两个基于双(4-吡啶-4-苯基)胺的钴、锌配合物的合成和晶体结构
2016-05-03张春丽郑和根
张春丽郑和根
(1宿州学院化学化工学院,宿州 234000) (2南京大学化学化工学院,配位化学国家重点实验室,人工微结构科学与技术协同创新中心,南京 210093)
张春丽1郑和根*,2
(1宿州学院化学化工学院,宿州234000) (2南京大学化学化工学院,配位化学国家重点实验室,人工微结构科学与技术协同创新中心,南京210093)
摘要:以双(4-吡啶-4-苯基)胺(BPPA)和4,4-(六氟)双(苯甲酸)(H2hfipbb)或对苯二甲酸(p-H2bdc)为配体,采用溶剂热法与M(NO3)2· 6H2O(M=Co、Zn)组装合成了2个新的配合物{[Co2(BPPA)(hfipbb)2·(H2O)3]·6H2O}n(1)、{[Zn2(BPPA)2(p-bdc)2]·H2O·DMF}n(2),利用元素分析、X射线粉末衍射(PXRD)、红外光谱等手段分别对它们进行了表征,并用X射线单晶衍射仪测定了配合物的单晶结构。X射线单晶衍射测定结果表明:配合物1和2均为二维结构。配合物1具有新的拓扑结构。配合物2的二维结构又可以组成三维框架结构。配合物1为正交晶系,Pnna空间群,a=2.640 9(2) nm,b=1.715 78(13) nm,c=1.504 11(11) nm,V=6.8154(9) nm3,Z=4,μ= 0.571 mm(-1),F(000)=2 592,Dc=1.245 g·cm(-3),Mr=1 383.76,R1=0.066 6,wR2=0.208 9(I>2σ(I));配合物2为单斜晶系,C2/c空间群,a= 2.796 70(9) nm,b=1.814 82(6) nm,c=2.303 13(7) nm,β=102.136 0(10)°,V=11.428 3(6) nm3,Z=8,μ=0.906 mm(-1),F(000)=4 944,Dc= 1.391 g·cm(-3),Mr=1 196.85,R1=0.039 8,wR2=0.106 4(I>2σ(I))。此外,热重分析表明配合物1和2均具有良好的热稳定性。
关键词:钴配合物;锌配合物;合成;晶体结构
国家自然科学基金(No.21371092)、安徽高校省级自然科学研究重点项目(No.KJ2015A203)和宿州学院自旋电子与纳米材料安徽省重点实验室开放课题(No.2014YKF51)资助。
*通信联系人。E-mail:zhenghg@nju.edu.cn;会员登记号:S060015914M。
The design and synthesis of solid-state complexes known as metal-organic frameworks (MOFs) is at the forefront of modern material chemistry. In the past decade, these materials have gained immense attention which is due to their ability to form intriguing architectures, topologies and their potential applications in materials, such as catalysis[1-2], gas adsorption[3-5], luminescence[6-14], magnetism[15-19]. Until now, Chemists have been working on designing and synthesizing coordination polymers with novel and fascinating topological structures[20-21], as well as some functional properties.
Although these supramolecular structures show beautiful and interesting structures, the structures can be hardly predicted because they are formed by very complicated self-assembly. Thus, modification of the structure seems to be rather difficult. It is necessary to develop a simple rationale for the construction of channels with a bent ligand that will assist a facile modification of the structure. Among them, flexible multidentate ligands with arene core and imidazole nitrogen donors have been found to be the most useful organic building blocks for constructing MOFs with versatile topologies such as cages, honeycombs, interpenetrating networks, and multidimensional frameworks architectures[22-24]. The carboxylate functional group having different coordination modes has been extensively used for constructing polynuclear clusters. Therefore, if the carboxylate and imidazole ligands were used, it can form coordination polymers with various intriguing structural motifs by self-assembly.
A large number of mixed-ligand MOFs have been reported[25-27], most of which are selected N-containing ligands and carboxylic acids. It is well known that combining mixed-ligand in a complex offers greater tunability of the structural framework than using a single ligand. Bis(4-(pyridin-4-yl)phenyl)amine (BPPA) is a V-shaped flexible polypyridyl ligand, carboxylic acids have been demonstrated excellent coordinating ability, giving various novel complexes with the structure aberrancy. 4,4′-(hexafluoroisopropylidenene) bis(benzoic acid) (H2hfipbb) is a bent linker, and its complexes may have novel networks. To test the ability of BPPA to give novel structures and topologies, we have selected two co-ligands consisting of BPPA, H2hfipbb and BPPA, p-H2bdc, different bivalent metal salts, to synthesize two new complexes with unusual structures, namely, {[Co2(BPPA)(hfipbb)2·(H2O)3]·6H2O}n(1) and {[Zn2(BPPA)2( p-bdc)2]·H2O· DMF}n(2). In addition, we described the syntheses and structures in detail.
