Nutritional composition,in vitro antioxidant and anti-diabetic potentials of Breynia retusa(Dennst.)Alston
2016-05-24RajanMuruganJaganathanPrabuRahulChandranThangarajanSajeeshMurugaiyanIniyavanThangarajParimelazhagan
Rajan Murugan,Jaganathan Prabu,Rahul Chandran,Thangarajan Sajeesh,Murugaiyan Iniyavan,Thangaraj Parimelazhagan
Bioprospecting Laboratory,Department of Botany,Bharathiar University,Coimbatore 641 046,Tamil Nadu,India
Abstract
Keywords:Antioxidant;Alpha-amylase;Breynia retusa;Phenolic comounds;Vitamins
1.Introduction
Diabetes mellitus is a metabolic disorder characterized by chronic hyperglycaemia leading to a progressive generation of oxidation products derived from glucose.Glucose is oxidized by the transition-metal-dependent reaction to form enediol radical anion that is converted into reactive ketoaldehydes and to superoxide anion radicals.These superoxide anion radicals undergo dismutation to produce hydrogen peroxide radicals and it may lead to production of extremely reactive hydroxyl radicals in the presence of transition metals[1,2].These reactive oxygen species affects the metabolism of carbohydrates,proteins,fat,electrolytes and water in the human body due to increased oxidative stress.Recent evidences suggest that oxidative stress and changes in the formation of reactive species play major roles in the onset of diabetic complications[3].Nowadays,several drugs are commercially available and used to treat diabetic complications.However,some of the standard drugs such as sulfonylureas,biguanides and alpha glucosidase inhibitors such as acarbose,glinides and miglitol cause side effects nausea,liver disorders,flatulence,abdominal pain,renal tumours,low blood glucose,dark urine and hepatic injury[4–6].Therefore,new antioxidant and anti-diabetic drugs from medicinal plants are needed to replace these synthetic drugs.
Currently,the consumption of fruits and vegetables has been used worldwide for health benefits because of the presence of nutritive and non-nutritive chemicals from medicinal plants which protect the humans from oxidative stress related disorders.Although the wild fruits are nutritious,more consumption of such fruits is hazardous to our body,therefore before eating it must be checked whether it contains any anti-nutritional factors.The anti-nutritional factors such as trypsin inhibitors and tannins inhibit the protein digestion,growth,iron and zinc absorption[7].Natural antioxidants from edible plants are source for dietary components to promote healthy life.For example, α-amylase and α-glucosidase inhibitors are considered as one of the effective measures for regulating type II diabetes by preventing the glucose oxidation[8].
Breyniaretusa(commonly known as Cup Saucer plant)belongs to the family Euphorbiaceae.MaceratedB.retusaleaf juice is used to cure body pain,skin inflammation,hyperglycaemia,diarrhoea and diuretic.The fruits have been used for dysentery and twigs used for toothache[9,10].Young leaves are cooked and used as poultice to hasten suppuration[11,12].Literatures survey showed thatB.retusaleaves have good total phenolic contents,antioxidant[13]and anti-diabetic activity[14,15].These studies can be taken as a strong platform to carry out antioxidant and anti-diabetic potentials inB.retusa.Hence,the present investigation on leaf and fruit was undertaken to study the nutritional composition,anti-nutritional,total phenolic content,antioxidant and anti-diabetic properties ofB.retusa.
2.Materials and methods
2.1.Chemicals
All the chemicals used in the study were of analytical grade.N α-Benzoyl-DL-arginine-4-nitroanilide hydrochloride(BAPNA),2,2-diphenyl-1-picryl-hydrazyl(DPPH),2,2'azinobis(3-ethylbenzothiazoline)-6-sulfonic acid diammonium salt(ABTS)and 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid(Trolox)were purchased from Sigma chemicals Co(St.Louis,MO,USA).All the other reagents were obtained from Himedia Laboratories(Mumbai,Maharashtra,India).
2.2.Collection and identification of plant materials
The fresh leaves and fruits ofB.retusawere collected from Kotagiri hills,The Nilgiris during the month of October,2014.The taxonomic identity of the plant was confirmed by Botanical Survey of India,Southern circle,Coimbatore,Tamil Nadu,India.(No.BSI/SRC/5/23/2015/Tech/531).The plant materials were washed under running tap water to remove the surface pollutants and the whole plants were air dried under shade.The dried samples were powdered and used for further studies.
2.3.Nutritional evaluation of B.retusa
The leaf and fruit samples were quantitatively analyzed for starch,total carbohydrates,total free aminoacids[16]and total proteins[17]using spectrophotometric(Shimadzu,Japan)methods.Ash and crude fibre content were obtained by the method of Sadasivam and Manickam[18]and minerals(K,Na,Cl,Ca,S,Cu,Fe,Mg,Mn,Zn,Mo,P and I)were estimated according to the method of AOAC[19].
2.4.Determination of trypsin inhibitor activity and tannin contents
Trypsin inhibition activity was evaluated in powdered leaf and fruit samples on a synthetic substrate BAPNA.A trypsin inhibitor unit(TIU)was defined in terms of trypsin units inhibited per mg of protein.Tannin content was determined by a spectrophotometric method and the results were expressed as tannic acid equivalents from a standard curve of different concentrations of tannic acid[16].
