Characterization of volatiles Tribolium castaneum(H.)in flour using solid phase microextraction–gas chromatography mass spectrometry(SPME–GCMS)
2016-05-24YonghaoNiuGilesHardyManjreeAgarwalLeiHuaYonglinRen
Yonghao Niu,Giles Hardy,Manjree Agarwal,c,Lei Hua,Yonglin Ren,c,∗∗
a College of Plant Protection,Northwest A&F University,Yangling,Shaanxi 712100,China
b School of Veterinary and Life Sciences,Murdoch University,90 South Street,Murdoch,WA 6150,Australia
c Australia Cooperative Research Centre for National Plant Biosecurity,LPO Box 5012,Bruce,ACT 2617,Australia
Abstract
Keywords:Flour;Tribolium castaneum(Herbst);Volatile organic compounds;Solid phase microextraction(SPME);Gas chromatography–mass spectrometry
1.Introduction
Flour is an important food ingredient for humans.It contains some basic nutrients such as fibre,protein and vitamins[1].Although flour is a central component of human diet,there have been few reports concerning the volatile organic compounds(VOCs)in this staple food until recently[2].Identification of the VOCs released by flour can help us know the components of its flavour and judge its quality over time during storage[3].
Triboliumcastaneum(Herbst)is one of the most common insects affecting stored cereal grains,beans,nuts and other durable agricultural products all over the world[4,5].The presence of this insect in flour,not only causes direct damage,but also results in the deterioration of grain quality,loss of feeding value for stock,and hygiene problems like off-odour damage[6].
Reliable and simple methods to detect the existence of pest infestations in stored products are critically important throughout the supply chain to ensure,for example,the maintenance of grain quality during domestic storage and compliance with international quarantine requirements[7].The typical approaches for detecting insects in stored grain are based on collecting representative samples of grain from stacks,trucks and rail bogies,and manually inspecting these samples for adult insects by sieving,flotation and Berlese-funnels[8].These techniques can easily trap or detect adult insects but are not suitable for immature insects.X-ray imaging and near infrared reflectance(NIR)spectroscopy have been studied for the detection of stored grain insects as they can detect hidden insects[9].However,the operation of these technologies is relatively complicated and there has been no success withinsitudetection.
A potential detection method is to analyze the air within a grain mass for specific volatile compounds(VOCs)released by insects.In recent years,headspace solid phase microextraction(HS-SPME)in conjunction with gas chromatography–mass spectrometry(GC–MS)has been employed to examine volatile compounds from stored product insects in grain[10].It has been widely used to isolate and identify the VOCs of stored grain and has also been used to detect the aggregation pheromone and other volatile metabolites of the lesser grain borerRhyzoperthadominica(F.)andT.castaneum[11].SPME–GCMS has also been used to determine ongoing spoilage based on the production of unique VOCs by insects to communicate with their own species[12].
Based on previous studies[13],the goal of this study was to screen samples of healthy(non-insect-contaminated)flour,samples ofT.castaneumadults and samples of flour infested withT.castaneumadults using a non-polar column at 25℃ to establish if there were clearly identifiable compounds unique to flour alone,infested flour and the contaminating insects,which would indicate that HS-SPME–GCMS could be used as a way of identifying an infestation ofT.castaneumin flour.If this were the case,this technique might also be used to gauge the quality of stored products.
2.Materials and methods
2.1.Preparation of samples for analysis
The flour was made from harvested wheat (Australia Standard Wheat I)(2011–2012)with a moisture content of 11.5%(w/w).The wheat had initially been sealed in glass jars(4 L)and held first at−4℃ for one week to kill any pests,and then at 4℃ until milling.A coffee grinder was used for that purpose and the flour produced was also stored in jars at 4℃.
T.castaneum(MUWTC-8)insects were supplied by School of Veterinary and Life Sciences,Murdoch University,Perth,Australia.Mixed age populations were produced by adding around 200 adults to 350 g wheat flour contained in 500 mL jars which were closed with a mesh lid.The cultures were then incubated for 4–5 weeks at 30±1℃ and 65% relative humidity(RH)in constant darkness to produce adults.Newly emerged adults were then harvested as required.
The samples prepared for assay were 70 g flour,or 50T.castaneumadults alone or 70 g flour plus 50T.castaneumadults sealed in a 100 mL Erlenmeyer flask.Each sample was replicated 3 times and all samples were conditioned for 24 h at 25℃ in a constant temperature room prior to examination.