1 Experimental
1.1 Materials and measurement
All the chemicals were of AR grade and used without further purification. Elemental analyses were performed on an Elementar Vario MICRO Elemental Analyzer. Powder X-ray diffraction (PXRD) patterns were collected in the 2θ range of 5°~50°with a scan speed of 0.1°·s-1on a Bruker D8 Advance instrument using a Cu Kα radiation (λ=0.154 056 nm) at room temperature. Thermogravimetric analyses (TGA) were performed on a Perkin-Elmer thermal analyzer under nitrogen with a heating rate of 10℃·min-1.
1.2 Synthesis of complexes
{[Co2(BPPA)(hfipbb)2·(H2O)3]·6H2O}n(1): Co(NO3)2·6H2O (29.1 mg, 0.1 mmol), BPPA (32.3 mg, 0.1 mmol) and H2hfipbb (39.2 mg, 0.1 mmol) were added in 6 mL mixing solution of H2O/DMF (2∶4, V/V). The final mixture was placed in a Parr Teflon-lined stainless steel vessel (15 mL) and heated at 90℃for 72 h under autogenous pressure. After being cooled to room temperature at 5℃·h-1, purple block crystals were obtained. The yield of the reaction was ca. 63% based on BPPA ligand. Anal. Calcd. for C56H51Co2F12N3O17(%): C, 48.56; H, 3.69; N, 3.04. Found (%): C, 48.63; H, 3.60; N, 3.10.
