双歧杆菌三联活菌散对特应性体质患儿毛细支气管炎后再发喘息的预防作用
2016-05-23任明星薛国昌沈琳娜张黎雯宋月娟夏雪霞
任明星,薛国昌,沈琳娜,张黎雯,宋月娟,夏 欢,夏雪霞
·临床医学·
双歧杆菌三联活菌散对特应性体质患儿毛细支气管炎后再发喘息的预防作用
任明星,薛国昌,沈琳娜,张黎雯,宋月娟,夏欢,夏雪霞
[摘要]目的观察双歧杆菌三联活菌散对特应性体质患儿毛细支气管炎后再发喘息的预防作用,及对嗜酸性粒细胞(EOS)和转化生长因子β1(TGF-β1)水平的影响。方法经监护人知情同意,并签署知情同意书后,采用数字表法将60例毛细支气管炎患儿随机分为治疗组30例,常规治疗组30例,并设健康对照组25例;常规治疗组予毛细支气管炎常规治疗,治疗组予常规治疗外,加用双歧杆菌三联活菌散治疗2个月。于急性期及口服双歧杆菌三联活菌散2月后检测EOS和TGF-β1水平。结果(1)治疗组患儿6月内再次喘息发作次数(0.67±0.13)明显少于常规治疗组(1.27±0.17),差异有统计学意义(P<0.05)。(2)治疗组和常规治疗组患儿急性期EOS[(0.72±0.13)×109/L和(0.70±0.13)×109/L]均高于健康对照组[(0.16±0.09)×109/L],差异有统计学意义(P<0.05);治疗组和常规治疗组患儿急性期TGF-β1[(1.20±0.13) ng/L和(1.22±0.11) ng/L]均低于健康对照组[(1.45±0.13) ng/L],差异有统计学意义(P<0.05)。口服双歧杆菌三联活菌散2月后,治疗组EOS[(0.27±0.12)×109/L]低于常规治疗组[(0.36±0.14)×109/L],差异有统计学意义(P<0.05)。治疗组TGF-β1水平[(1.41±0.09) ng/L]高于常规治疗组[(1.34±0.10) ng/L],差异有统计学意义(P<0.05)。结论口服双歧杆菌三联活菌散能降低特应性体质毛细支气管炎患儿再发喘息次数并上调患儿EOS和TGF-β1水平。
[关键词]毛细支气管炎;双歧杆菌三联活菌散;嗜酸性粒细胞;转化生长因子β1
[作者单位]214062江苏 无锡,苏州大学附属无锡市第九人民医院儿科
毛细支气管炎又称喘憋性肺炎,是2岁以内婴幼儿最常见的下呼吸道感染。流行病学资料显示毛细支气管炎是婴幼儿发展成为支气管哮喘的高危因素,尤以特应性体质患儿为重,尚无特殊预防措施,目前已有报道应用益生菌来防治儿童支气管哮喘[1]。因毛细支气管炎与支气管哮喘有相似的免疫学异常表征[2],本文采用双歧杆菌三联活菌散辅助治疗特应性体质毛细支气管炎患儿,观察其对毛细支气管炎后再发喘息的预防作用及对嗜酸性粒细胞(eosinophil, EOS)和转化生长因子β1(transforming growth factor-beta1, TGF-β1)水平的影响,现报道如下。
1对象与方法
1.1研究对象
2013年5月至2014年12月我院收治的毛细支气管炎住院患儿,诊断标准参照《儿科学》第8版[3],且符合特应性体质的诊断标准[4]。入组前无糖皮质激素治疗史,无先天性心脏病、肝肾功能不全及其他疾病史,胸部X线检查无片状浸润影。共60例,其中男33例,女27例,年龄3~20个月。经监护人知情同意并签署知情同意书,采用数字表法,随机抽样分为2组,治疗组30例,常规治疗组30例。另选取同期我院儿童保健门诊健康体检儿童25例作为健康对照组,年龄3~20个月,男18例,女12例。治疗组、常规治疗组和健康对照组的一般情况间差异无统计学意义(P>0.05) ,有可比性。
1.2方法
1.2.1治疗方法治疗组和常规治疗组均给予常规治疗,用药及疗程无差异,具有可比性。治疗组入院后即加服双歧杆菌三联活菌散(商品名:培菲康;上海信谊药厂有限公司生产;规格:每包2 g,每克含双歧杆菌、乳酸杆菌和肠球菌活菌数不低于1.0×107cfu;批号:国药准字S10970104),1 g,2次/d,温水冲服,疗程共2个月。