2.2.HS-SPME–GCMS equipments and methods
Samples were held in Erlenmeyer flasks(100 mL)with ground glass joints(Crow Scientific,NSW,Australia,Cat.No.FE100/3)fitted with half hole septa(Alltech Associates,P/N 6526),and obtain a sealed system suitable for sampling of the headspace.
The SPME fibre of 50/30μm Divinylbenzene/Carboxen/Polydimethylsiloxane(DVB/CAR/PDMS)(Sigma–Aldrich Australia,Cat.No.57348-U)was used based on Niu et al.(2012),optimized HS-SPME–GC method for detection of stored grain insects.The fibre was conditioned before use as per manufacturer’s recommendations.The sample’s extraction time and desorption time was 4 h and 5 min respectively.
The profiles of the VOCs extracted by HS-SPME were analyzed with an Agilent 6890 GC manufactured by Agilent Technology(Palo Alto,CA,USA)with a Flame Ionization Detector(FID).Anon-polar Rxi®-5 ms column was used which was 30 m×0.25 mm×0.25μm in size.The carrier gas used was hydrogen at a constant speed of 40 mL/min in the split-less mode.The GC inlet and FID temperature was 250℃.To obtain the best possible GC conditions,the oven temperature was set at 45℃ for 5 min,then increased at 5℃/min to 250℃ and held at each increment for 5 min.The total run was therefore 51 min and each flask was sampled three times.
An Agilent 6890 GC was used for GC–MS.This was combined with an Agilent 5793 Network mass selective detector(MSD).The GC inlet(at splitless mode),interface and MS source temperatures were 250℃,250℃ and 230℃ respectively.The carrier gas used was helium at a constant airflow of 1.0 mL/min.The column and oven temperatures were the same as those used above.The ionization potential was 70 eV and scanning was from 35 to 500 atomic mass units.The volatiles were identified by comparison of the mass spectrum with the NIST08 mass spectra library.
The GC-FID data including retention time,peak height and peak area were collected and integrated by the chromatography software,Agilent Chemistation,and then exported to Microsoft Excel for further analysis.The GC–MS data were collected by Agilent Chemistation and then exported to Analyzer Pro®and Pancho Data Analysis(Version 2.7.0.0 Manual)for further analysis.
2.3.Statistical analysis
The variations(standard deviations of mean)of VOCs concentrations,the replicate samples and injections in comparison with average readings were analyzed by Microsoft Excel 2007.
3.Results
3.1.GC-FID analysis
Fig.1 shows representative chromatograms gained from the three test samples of flour,T.castaneumandT.castaneuminfested flour under the same experiment conditions by GCFID.The results showed that the chromatograms of flour alone andT.castaneuminfested flour were almost the same.The compounds,2-methyl-1,3-benzenediol,4-ehtyl-1,3-benzenedioland 1-hexadecene were the main ones detected in theT.castaneumsample.However,these were not detected in theT.castaneuminfested flour.Therefore,GC-FID alone cannot distinguish between healthy flour andT.castaneuminfested flour under the experiment conditions that was used.

Fig.1.Representative chromatograms of VOCs from the 3 samples investigated:(A)Wheat flour,(B)T.castaneum adults and(C)T.castaneum infested flour.The specific compounds identified in Chromatogram(B)are:(1)2-methyl-1,3-benzenediol,(2)4-ehtyl-1,3-benzenediol,(3)1-hexadecene.
3.2.GC–MS analysis
Seventy-one VOCs were identified by GC–MS from one or more of the three samples.Twenty-seven VOCs were detected from healthy flour,32 VOCs fromT.castaneumand 39 VOCs fromT.castaneuminfested flour(Table 1).