{[Zn2(BPPA)2(p-bdc)2]·H2O·DMF}n(2): Zn(NO3)2· 6H2O (29.7 mg, 0.1 mmol), BPPA(32.3 mg, 0.1 mmol) and p-H2bdc (16.6 mg, 0.1mmol) were added in 6 mL mixing solution of H2O/DMF (3∶3, V/V). The final mixture was placed in a Parr Teflon-lined stainless steel vessel (15 mL) heated at 85℃for 72 h under autogenous pressure. After being cooled to room temperature at 5℃·h-1, yellow block crystals were obtained. The yield of the reaction was ca. 60% based on BPPA ligand. Anal. Calcd. for C63H51N7O10Zn2(%):C, 63.17; H, 4.26; N, 8.19. Found (%): C, 63.10; H, 4.28; N, 8.21.
1.3 Structure determination of single crystal
Single-crystal X-ray diffraction measurements were carried out on a Bruker Smart APEXⅡCCD diffraction. Crystallographic data were collected using a graphitemonochromatic Mo Kα radiation (λ=0.071 073 nm). Empirical absorption corrections by SADABS was applied to the intensity data[28]. The structures were determined by direct methods, and non-hydrogen atoms were refined anisotropically on F2by the fullmatrix least-squares techniques using SHELXL-97 program package[29]. Six lattice water molecules were removed using PLATON[30]. Crystallographic data and structure refinements of complexes 1 and 2 are listed in Table 1. The selected bond lengths and bond angles of complexes are listed in Table 2.
CCDC: 1434213, 1; 1434214, 2.
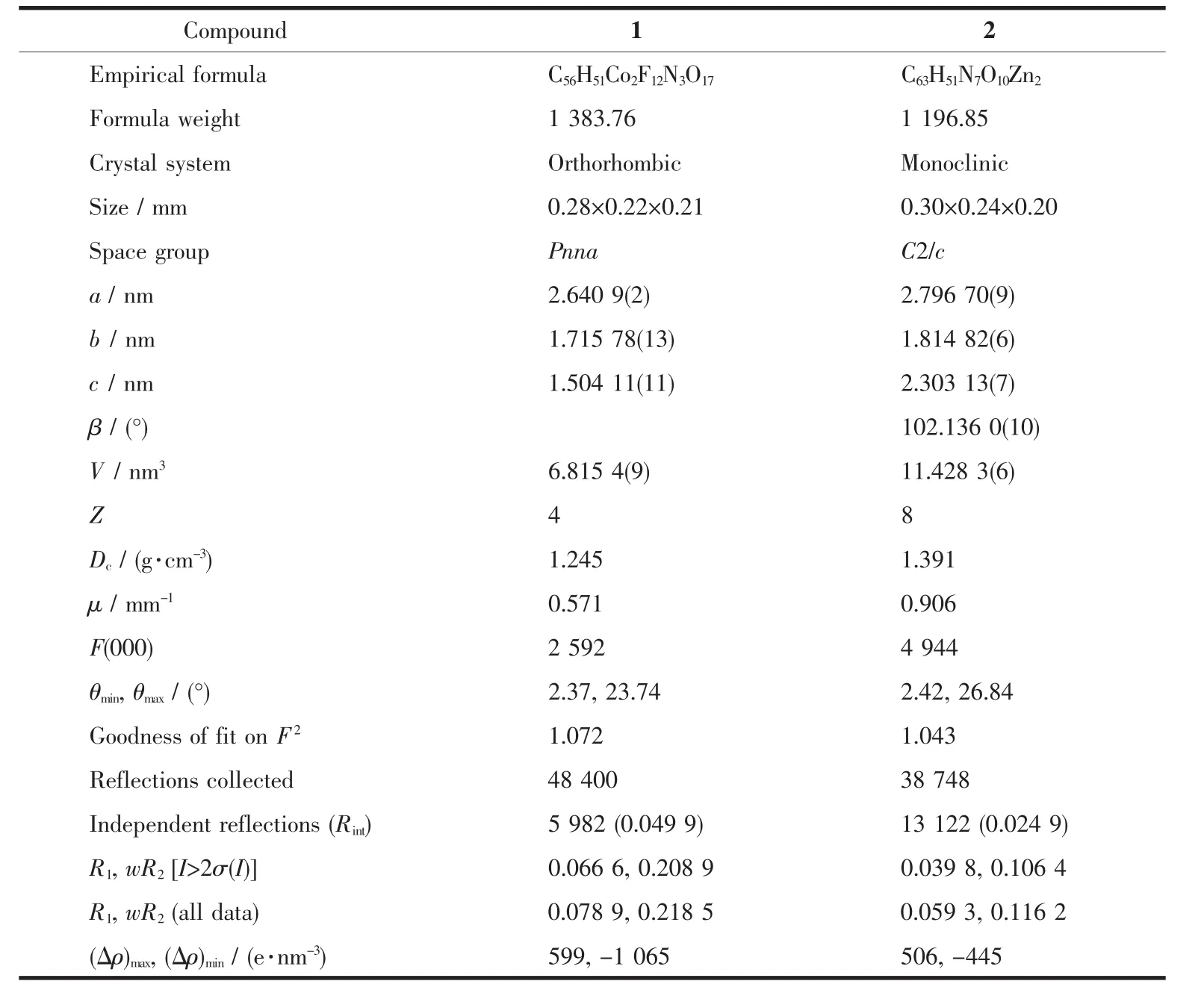
Table1 Crystallographic data and structure refinement parameters of 1 and 2
