.48(2012)7022-7024.
[84]P.Paik,A.Gedanken,Y.Mastai,Chiral-mesoporous-polypyrrole nanoparticles:Its chiral recognition abilities and use in enantioselective separation,J.Mater.Chem.20(2010)4085-4093.
[85]C.Casado,J.Castán,I.Gracia,M.Yus,A.Mayoral,V.Sebastián,P.López-Ram-de-Viu,S.Uriel,J.Coronas,L-and D-proline adsorption by chiral ordered mesoporous silica,Langmuir 28(2012)6638-6644.
[86]S.Fireman-Shoresh,S.Marx,D.Avnir,Enantioselective sol-gel materials obtained by either doping or imprinting with a chiral surfactant,Adv.Mater.19(2007)2145-2150.
[87]W.Liu,Z.Zhu,K.Deng,Z.Li,Y.Zhou,H.Qiu,Y.Gao,S.Che,Z.Tang,Gold nanorod@chiral mesoporous silica core-shell nanoparticles with unique optical properties,J.Am.Chem.Soc.135(2013)9659-9664.
[88]J.H.Jung,S.-J.Moon,J.Ahn,J.Jaw orski,S.Shinkai,Controlled supramolecular assembly of helical silica nanotube-graphene hybrids for chiral transcription and separation,ACS Nano 7(2013)2595-2601.
[89]J.Ren,J.Wang,J.Wang,E.Wang,Colorimetric enantiorecognition of oligopeptide and logic gate construction based on DNA aptamer-ligand-gold nanoparticle interactions,Chem.Eur.J.19(2013)479-483.
[90]X.Shen,A.Asenjo-Garcia,Q.Liu,Q.Jiang,F.Favier Garcia de Abajo,N.Liu,B.Ding,Three-dimensional plasmonic chiral tetramers assembled by DNA origami,Nano Lett.13(2013)2128-2133.
[91]B.Liu,Y.Cao,Y.Duan,S.Che,Water-dependent optical activity inversion of chiral DNA-silica assemblies,Chem.Eur.J.19(2013)16382-16388.
[92]L.A.Yatsunyk,O.Mendoza,J.-L.Mergny,“Nano-oddities”:Unusual nucleic acid assemblies for DNA-based nanostructures and nanodevices,Acc.Chem.Res.47(2014)1836-1844.
[93]J.Choi,T.Majima,Conformational changes of non-B DNA,Chem.Soc.Rev.40(2011)5893-5909.
[94]A.D'Urso,A.Mammana,M.Balaz,A.E.Holmes,N.Berova,R.Lauceri,R.Purrello,Interactions of atetraanionic porphyrin with DNA:From a Z-DNAsensor to aversatile supramolecular device,J.Am.Chem.Soc.131(2009)2046-2047.
杂志排行
Chinese Journal of Chemical Engineering的其它文章
- Scoping biology-inspired chemical engineering☆
- Review on the nanoparticle fluidization science and technology☆
- Multi-functional forward osmosis draw solutes for seawater desalination☆
- Process engineering in electrochemical energy devices innovation☆
- In-situ design and construction of lithium-ion battery electrodeson metal substrates with enhanced performances:A brief review☆
- Developments in the understanding of gas-solid contact efficiency in the circulating fluidized bed riser reactor:A review☆