2.5.Preparation of plant extracts
The powdered plant materials of leaf and fruit ofB.retusa(100 g)were packed in small thimbles separately and extracted successively with organic solvents(400 mL)such as petroleum ether,chloroform,ethyl acetate and methanol in the increasing order of polarity using Soxhlet apparatus.The different solvent extracts were concentrated by rotary vacuum evaporator(Yamato RE300,Japan)and then air dried.The dried extract obtained with each solvent was weighed and percentage yield was calculated by the following formula:
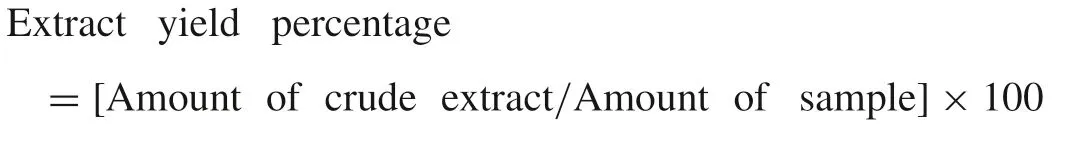
The extracts obtained were used for the assessment of various antioxidant assays and other analysis(1 mg/mL of respective organic solvents).
2.6.Quantification of total phenolics,flavonoids,vitamin C and E contents
The total phenolic content was determined according to the method described by Siddhuraju and Becker[20]and the results were expressed as gallic acid equivalents(GAE).Total flavonoids in the extracts were estimated as rutin equivalents according to the method of Zhishen et al.[21].The vitamin C content in leaf and fruit extracts was estimated based on the method of Sadasivam and Manikam[16]and the results were expressed as milligrams of ascorbic acid equivalents per gram extract.The vitamin E content was determined by Prieto et al.[22]method and α-tocopherol was used as standard.
2.7.In vitro antioxidant assays
2.7.1.DPPH•scavengingassay
The DPPH radical was used to measure the free radical scavenging activity of plant extracts by the method of Blois[23].Different concentrations of leaf and fruit extracts were taken and 3 mL of a 0.1 mmol/L methanolic solution of DPPH was added to the aliquots of different extracts of plant sample and standards.DPPH solution(3 mL)along with methanol(100μL)was used as a negative control.All the reaction mixtures were incubated for 20 min at 27℃.DPPH radical inhibition by the plant samples was measured at 517 nm against the blank(methanol).The results were expressed as inhibitory concentration at 50 percentage of DPPH radical scavenging by plant extracts(μg/mL).
2.7.2.ABTS•+scavengingassay
The total antioxidant activity ofB.retusaextracts was determined by ABTS radical cation scavenging assay by Re et al.[24]method.7 mmol/L ABTS(stable radical)aqueous solution and 2.4 mmol/L potassium persulfate was used to produce ABTS radical cation in the dark at 12–16 h.Prior to assay,ABTS solution was diluted in ethanol(1:89 v/v)to give an absorbance of 0.700±0.02 at 734 nm.Triplicates of 10μL sample and aliquots of trolox(concentration 0–15μmol/L)was added to 1 mL of diluted ABTS solution.The reaction mixture was incubated at 30℃ exactly 30 min and the absorbance was measured at 734 nm against the ethanol(blank).The results were expressed asμmol/L Trolox equivalents antioxidant capacity(TEAC)/g extract.
2.7.3.Phosphomolybdenumassay
The total antioxidant activity of leaf and fruit samples was determined by the green phosphomolybdenum assay by the method of Prieto et al.[22].250μL of sample and standard(1 mmol/L ascorbic acid in DMSO)was added to 3 mL of reagent solution(0.6 mol/L sulphuric acid,28 mmol/L sodium phosphate and 4 mmol/L ammonium molybdate).The reaction mixture was incubated at 95℃ for 90 min.The absorbance of the mixture was measured at 695 nm against the reagent blank(reaction mixture).The results reported are mean values and expressed as gram of ascorbic acid equivalents(AAE)/100 g extract.
2.7.4.Ferricreducingantioxidantpower(FRAP)assay
The ferric reducing ability of different extracts of leaf and fruitwas estimated by the method of Pulidoetal.[25].The FRAP reagent was prepared by mixing 2.5 mL of 10 mmol/L TPTZ in 40 mmol/L HCl,2.5 mL of 20 mmol/L FeCl3·6H2O and 25 mL of 0.3 mol/L acetate buffer(pH 3.6).900μL of FRAP reagent was mixed with 10μL of aliquots of plant extracts and incubated at 37℃.After incubation,ferric reducing ability of plant extracts was measured at 595 nm.The results were expressed as μmol/L Fe(II)equivalents/mg extract.
2.7.5.Metalionchelatingactivity
The chelating activity ofB.retusawas determined by the method of Dinis et al.[26].500μL of samples were added to 100μL solution of 2 mmol/L FeCl2.The reaction was initiated by the addition of 400μL of 5 mmol/L ferrozine and incubated at room temperature for 10 min.Absorbance of the samples was then measured spectrophotometrically at 562 nm against the blank(deionized water).The metal chelating capacities of the extracts were expressed as mg EDTA equivalents/g extract.