1.2.2标本采集所有患儿于入院时(急性期)及口服双歧杆菌三联活菌散2个月后(疗程结束时)抽取空腹静脉血3 ml,3 000 r/min(r=1.5 cm),离心10 min,留取血清-70 ℃冻存备测。对照组儿童采血及保存同上。
1.2.3检测方法外周血EOS计数常规采集无名指指端血,由本院检验科实验室计数。TGF-β1采用双抗体夹心ELISA 法,试剂盒购自美国R&D公司,严格按说明书操作。
1.2.4临床疗效观察记录2组患儿急性期病程、喘息再次发作次数及可能出现的不良反应。随访6个月。
1.3统计学处理
使用SPSS 16.0统计软件进行统计分析。计量资料以均数±标准差(x±s)表示,2组间比较采用t检验,多组间比较采用单因素方差分析,多重比较采用LSD法。计数资料采用χ2检验。P<0.05为差异有统计学意义。
2结果
治疗组和常规治疗组患儿急性期病程及6月内再次喘息发作次数的比较,治疗组急性期病程与对照组比较差异无统计学意义(P>0.05);治疗组6月内再次喘息发作次数明显少于对照组,差异有统计学意义(P<0.05)。见表1。治疗组和对照组患儿急性期EOS均高于健康对照组,差异有统计学意义(P<0.05);治疗组和对照组患儿急性期TGF-β1均低于健康对照组,差异有统计学意义(P<0.05)。口服双歧杆菌三联活菌散2个月后,治疗组EOS低于对照组,差异有统计学意义(P<0.05)。治疗组TGF-β1水平高于对照组,差异有统计学意义(P<0.05)。见表2。全部病例无皮疹、腹泻、呕吐等不良反应。
表1治疗组和常规治疗组患儿急性期病程及6个月内
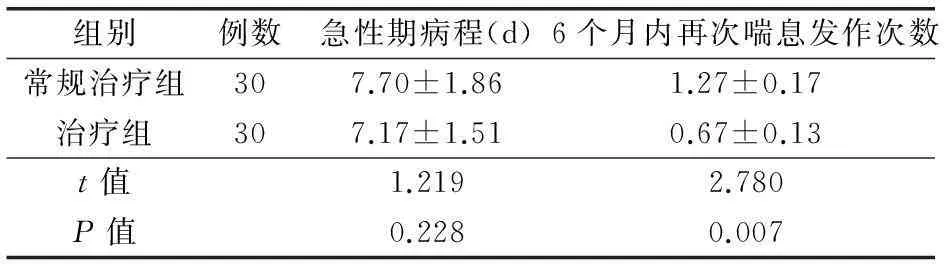
再次喘息发作次数的比较

表2 3组EOS、TGF-β1水平比较
注:与健康对照组比较aP<0.05,与常规治疗组比较bP<0.05。EOS为嗜酸性粒细胞,TGF-β1为转化生长因子
3讨论
毛细支气管炎患儿气道炎症是气道内炎性细胞释放的多种细胞因子、炎性递质与气道内细胞受体共同作用的结果,临床表现为反复喘息发作和呼吸道高反应性,长期随访观察发现约30%的患儿发展成为哮喘,尤其是特应性体质的患儿[5-6]。益生菌是指当足量补充时,对宿主健康有益的活的微生物。目前大量动物实验表明应用益生菌可抑制哮喘模型小鼠气道高反应性和过敏性气道炎症反应,并与Th1/Th2平衡和Th17细胞/调节性T细胞(Treg)平衡关系密切[7-8]。本研究发现治疗组6月内再次喘息发作次数明显少于对照组,差异有统计学意义。因此笔者认为较长时间(2个月)口服双歧杆菌三联活菌散可能有预防毛细支气管炎喘息再次发作的作用。
嗜酸性粒细胞是参与I型变态反应的主要细胞之一,第一次接触过敏原时机体产生的免疫球蛋白IgE与嗜酸性粒细胞结合并使之致敏,当再次接触相同的过敏原时,这些致敏的嗜酸性粒细胞就会在趋化因子的作用下通过血管内皮细胞向炎性区域趋化并释放出大量的炎性介质,并拌有炎性细胞浸润、气道上皮损伤、纤毛脱落、腺体增生、黏液高分泌、平滑肌增生使气道处于高反应状态。TGF-β1主要由淋巴细胞和单核细胞产生,是一个多效性细胞因子,在哮喘中不仅能促进机体对过敏原的免疫耐受,而且在不可逆的气道重构中也发挥着重要作用[9]。动物实验表明大鼠气道可通过旁分泌等方式分泌TGF-β1,从而介导T调节细胞抑制气道炎症高反应[10]。TGF-β1作为一种免疫应答负性调控因子,可抑制淋巴细胞增殖及功能,并抑制巨嗜细胞激活,可能具有关闭免疫应答信号的作用[11]。本研究发现治疗组、对照组患儿急性期EOS水平高于健康对照组,TGF-β1水平低于健康对照组,这与文献报道的一致,提示EOS和TGF-β1参与了毛细支气管炎的发病机制。