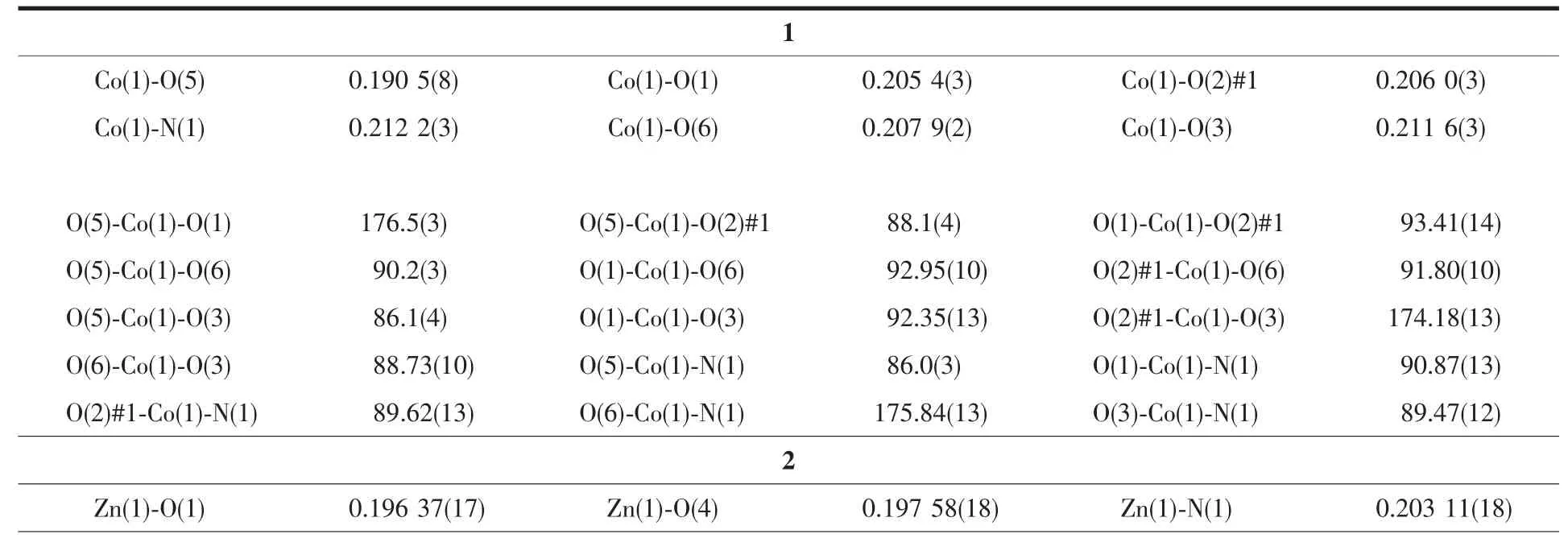
Table2 Selected bond lengths (nm) and bond angles (°) of 1 and 2

Continued Table 2
2 Results and discussion
2.1 Crystallographic description
2.1.1 Crystal structure description of complex 1
As shown in Fig.1, single crystal X-ray analysis reveals that complex 1 is a two-dimensional supramolecular framework, crystallizing in orthorhombic crystal system within Pnna space group. The asymmetric unit contains two Cocations, one BPPA ligand, two deprotonated hfipbb2-ligands, three coordinated water molecules and six free water molecules. Each Coatom is six coordinated by one nitrogen atom from one BPPA ligand, two oxygen atoms from two water molecules, and three oxygen atoms from three hfipbb2-ligands. The bond length of Co-N is 0.212 2 (3) nm and Co-O (6) bond length in the axis direction is 0.208 2(2) nm; Co-O bond lengths {O(1), O(2)#1, O(3), O(5)} are from 0.190 5(8) to 0.211 6(3) nm. The bond angles of N-Co-O are in the range of 86.0(3)°~175.84(13)°. The bond angles of O-Co(1)-O are in the range of 86.1(4)° ~176.5(3)°. It is interesting that neighboring two Cocations are bridged by two carboxylate groups from two different hfipbb2-ligands and an oxygen in coordinated water to form binuclear building block with distance of Co…Co