2.7.6.Lipidperoxidationinhibitionassay
A modified thiobarbituric acid-reactive substances(TBARS)assay was used to measure the lipid peroxide formed,using goat liver homogenates[27].Malondialdehyde(MDA),a secondary end product of the oxidation of polyunsaturated fatty acids,reacts with two molecules of TBA yielding a pinkish red chromogen.Liver homogenate(500μL of 10%,v/v in phosphate-buffered saline pH 7.4)and 500μL of sample were added to a test tube and made up to 1.0 mL with distilled water.Then,50μL of FeSO4(0.075 mol/L)and 20μL ofLascorbic acid(0.1 mol/L)were added and incubated for 1 h at 37℃ to induce lipid peroxidation.Thereafter,0.2 mL of EDTA(0.1 mol/L)and 1.5 mL of TBA reagent(1.5 g TBA,60 g TCA and 5.2 mL 70% HClO4in 800 mL of distilled water)were added in each sample and heated for 15 min at 100℃.After cooling,samples were centrifuged for 10 min at 3000×gand absorbance of supernatant was measured at 532 nm.Inhibition(%)of lipid peroxidation was calculated using the equation:
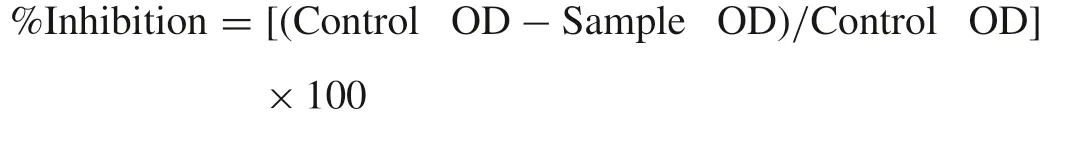
2.8.In vitro anti-diabetic assay
2.8.1. α-Amylaseinhibitionassay
B.retusaextracts(250μL)and 0.02 mol/L sodium phosphate buffer(pH 6.9 with 0.006 mol/L NaCl)containing porcine pancreatic α-amylase enzyme(0.5 mg/mL)was incubated at 25℃ for 10 min.250μL of 1% starch solution in 0.02 M sodium phosphate buffer(pH 6.9 with 0.006 mol/L NaCl)was added to the reaction mixture after incubation.Subsequently,the reaction mixture was incubated at 25℃ for 10 min,followed by addition of 2 mL of dinitrosalicylic acid(DNS).Finally the reaction was stopped by incubation of boiling water bath for 5 min and cooled to room temperature.The reaction mixture was diluted with 10 mL distilled water and the absorbance was measured at 540 nm.The mixture of all other reagents and the enzyme except the test sample was used as a control and the results of αamylase inhibition activity were expressed in terms of inhibition percentage[28].
2.8.2. α-Glucosidaseinhibitionassay
B.retusaextracts(1 mL)and1 mL of α-glucosidase(1 U/mL)in 0.1 mol/L phosphate buffer(pH 6.9)solution was incubated at 25℃ for 10 min.Then,1 mL of 5 mmol/Lp-nitrophenyl α-D-glucopyranoside in 0.1 mol/L phosphate buffer(pH 6.9)solution was added.Reaction mixtures were incubated at 25℃ for 10 min and the absorbance was taken at 405 nm by a spectrophotometer.The mixture of all other reagents and the enzyme except the test sample was used as a control and the results of α-glucosidase inhibition activity were expressed in terms of inhibition percentage[28].
2.9.Statistical analyses
All the experiments were done in triplicates and the results were expressed as Mean±SD.The data were statistically analyzed using one way ANOVA by Duncan’s test for all the studies.Mean values were considered statistically significant whenp<0.05.
3.Results and discussion
3.1.Nutrition composition of leaves and fruits
The aim of the present study was to assess the nutrient value ofB.retusaleaves and fruits which are widely consumed by the local Kurumba tribes of the Kotagiri hill region,Tamil Nadu.Proximate compositions ofB.retusaleaf and fruit are shown in Table 1.The starch content was high in fruit(3.67±0.10 g starch equivalents/100 g sample)while in leaf extracts a high total carbohydrate content was found(14.08±0.27 g glucose equivalents/100 g sample).The total protein content was higher in fruit(3.85±0.61 g BSA equivalents/100 g sample)as compared to leaves(2.65±0.09 g BSA equivalents/100 g sample).However,previous studies showed that carbohydrates and proteins contents were found to be 9.1±0.4 mg/g sample and 33.5±1.4 mg/g for the samples in the macerated leaves,respectively[14].Moreover,fruit was also having high amounts of free amino acids(52.25±1.76 g leucine equivalents/100 g sample).The ash content of fruit was determined as 9.50% and that of leaf as 19.04%.The crude fibre was found to be high in leaf(0.52%)and in fruit as0.40%.Mineral analysis showed that leaf and fruits are rich in K,Na,Cl,Ca,S,Cu and Fe as shown in Table 2.The minerals Cl(1020 ppm),Ca(4040 ppm),S(2005 ppm)and Cu(3800 ppm)were found higher in the fruits.From the results obtained,total starch,total proteins,total free amino acids and minerals were found higher in wild edible fruits as compared to the leaves ofB.retusa.Further,dietary compounds from plants have played a crucial role in the treatment of diabetes by inhibiting the free radical generation[29].Therefore,nutritional rich plant ofB.retusacan be recommended as a dietary supplement.