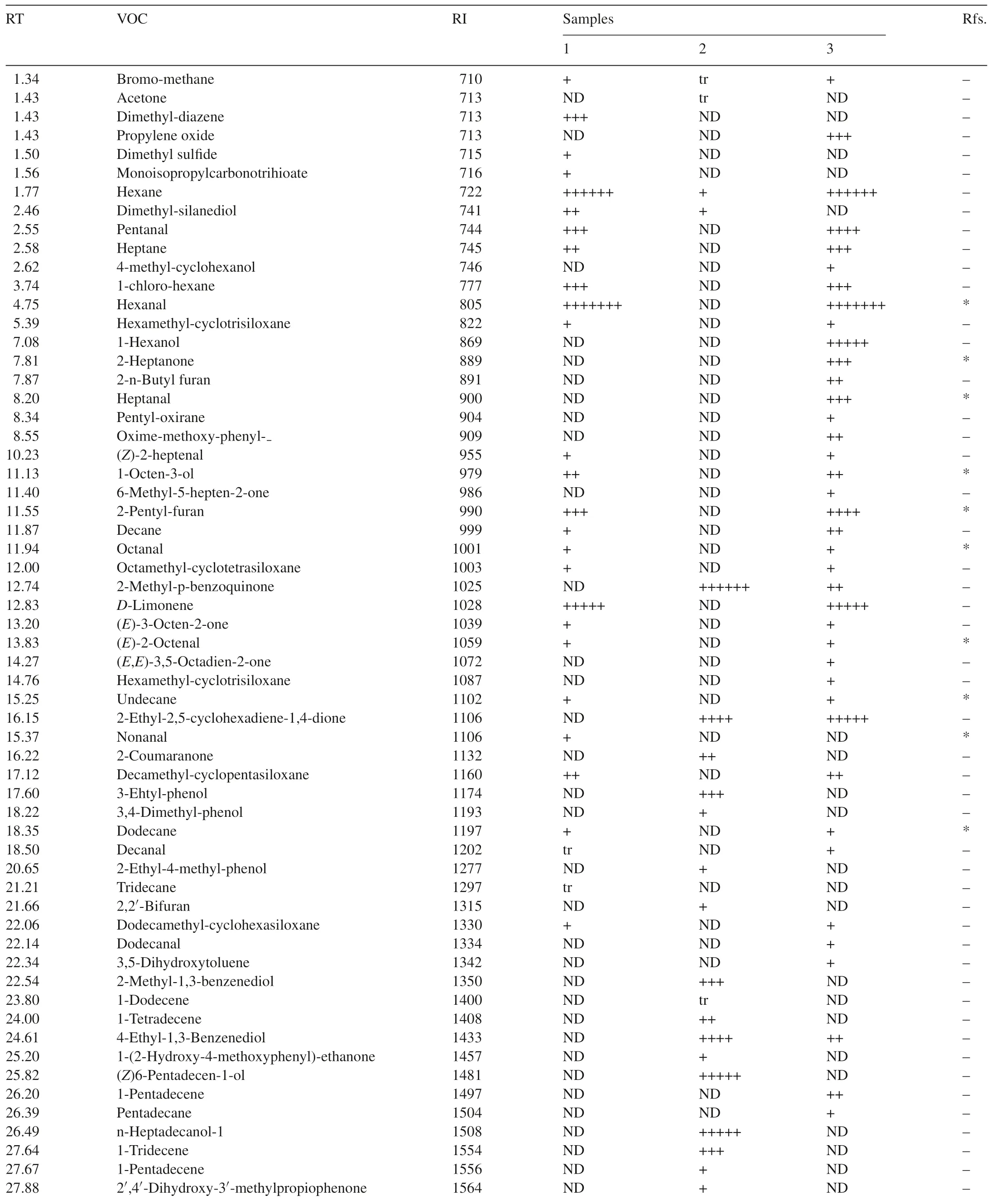
Table 1Compounds from flour,T.castaneum and T.castaneum infested flour determined by GC–MS.