本研究还发现口服双歧杆菌三联活菌散2个月后治疗组患儿IgE水平低于对照组,TGF-β1水平高于对照组,差异均有统计学意义。这表明长期(2个月)口服双歧杆菌三联活菌散有下调EOS,上调TGF-β1的作用。其机制可能有:(1)双歧杆菌三联活菌散诱导Th1细胞活性,抑制Th2细胞活性,使B细胞产生IgE减少,导致肥大胞脱颗粒和释放嗜酸细胞阳离子蛋白等碱性蛋白减少,从而下调嗜酸性粒细胞。 (2)诱导Treg细胞分化,进而上调TGF-β1水平。TGF-β1主要由Treg细胞分泌。Feleszko等[12]发现鼠李糖乳酸杆菌GG(lactobacillus rhanmosus GG,LGG)能够抑制哮喘主要是通过增加Treg数量完成的。Martinez等[13]给患有过敏性疾病患儿服用含有乳酸杆菌gassed CECT5714和乳酸杆菌coryniformis CECT571l的奶制品,每天至少1×106cfu/g,持续3个月,结果发现Treg数量增加,血清IgE含量下降。
本研究发现治疗组6个月内再次喘息发作次数明显少于对照组,因此,推测治疗组6个月内再次喘息发作次数少于对照组可能与上述机制有关,但需要进一步扩大样本量及延长随访时间。
[参考文献]
[1]Shu CL, Yao HY, Shao YC, et al. Reduced medication use and improved pulmonary function with supplements containing vegetable and fruit concentrate, fish oil and probiotics in asthmatic school children: a randomised controlled trial[J]. Br J Nutr, 2013, 110(1): 145-55.DOI:10.1017/s0007114512004692.
[2]李宾,吴福玲,冯学斌,等.呼吸道合胞病毒毛细支气管炎与支气管哮喘的相关性研究[J].临床儿科杂志, 2012, 30(2):116-119.DOI:10.3969/j.issn.1000-3606.2012.02.005.
[3]王卫平.儿科学[M] .第8版. 北京:人民卫生出版社,2013:269-271.
[4]胡亚美,江载芳.诸福棠实用儿科学[M] .第7版. 北京:人民卫生出版社,2003:624.
[5]李福,罗文娟,赵晓瑞,等.儿童支气管哮喘发病的危险因素分析[J]. 临床儿科杂志, 2013, 31:(4): 384.DOI:10.3969/j.issn.1000-3606.2013.04.022.
[6]张秀秀,曲书强.毛细支气管炎患儿外周血中IL-17和IL-23水平变化及意义[J]. 中国儿童保健杂志,2014,22:(1):65-67.
[7]Ha JK, Young JK, Seung HL, et al. Effects of Lactobacillus rhamnosus on allergic march model by suppressing Th2, Th17, and TSLP responses via CD4(+)CD25(+)Foxp3(+) Tregs[J]. Clin Immunol(Orlando, Fla.), 2014,153(1): 178-186.DOI:10.1016/j.clim.2014.04.008.
[8]Soichi Tanabe. The effect of probiotics and gut microbiota on th17 cells[J]. Methods Mol, 2013,32(5-6): 511-525.DOI: 10.3109108830185.2013.839665.
[9]Mantel PY, Schmidt-Weber CB. Transforming growth factor-beta: recent advances on its role in immance tolerance[J]. Methods Mol Biol, 2011, 677: 303-338.DOI:10.1007/978-1-60761-869-0-21.