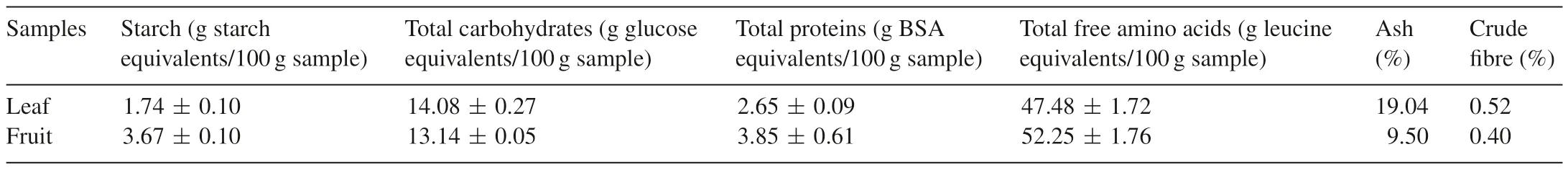
Table 1Proximate composition of B.retusa.
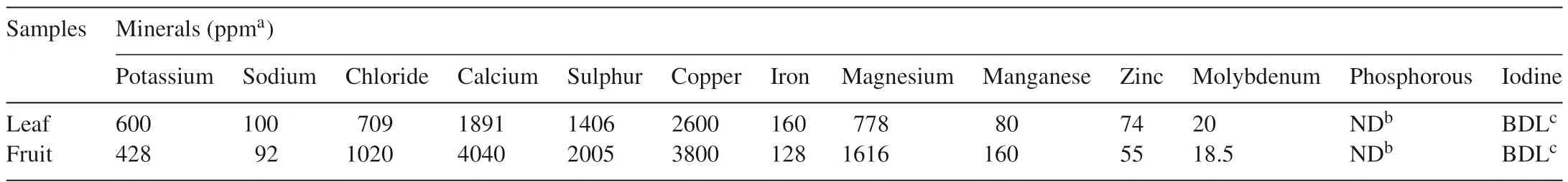
Table 2Mineral composition of B.retusa.
3.2.Anti-nutrient contents of B.retusa
The presence of anti-nutritional factors can limit nutrition and food qualities of plants[30].Anti-nutrients such as trypsin inhibitors ingested in significant amounts disrupt the digestive process and may lead to cause many digestive diseases.The major effect of tannin is growth depression by reducing the digestibility of protein and carbohydrate metabolism.The antinutrient contents ofB.retusashowed good trypsin inhibiting activity and lower amount of tannins(Table 3).Leaves and fruit samples showed 14.08±0.03 and 13.14±0.034 TIU/mg protein respectively.Moreover,tannin contents of leaf and fruit were 0.011±0.001 and 0.028±0.001 mg TAE/g for the samples,respectively.From the results,lower anti-nutrient content ofB.retusaleaves and fruits should not pose a problem to human digestion andB.retusacould be used as a food ingredients.
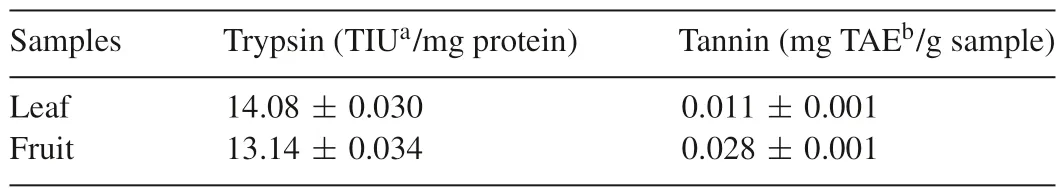
Table 3Anti-nutrient content of B.retusa.
3.3.Extractability,total phenolic and flavonoid contents of B.retusa
Soxhlet extraction is a standard method for the extraction of bioactive compounds from plant sources.The extract yield percentage ofB.retusais shown in Fig.1.The fruit methanol extract(15.89%)showed higher recovery percentage while petroleum ether showed moderate yield percentage(12.36%).Considering the leaf extracts,methanol extract(6.12%)showed higher recovery percentage than other solvent extracts.
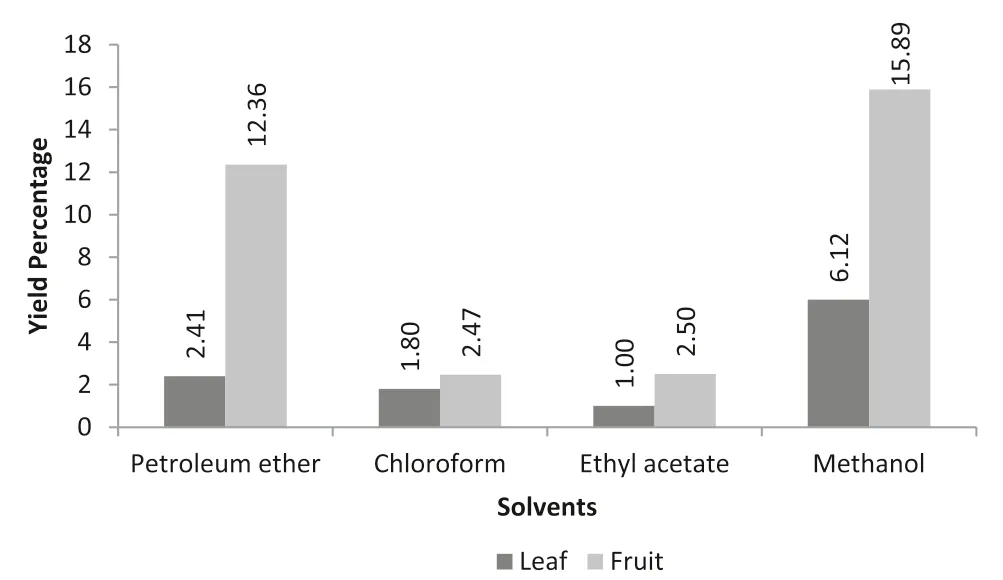
Fig.1.Extract yield percentage.