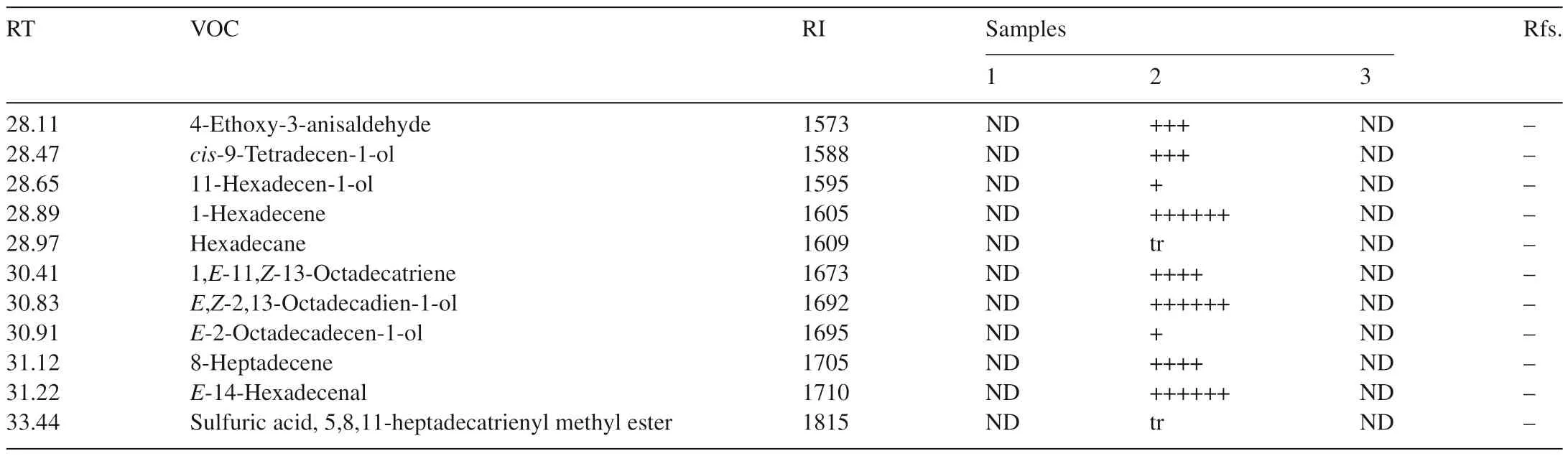
Table 1(Continued)
3.3.VOCs from flour and flour infested with T.castaneum
Five special VOCs were detected only in flour alone and cannot be detected in flour infested byT.castaneum,these were dimethyl-diazene,dimethyl sulfide,monoisopropylcarbonotrihioate,nonanal and tridecane.21 compounds were found in both of flour alone andT.castaneuminfested flour(Fig.2).
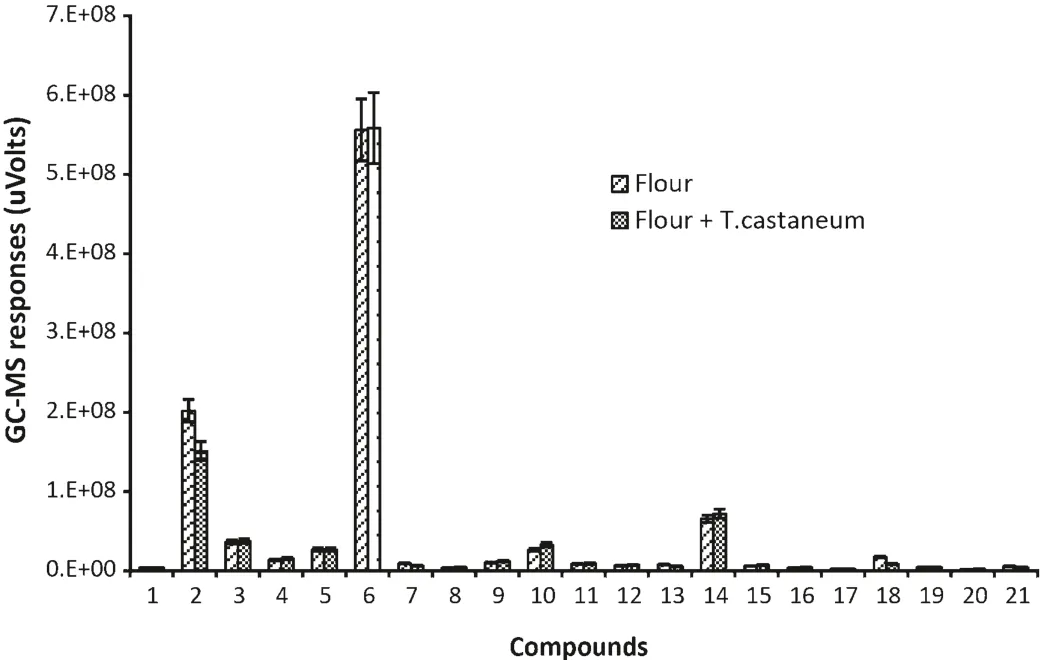
Fig.2.GC–MS responses for VOCs common to both healthy wheat flour and T.castaneum infested flour.(1)Bromo-methane;(2)hexane;(3)pentanal;(4)heptane;(5)1-chloro-hexane;(6)hexanal;(7)hexamethyl-cyclotrisiloxane;(8)(Z)-2-heptenal;(9)1-octen-3-ol;(10)2-pentyl-furan;(11)decane;(12)octanal;(13)octamethyl-cyclotetrasiloxane;(14)D-limonene;(15)(E)-3-octen-2-one;(16)(E)-2-octenal;(17)undecane;(18)decamethyl-cyclopentasiloxane;(19)dodecane;(20)decanal;(21)dodecamethyl-cyclohexasiloxane.(The data were alalysed with a variation less than 9%(SD)between three replicates and the duplicate injections,bars represent standard deviations of the mean.)