[10] Burchell JT, Wiketrom ME, Stumbles PA, et al. Attenuation of allergen-induced airway hyperresponsiveness is mediated by airway regulatory T cells[J]. Am J Physiol Lung Cell Mol Physiol, 2009, 296:307-319.DOI:10.1152/ajplung.00521.
[11] Mclaren JE,Michael DR,Ashlin TG,et al.Cytokiness,macrophage lipid metabolism and foam ceils implications for cardiovascular disease therapy [J]. Prog Lipid Res,2011,50(4): 331-347.DOI:10.1016/j.plipres.2011.04.002.
[12] Feleszko W,Jaworska J,Rha RD,et al.Probiotic-induced suppression of a Llergic sensitization and airway inflammation is associated with an increase of Tregulatory-dependent mechanisms in a murine model of asthma[J].Clin Exp Allergy,2007,37(4):498-505.
[13] Martinez CA,Sierra S,Lara VF,et al.A probiotic dagy product containing L.gasseriCECT5714 and L.coryniformis CECT5711 inducesimmunological changes in cMldrens uffering from allergy[J].Pediatr Allergy Immunol,2009,20(6):592-600.
(本文编辑:张阵阵)
Effects of live trigeminal bifidobacterium, lactobacillus and enterococcus powder on the recurrence of wheezing in atopic children with bronchiolitis
Ren Mingxing, Xue Guochang, Shen Linna, Zhang Liwen, Song Yuejuan, Xia Huan, Xia Xuexia
(Department of Pediatrics, Nineth People′s Hospital of Wuxi, Affiliated to Suzhou University, Wuxi 214062, China)
[Abstract]ObjectiveTo observe the effects of live trigeminal bifidobacterium, lactobacillus and enterococcus powder on the recurrence of wheezing, and the levels of peripheral blood eosinophil (EOS) and serum transforming growth factor-beta 1(TGF-β1) in atopic children with bronchiolitis.Methods Sixty atopic children with bronchiolitis were randomly divided into the therapy group (30 cases) and the conventional treatment group (30 cases) and another 25 healthy children were recruited as the healthy control group. The conventional treatment group was given routine therapy, and the therapy group received live trigeminal bifidobacterium, lactobacillus and enterococcus, in addition to routine therapy for 2 months. The levels of EOS and TGF-β1 were detected at the acute stage and 2 months after receiving trigeminal bifidobacterium, lactobacillus and enterococcus.Results(1)The recurrent rate of wheezing after medication for the therapy group (0.67±0.13) was significantly lower than that for the conventional treatment group (1.27±0.17), with statistical significance (P<0.05).(2)The levels of EOS of the therapy group [(0.72±0.13)×109/L] and the conventional treatment group [(0.70±0.13)×109/L] at the acute stage were markedly higher than those of the healthy control group [(0.16±0.09)×109/L], also with statistical significance (P<0.05). The levels of TGF-β1 of the therapy group [(1.20±0.13) ng/L] and the conventional treatment group(1.22±0.11) at acute stage were all considerably lower than those of the control group [(1.45±0.13) ng/L], with statistical significance (P<0.05). The level of EOS in the therapy group [(0.27±0.12)×109/L] 2 months after medication of oral live trigeminal bifidobacterium, lactobacillus and enterococcus powder was lower than that in the conventional treatment group [(0.36±0.14)×109/L], also with statistical significance (P<0.05). The level of TGF-β1 of the therapy group [(1.41±0.09) ng/L] 2 months after medication was markedly higher than that of the conventional treatment group [(1.34±0.10) ng/L], also with statistical significance (P<0.05).ConclusionOral medication of live trigeminal bifidobacterium, lactobacillus and enterococcus powder for 2 months could obviously reduce the recurrent rate of wheezing within 6 months after the onset of bronchiolitis and could also up-regulate the levels of EOS and TGF-β1 in atopic children with bronchiolitis.
[Key words]Bronchiolitis; Live trigeminal bifidobacterium, lactobacillus and enterococcus powder; Eosinophil; Transforming growth factor-beta 1
(收稿日期:2015-10-11)
[中图分类号]R735.2
[文献标识码]A[DOI]10.3969/j.issn.1009-0754.2016.02.008
·论著·