Leaf and fruit extracts were quantitatively analyzed for total phenolics,flavonoids,vitamin C and E contents as shown in Table 4.The total phenolic content was found to be higher in methanol extract of leaf(81.05±0.42 g GAE/100 g extract)while in fruit methanol extract(53.02±0.95 g GAE/100 g extract)also showed appreciable amount of total phenolic contents.Moreover,the ethyl acetate extract of fruit was found to be higher in flavonoid contents(259.33±7.37 g RE/100 g extract)as compared to other solvent extracts.Leaf ethyl acetate extract also showed moderate flavonoid content(208.94±50.03 g RE/100 g extract).Similarly,Kripa et al.[14]reported that macerated extract ofB.retusaleaves have lower amount of total phenolic content(25.09±0.2 mg/mL)and flavonoid content(6.00±0.11 mg/mL).The results revealed maximum quantity of vitamin C inB.retusafruit methanol extract(4.48±1.10 g AAE/100 g extract)whereas lower in the petroleum ether extract of fruit(0.36±0.58 g AAE/100 g extract).Vitamin E content was highest in leaf methanol extract(38.15±2.78 g TE/100 g extract).
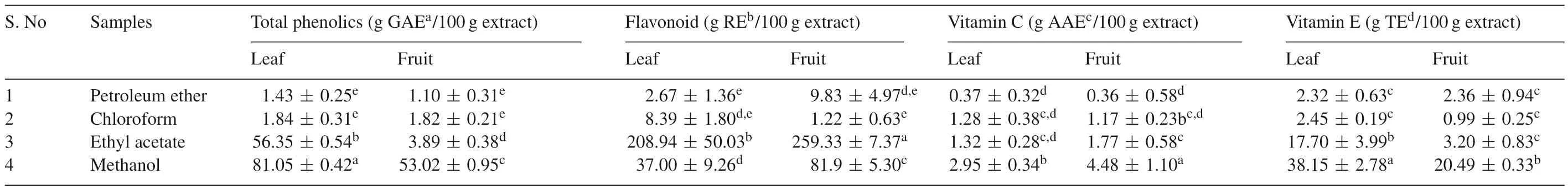
Table 4Total phenolic,fa lvonoid,vitamin C and vitamin E contents of B.retusa.
For our experiments,petroleum ether,chloroform,ethyl acetate and methanol have been used to extract the lipophilic compounds(oils and fatty acids),pigments(chlorophyll)and polyphenolics(phenolics and flavonoids).Among the different solvent extracts,methanol extract of leaf and fruit showed higher amount of total phenolics,vitamin C and E than low polar solvents.Further,methanol extracts have been proven effective solvent to extract phenolic compounds than other polar solvents[20].Our results demonstrate that Soxhlet method is an effective method for extraction of phenolics,flavonoids and vitamins as compared to maceration in correspondence to Murugan and Parimelazhagan[31].It has been shown that phenolics,flavonoids,vitamins C and E possess antioxidant and anti-diabetic activity[8,32].Therefore,bioactive compounds ofB.retusamay have anti diabetic activity.
3.4.DPPH•scavenging activity
Antioxidant activity of samples was estimated using the DPPH radical assay.The DPPH•assay is a well accepted method to evaluate the free radical scavenging activity of plant extracts because of its simple,rapid,sensitive and reproducible procedure[33].The results of DPPH assay are expressed in IC50values where low values are an indication of high antioxidant activity.Among different solvent extracts ofB.retusastudied,the methanol extract of leaf(2.42μg/mL)in contrast to fruit methanol extract(4.24μg/mL)showed better DPPH radical scavenging activities as compared to other solvent extracts and are shown in Fig.2.Similarly,Sudhanshu et al.[34]has also been reported to have anti-DPPH•activity inB.retusaextracts.From this,it is clear that methanol extracts showed better radical scavenging activity by reducing the stable DPPH radical to a yellowish diphenylpicrylhydrazine derivative.It also exhibited good antioxidant activity comparable to that of quercetin(4.70μg/mL)and BHT(31.12μg/mL)which were used as reference compounds.
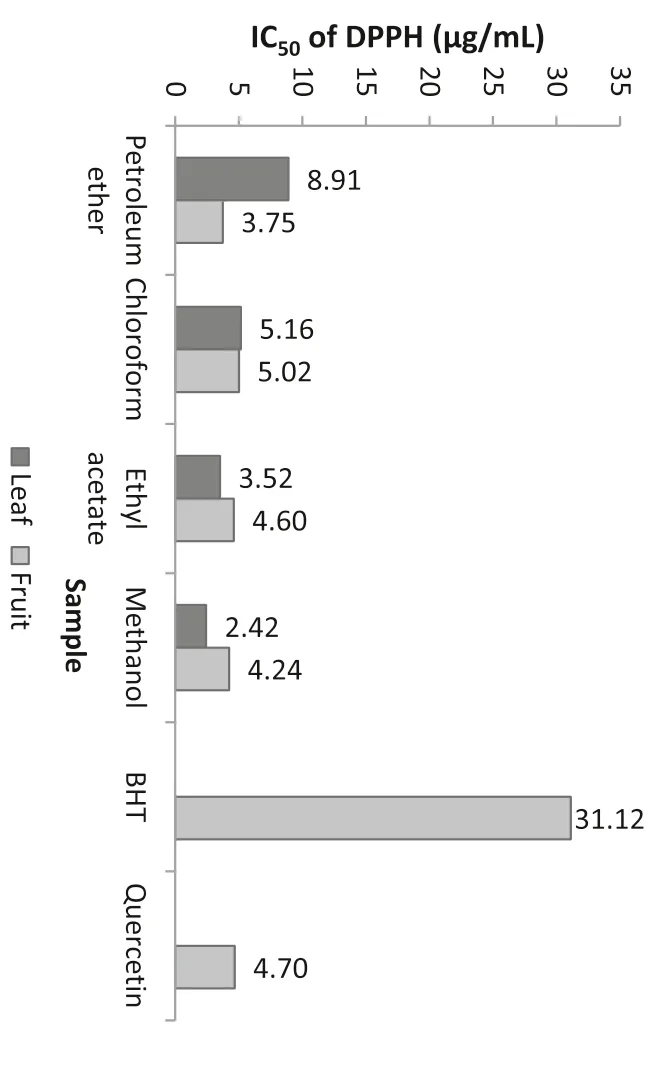
Fig.2.DPPH radical scavenging activity.