being 0.355 66(7) nm. The adjacent binuclear building blocks are connected by BPPA and hfipbb2-ligands to form an intriguing 2D network, as shown in Fig.2. To better analyze the structure of complex 1, from the topological perspective, the binuclear Cosecond building units (SBUs) can be regarded as 6-connected nodes with BPPA and hfipbb2-ligands as linkers. Complex 1 represents 6-connectednet with Point Symbol of (411.64). Interestingly, it is a new topology (Fig.3).
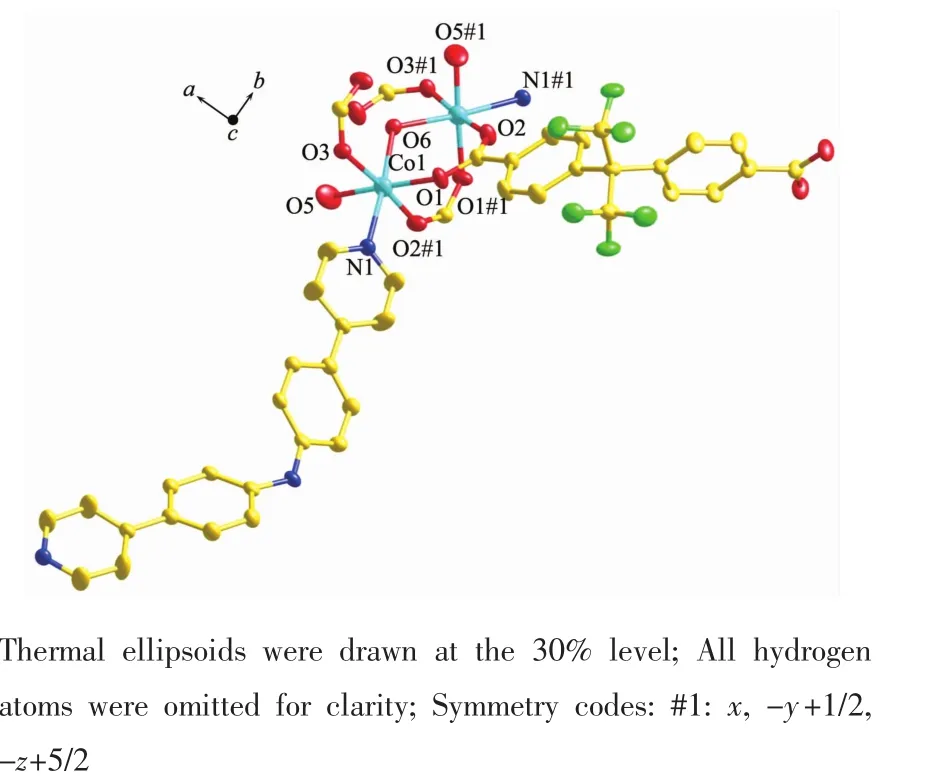
Fig.1 Coordination environment of the Cocation in complex 1
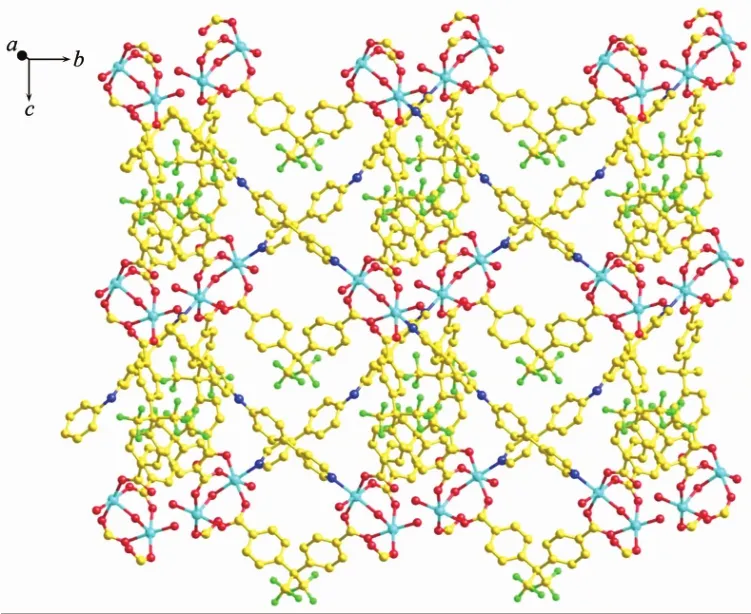
Fig.2 2D framework of complex 1
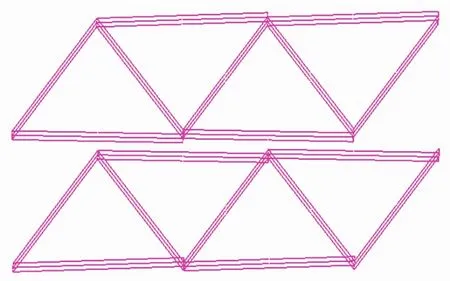
Fig.3 Schematic view of new topology of complex 1
2.1.2 Crystal structure description of complex 2
As shown in Fig.4, single crystal X-ray analysis reveals that complex 2 is a two-dimensional supramolecular framework, crystallizing in monoclinic crystal system within C2/c space group. The asymmetric unit contains two independent Zncations, two BPPA ligands, two p-bdc ligands, one free water and one DMF molecules. Each Znatom is four coordinated by two nitrogen atoms from two BPPA ligands, and two oxygen atoms from two p-bdc ligands. The bond lengths of Zn-N are in the range of 0.202 39(18)~ 0.207 