3.4.VOCs from T.castaneum and T.castaneum infested flour
The main compounds fromT.castaneumwere identified as 2-methyl-p-benzoquinone,4-ethyl-1,3-benzanediol and 2-ethyl-2,5-cyclohexadiene-1,4-dione(Fig.3).These three compounds can also be detected and identified inT.castaneuminfested flour but at a very low trace level and often overlapped and dwarfed by other compounds.
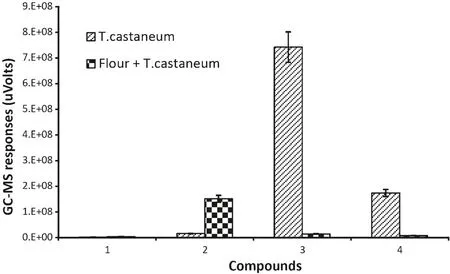
Fig.3.GC–MS responses for VOCs common to both T.castaneum and T.castaneum infested flour.(1)Bromo-methane;(2)hexane;(3)2-methylp-benzoquinone;(4)2-ethyl-2,5-cyclohexadiene-1,4-dione.(The data were analyzed with a variation less than 9%(SD)between three replicates and the duplicate injections,bars represent standard deviations of the mean.)
3.5.VOCs from T.castaneum infested flour
There were 16 compounds identified only inT.castaneuminfested flour(Fig.4).Some compounds which come fromT.castaneum,such as 1-pentadecene,have been known as common semi chemicals of flour beetles[11].Other compounds,like(E,E)-3,5-octadien-2-one were previously detected from healthy flour[3].However,there were some unique compounds detected in the current study which were just found inT.castaneuminfested flour,such as,propylene oxide,1-hexanol and 2-ethyl-2,5-cyclohexadiene-1,4-dione.The functions of these compounds are currently unknown.
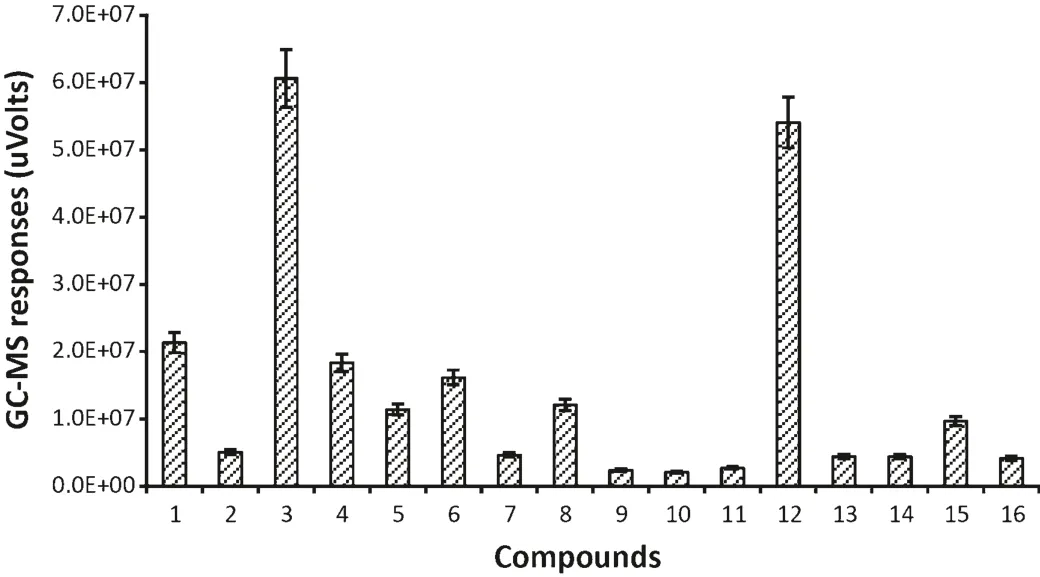
Fig.4.GC–MS responses for VOCs from T.castaneum infested flour.(1)Propylene oxide;(2)4-methyl-cyclohexanol;(3)1-hexanol;(4)2-heptanone;(5)2-n-butyl furan;(6)heptanal;(7)pentyl-oxirane;(8)oxime-methoxy-phenyl-;(9)6-methyl-5-hepten-2-one;(10)(E,E)-3,5-octadien-2-one;(11)hexamethylcyclotrisiloxane;(12)2-ethyl-2,5-cyclohexadiene-1,4-dione;(13)dodecanal;(14)3,5-dihydroxytoluene;(15)1-pentadecene;(16)pentadecane.(The data were analyzed with a variation less than 9%(SD)between three replicates and the duplicate injections,bars represent standard deviations of the mean.)