3.5.ABTS•+scavenging activity
ABTS is a radical cation assay commonly used to test radical scavenging activity of plant extracts.The ABTS cation radicals were generated from oxidation of ABTS based on the method of Re et al.[24].The results were expressed asμmol/L TEAC/g extract.The results of ABTS•+scavenging activities of leaf and fruit extracts ofB.retusaare shown in Table 5.The ethyl acetate extract of leaf showed high radical scavenging activity(7195.45±44.31μmol/L TEAC/g extract).Moreover,fruit ethyl acetate extract(978.74±18.65μmol/L TEAC/g extract)also showed better total antioxidant activity when compared to other solvent extracts.Many plant extracts showed higher total antioxidant activity because of their higher phenolic contents used as potential nutraceutical[35,36].Thus,due to the total antioxidant activity ofB.retusait might be used as a natural additive with strong free radical scavenger potential.
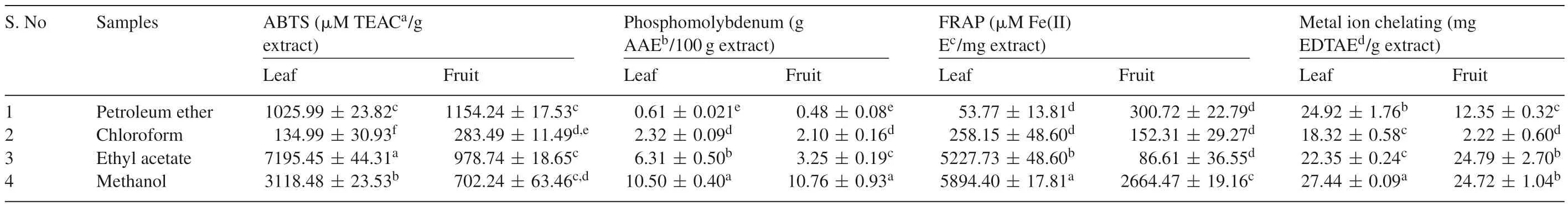
Table 5Antioxidant activity of B.retusa.
3.6.Phosphomolybdenum assay
The molybdenum reduction assay is an essential method to determine the antioxidant potential of plant extracts.The total antioxidant capacity of different solvent extracts ofB.retusawere analyzed and shown in Table 5.Among different solvent extracts used,higher phosphomolybdenum reduction was observed by leaf and fruit methanol extracts and was 10.50±0.40 and 10.76±0.93 g AAE/100 g extract respectively.The lower antioxidant capacities of the fruit extracts were found to be 0.48±0.08 g AAE equivalents/100 g extract.
From the obtained results,phenolic compounds(antioxidants)in the leaf and fruit extracts interfere with Mo(VI)to Mo(V)reduction and produce the green phosphate/Mo(V)complex[37].Since the phosphomolybdenum reduction assay particularly quantifies vitamin C content in plant samples[22],total antioxidant activity of methanol extracts may be due the presence vitamin C contents inB.retusa.
3.7.Ferric reducing ability
FRAP is simple and reliable test to measure the reducing potential of an antioxidant reacting with a Fe(III)-TPTZ complex producing a coloured Fe(II)-TPTZ complex by a antioxidant[38].A higher absorbance power indicates a higher ferric reducing ability of plant extracts(Table 5).Leaf ethyl acetate(5227.73±48.60μmol/L Fe(II)E/mg extract)and methanol extract(5894.40±17.81μoml/L Fe(II)E/mg extract)exhibited high ferric reducing ability,whereas the fruit methanol extract(2664.47±19.16μmol/L Fe(II)E/mg extract)showed only moderate ferric reducing ability.Siddhuraju and Becker[20]have reported that the ferric reducing ability of plant extracts mainly depends on phenolic contents of plant extracts.Thus,the ferric reducing ability ofB.retusaleaves reveals that polyphenolic compounds in the methanol extracts may have high affinity to the ferrous ions and thereby scavenge them through donating hydrogen atom.
3.8.Metal chelating activity
The metal chelating activities of leaf and fruit are shown in Table 5.The metal chelating activity is based on chelating of Fe2+ions by the ferrozine method and the results were expressed as mg EDTAE/g extract[26].Among the different extracts,methanol extract of leaf(27.44±0.09 mg EDTAE/g extract)showed highest scavenging ability.Metal chelating activity of fruit was found to be 24.72±1.04 mg EDTAE/g extract.Chelating agents may serve as secondary antioxidants because they reduce the redox potential thereby stabilizing the oxidized form of the metal ions[39].Our results suggest that phenolic compounds and flavonoids present inB.retusamay inhibit interaction between metal and lipid through formation of insoluble metal complexes with ferrous ion.