08(19) nm, and the bond lengths of Zn-O are in the range of 0.193 6(2)~0.197 6(2) nm. The bond angles of N-Zn-O are in the range of 99.19(7)°~ 125.81(8)°. The bond angles of O-Zn-O are in the range of 105.51(7)°~106.05(8)°. The bond angle of N-Zn-N are in the range of 106.42(8)°~107.92(8)°. Interestingly, the Zncations are bridged by BPPA and p-bdc ligands to form two kinds of zigzag chains respectively, and Zn…Zn…Zn angles are 180.00°and 106.71°. The combination of two kinds of 1D chain generates the 2D framework (Fig.5). Further, the 2D structure can assemble into 3D extended supramolecular framework (Fig.6).
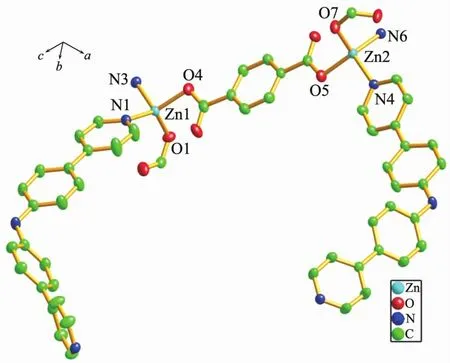
Thermal ellipsoids were drawn at the 30% level; All hydrogen atoms were omitted for clarityFig.4 Coordination environment of the Zncation in complex 2
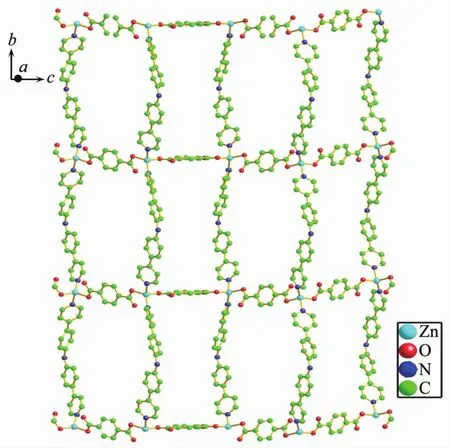
Fig.5 2D framework of complex 2
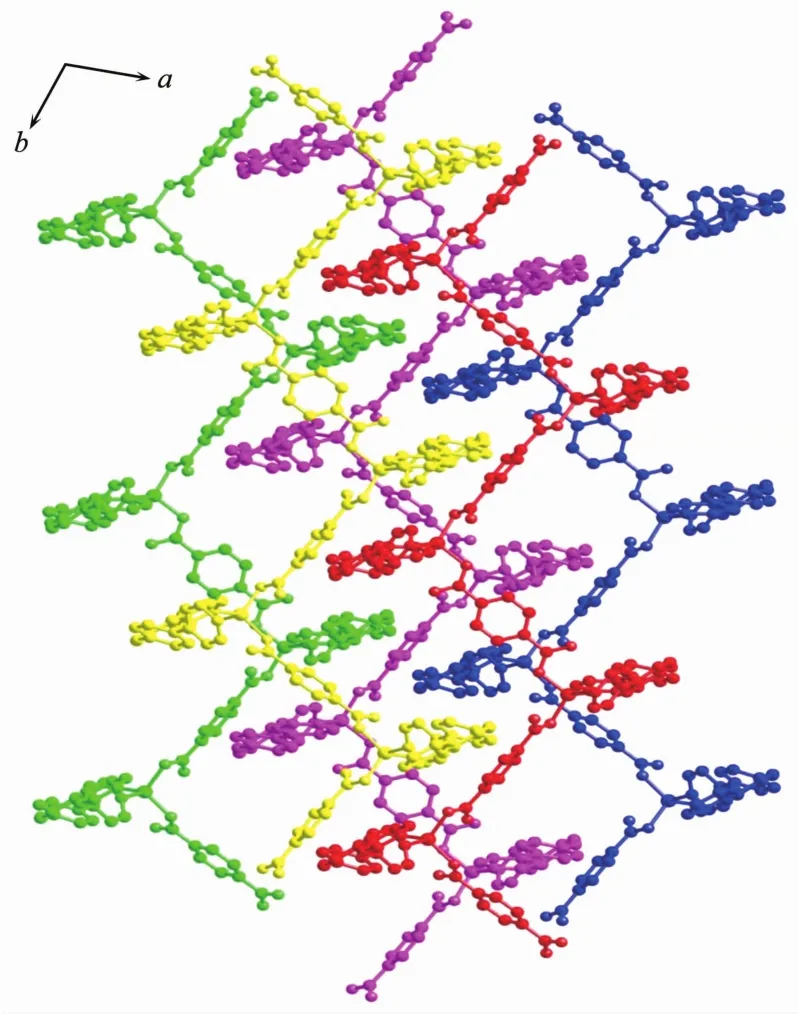
Fig.6 Schematic representation of the 3D framework of complex 2
2.2 Thermal stability and powder X-ray diffraction (PXRD)
To examine the thermal stabilities of complexes 1 and 2, TG analyses were carried out (Fig.7). The TGA study of complex 1 shows a weight loss of approximately 12.80% in the temperature range of 60 ~247℃, corresponding to the loss of the six lattice water and three coordinated water molecules (Calcd. 