4.Discussion
Senthilkumar et al.[14]were the first to detect 1-tridecene fromT.castaneum.They speculated that this compound was a sex pheromone of the species in accordance with a study of maleParastizopustransgariepinus(Koch)by Geiselhardt et al.[15].While1-tridecene was detected in the current study,two other previously reported volatiles,methyl-1,4-benzoquinone and ethyl-1,4-benzoquinone were not.These compounds were considered to be defensive secretions[12].However,2-methylp-benzoquinone and 4-ethyl-1,3-benzanediolwere detected for the first time under the current experiment conditions.
Nonanal has also been identified in healthy flour by Maeda et al.[3].The major VOCs in descending order of response level were hexanal,hexane,D-limonene,pentanal and 2-pentyl-furan.Again,2-heptanone and heptanal have previously been detected inT.castaneuminfested flour and hexanal has been detected in both flour andT.castaneuminfested flour[3].However,some compounds like heptane,pentanal and decamethyl-cyclopentasiloxane were identified for the first time in the present study.The quantity of the 21 compounds found in the samples of flour alone andT.castaneuminfested flour were almost same.This suggests that the presence ofT.castaneumhas little impact on the VOCs of flour.
Most compounds from flour andT.castaneumwere different.Hexane was the only compound that could be detected from all of three samples,but it was found at a very low level inT.castaneum.Most compounds found in healthy flour were detected inT.castaneuminfested flour.On the other hand,just a few compounds found fromT.castaneumalone could be detected inT.castaneuminfested flour.There were some specific compounds that were only detected inT.castaneuminfested flour.The common peak in the spectra of healthy flour andT.castaneuminfested flour was hexane,so the data for them in Table 1 were scaled relative to hexane for comparison.The equivalent levels of 2-ethyl-2,5-cyclohexadiene-1,4-dione inT.castaneumand Flour withT.castaneumcould be a compound to determineT.castaneuminfestations in flour(Table 1).ForT.castaneum,2-methyl-p-benzoquinonewas used as a scale for comparison.
5.Conclusion
Totally,71 different compounds were identified in flour,T.castaneumandT.castaneuminfested flour use HS-SPME coupled with GC–MS technique.However,most of compounds fromT.castaneuminfested flour are same as healthy flour.The compound 2-ethyl-2,5-cyclohexadiene-1,4-dione was only found inT.castaneuminfested flour(e.g.it is listed in Fig.4 and Table 1).This suggests that 2-ethyl-2,5-cyclohexadiene-1,4-dione is considered to be a very useful VOC for detectingT.castaneumin flour.
Ethical approval
All institutional and national guidelines for the care and use of laboratory animals were followed.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
We thank the Australian Government’s Cooperative Research Centre Program and the China Scholarship Council for financial support.We would also like to acknowledge the support of staff from the Postharvest Biosecurity and Food Safety Laboratory(Murdoch University)for providing technical assistance.
杂志排行
食品科学与人类健康(英文)的其它文章
- Effects of antimicrobial and antioxidant activities of spice extracts on raw chicken meat quality
- Nutritional composition,in vitro antioxidant and anti-diabetic potentials of Breynia retusa(Dennst.)Alston
- Phenolics extract of Tetrapleura tetraptera fruit inhibits xanthine oxidase and Fe2+-induced lipid peroxidation in the kidney,liver,and lungs tissues of rats in vitro
- Calcium intake,calcium homeostasis and health
- Bioactive peptides on endothelial function
- GUIDE FOR AUTHORS