3.9.Lipid peroxidation inhibition activity
Free radicals especially reactive oxygen species cause lipid peroxidation in cell membranes particularly through oxidation of polyunsaturated fatty acids[40].As shown in Fig.3,B.retusaextracts exhibited a strong inhibitory effect on lipid peroxidation and the inhibitory effect was concentration dependent.Lipid peroxidation inhibition was estimated by inhibition of malondialdehyde production by the antioxidants.In this assay,malondialdehyde was produced by reaction between ferrous sulphate and TBA.Invitromalondialdehyde can alter proteins,DNA,RNA and many other biomolecules[27].The methanol extract of fruit(52.19%)showed strong inhibition at high concentrations(500μg).On the other hand,quercetin(58.90%)exhibited higher inhibition than BHT(24.34%).Based on our study,it is suggested that dietary compounds from fruit methanol extract can interfere with Fe2+induced lipid peroxidation and inhibit the production of malondialdehyde thus preventing lipid peroxidation.
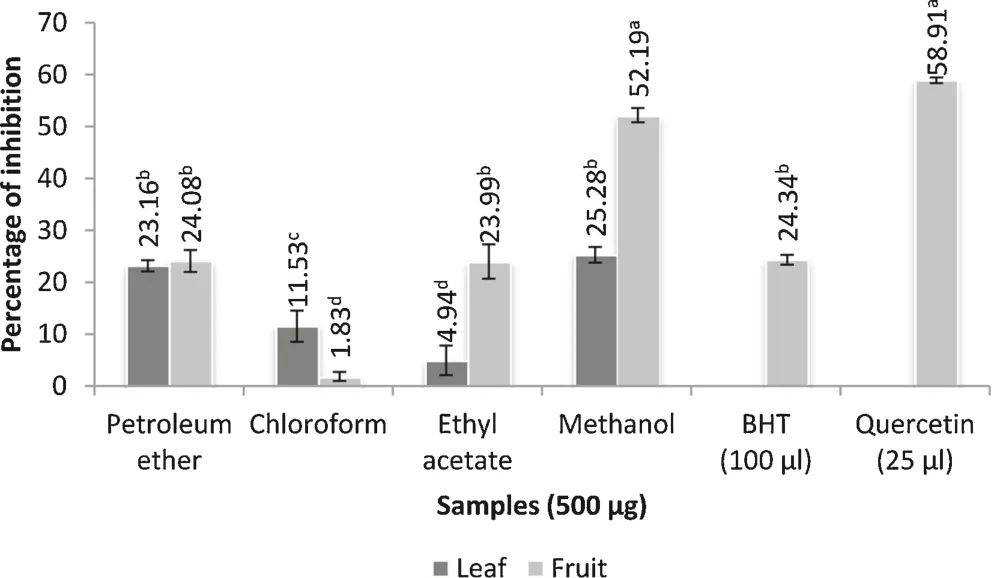
Fig.3.Lipid peroxidation inhibition assay.Values are mean of triplicate determination(n=3)±standard deviation;statistically significant at p<0.05 where a>b>c>d.
3.10.Anti-diabetic activity
Diabetes mellitus is a chronic disease associated with high concentrations of blood sugar which can cause serious complications.Consequently,the treatment is mainly focused on reducing fluctuations in blood sugar and subsequent complications[41].Recently,inhibitors of α-amylase and α-glucosidase,two digestive enzymes playing a central role in diabetes are used to estimate the anti-diabetic potential of plant extracts[8].These two enzymes are involved in the breakdown of starch into disaccharides and oligosaccharides finally liberating glucose which is later absorbed into the blood circulation.Inhibition of α-amylase and α-glucosidase would diminish the breakdown of starch in the gastro-intestinal tract.Therefore,the postprandial hyperglycaemia level may also be reduced[42].Hence,anti-diabetic potentials inB.retusaextracts were measured by inhibition of α-amylase and α-glucosidase.The inhibition of α-amylase and α-glucosidase enzyme activity ofB.retusacompared to the standard drug acarbose is presented in Fig.4a and b.Among the different solvent extracts analyzed,methanol extracts of leaf(96.32%)and fruit(92.81%)showed higher percentage of inhibition against α-amylase enzyme as compared with standard acarbose(84.12%).Moreover,the fruit methanol extract showed the most significant inhibition activity against α-glucosidase of 54.34% in a concentration-dependent manner,which is comparable with the standard acarbose(79.62%).Our results show thatB.retusahas strong inhibitory effects on α-amylase and αglucosidase enzymes which may delay the degradation of starch and oligosaccharides.In turn,decrease of glucose absorption might suppress the postprandial blood glucose level.A number of reports support our observation that polyphenols in plants acting as potent inhibitors of α-amylase and α-glucosidase enzymes which delay degradation of starch and oligosaccharides thus decreasing postprandial blood glucose[8,14,43].Our data suggest that the inhibitory activity against digestive enzymes might be due to presence of polyphenolic contents in the extracts ofB.retusa.