11.70%). Then the TG curve presents a platform and the framework starts to decompose at 384℃. The thermogravimetric curve of complex 2 indicates the loss of approximately 8.0% in the temperature range of 110~ 200℃(7.60% Calcd. for one DMF and one water molecule per unit formula), and the solvent-free framework begins to collapse at 356℃.
To confirm whether the crystal structures aretruly representative of the bulk materials, the powder X-ray diffraction (PXRD) patterns are recorded for complexes 1 and 2, and they are comparable to the corresponding simulated patterns calculated from the single-crystal diffraction data (Fig.8), indicating a pure phase of each bulky sample.
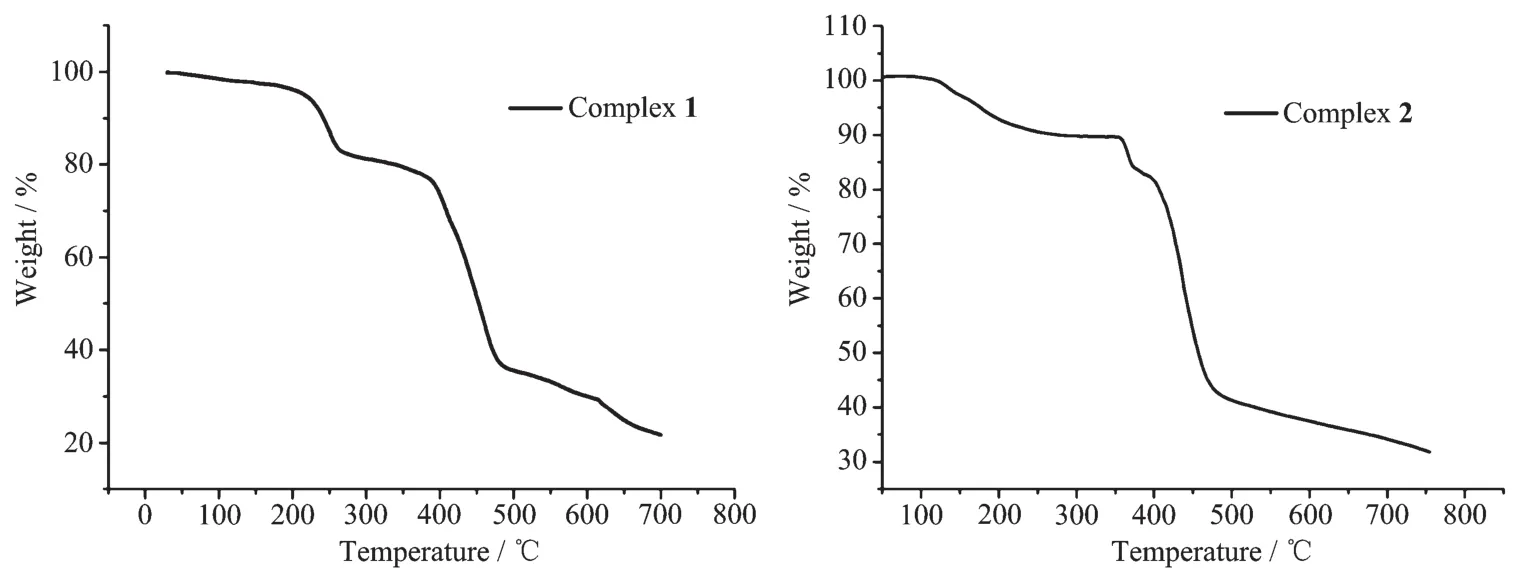
Fig.7 TG curves of complexes 1 and 2
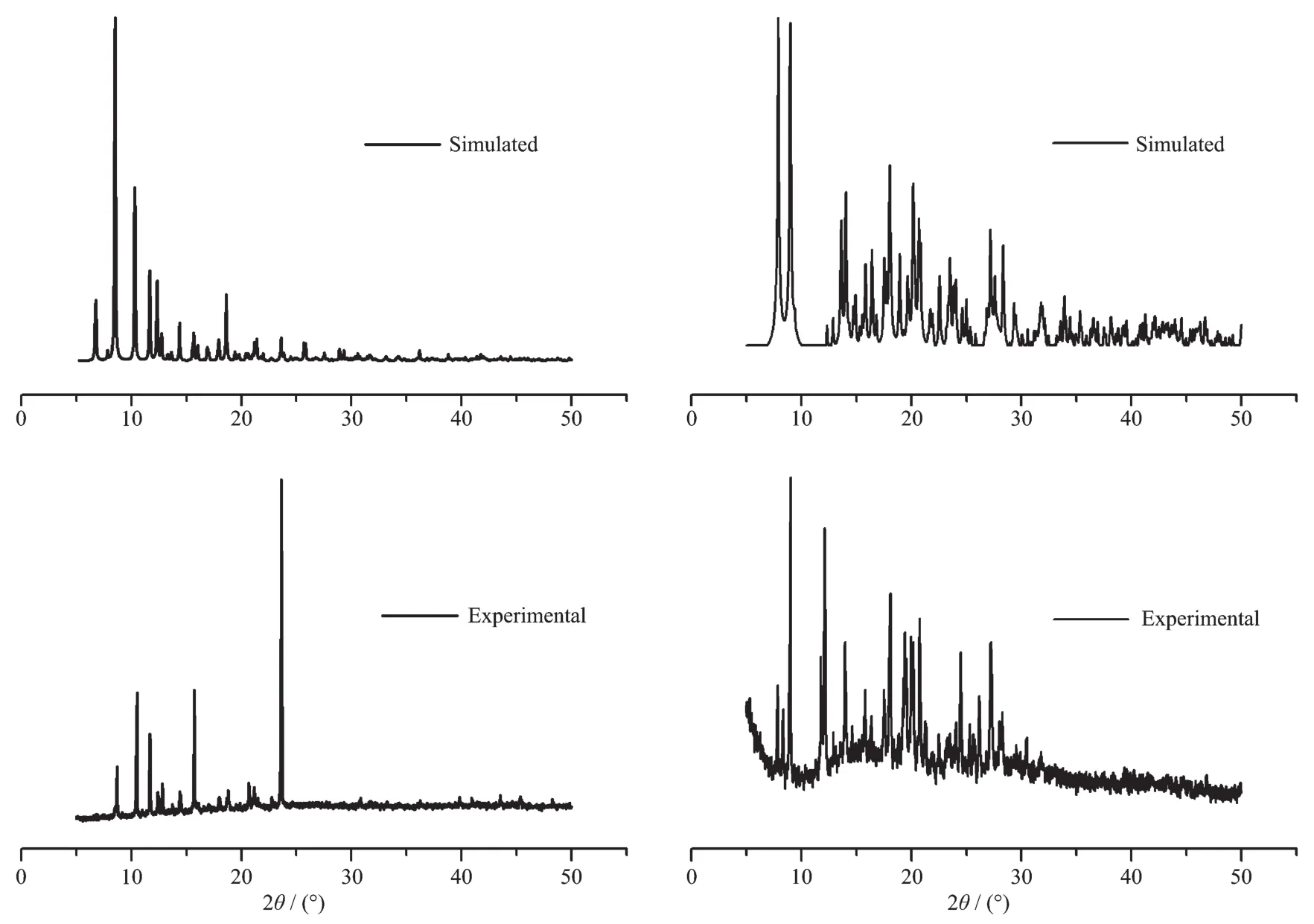
Fig.8 PXRD patterns of complexes 1 (left) and 2 (right)
3 Conclusions
In summary, two new complexes of unusual structures have been successfully synthesized by using two mixed-ligands consisting of BPPA and H2hfipbb/ p-H2bdc respectively. Complex 1 represents 6-connected new topology net with Point Symbol of (411.64). Complex 2 display an interesting 3D extended supramolecular framework. Furthermore, complexes 1 and 2 display better thermal stability.