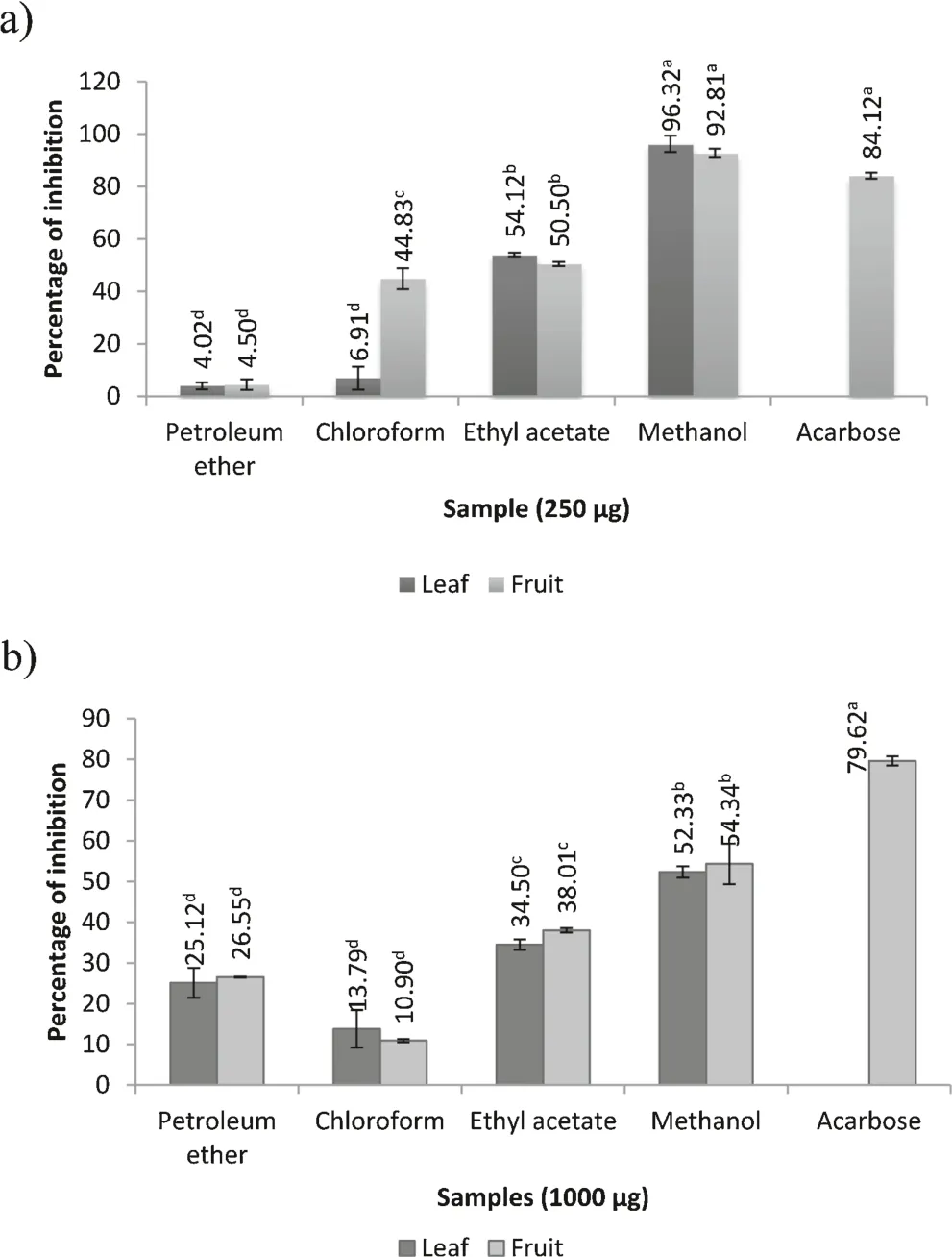
Fig.4.(a) α-amylase inhibition assay.(b) α-Glucosidase inhibition assay.Values are mean of triplicate determination(n=3)±standard deviation;statistically significant at p<0.05 where a>b>c>d.
4.Conclusion
The present study confirms the nutritional value ofB.retusaleaves as indicated by high total phenolic content that have the ability to reduce the oxidative stress related disorders by scavenging different free radicals.Chemical composition with strong antioxidant properties ofB.retusacorrelated to strong inhibition of α-amylase and α-glucosidase enzymes which might regulate the hyperglycaemia and other diabetic complications.Our results suggest that dietary compounds fromB.retusacan be used as a nutritional food supplement against disorders related to oxidative stress such as diabetes or others.Further studies will be conducted on identification of dietary compounds,molecular mechanisms involved in antioxidant activity and determination of their efficacy byinvivostudies.
Conflict of interest
We declare that we have no conflict of interest.
Acknowledgement
The authors are thankful to Dr.V.Narmatha Bai,the Head of the Department of Botany,Bharathiar University,Coimbatore,Tamil Nadu,India.
杂志排行
食品科学与人类健康(英文)的其它文章
- Bioactive peptides on endothelial function
- Calcium intake,calcium homeostasis and health
- Phenolics extract of Tetrapleura tetraptera fruit inhibits xanthine oxidase and Fe2+-induced lipid peroxidation in the kidney,liver,and lungs tissues of rats in vitro
- Characterization of volatiles Tribolium castaneum(H.)in flour using solid phase microextraction–gas chromatography mass spectrometry(SPME–GCMS)
- Effects of antimicrobial and antioxidant activities of spice extracts on raw chicken meat quality
- GUIDE FOR AUTHORS