References:
[1] Wang L H, Zeng Y, Shen A G, et al. Chem. Commun., 2015,51:2052-2055
[2] Roberts J M, Fini B M, Sarjeant A A, et al. J. Am. Chem. Soc., 2012,134:3334-3337
[3] O′Keeffe M, Yaghi O M. Chem. Rev., 2012,112:675-702
[4] Jiang L, Meng X R, Zhong D C, et al. Inorg. Chem., 2012, 51:1874-1880
[5] Qin L, Hu J S, Zhang M D, et al. Chem. Commun., 2012,48: 10757-10759
[6] Qin L, Li Y Z , Guo Z J, et al. Cryst. Growth Des., 2012,12: 5783-5791
[7] ZHANG Chun-Li(张春丽), QIN Ling(覃玲), XU Ji-Gui(徐基贵), et al. Chinese J. Inorg. Chem.(无机化学学报), 2013,29 (11):2347-2350
[8] Perry J J, Perman J A, Zaworotko M J. Chem. Soc. Rev., 2009,38:1400-1417
[9] Yang Q X, Huang L F, Zhang M D, et al. Cryst. Growth Des., 2013,13(2):440-445
[10]Liu X G, Wang H, Chen B, et al. Chem. Commun., 2015, 51:1677-1680
[11]ZHAO Xiu-Hua(赵秀华), ZHAO Ya-Yun(赵亚云), ZHANG Jie(张洁), et al. Chinese J. Inorg. Chem.(无机化学学报), 2014,30:633-639
[12]Yu J H, Yao R, Yuan L M, et al. Inorg. Chim. Acta, 2011, 376:222-229
[13]Hu J S, Shang Y J, Yao X Q, et al. Cryst. Growth Des., Cryst. Growth Des., 2010,10:2676-2684
[14]Hu J S, Shang Y J, Yao X Q, et al. Cryst. Growth Des., 2010,10:4135-4142
[15]ZHANG Chun-Li(张春丽), QIN Li(覃玲), YANG Qing-Xiang(杨清香), et al. Chinese J. Inorg. Chem.(无机化学学报), 2013,29(10):157-2161
[16]Uemura K, Yamasaki Y, Komagawa Y, et al. Angew. Chem. Int. Ed., 2007,6:6662-6665
[17]Yang Q X, Huang L F, Zhang M D, et al. Cryst. Growth Des., 2013,13:440-445
[18]Hu J S, Huang L F, Yao X Q, et al. Inorg. Chem., 2011,50: 2404-2414
[19]Qin L, Hu J S, Li Y Z, et al. Cryst. Growth Des., 2012,12: 403-413
[20]Zheng M X, Gao X J, Zhang C L, et al. Dalton Trans., 2015, 44:4751-4758
[21]Qin L, Ju Z M, Wang Z J, et al. Cryst. Growth Des., 2014, 14:2742-2746
[22]Wen L L, Lu Z D, Ren X M, et al. Cryst. Growth Des., 2009,9: 227-238
[23]Dobrzańska L, Lloyd G O, Raubenheimer H G, et al. J. Am. Chem. Soc., 2006,128:698-673
[24]Dobrzańska L, Lloyd G O, Raubenheimer H G, et al. J. Am. Chem. Soc., 2005,127:13134-13139
[25]Qin L, Hu J S, Zhang M D, et al. Chem. Commun., 2012,48: 10757-10759
[26]Zhang C L, Qin L, Zheng H G. Inorg. Chem. Commun., 2013, 36:192-194
[27]Zhang M D, Di C M, Qin L, et al. Cryst. Growth Des., 2012, 12:3957-3963
[28]SMART and SAINT, Siemens Analytical X-ray Instrument Inc., Madison, WI, 1996.
[29]Sheldrick G M. SHELXL-97, Program for the Refinement of Crystal Structure, Universirty of Göttingen, Germany, 1997.
[30]Spek A L. Acta Crystallogr., 2009,D65:148-155
Syntheses, Crystal Structures of Two Cobalt/ZincComplexes Based on Bis(4-(pyridin-4-yl)phenyl)amine
ZHANG Chun-Li1ZHENG He-Gen*,2
(1School of Chemistry and Chemical Engineering, Suzhou University, Suzhou, Anhui 234000, China)
(2State Key Laboratory of Coordination Chemistry, Collaborative Innovation Center of Advanced Microstructures,
Nanjing University, School of Chemistry and Chemical Engineering, Nanjing 210093, China)
Abstract:Two complexes {[Co2(BPPA)(hfipbb)2·(H2O)3]·6H2O}n(1) and {[Zn2(BPPA)2(p-bdc)2]·H2O·DMF}nwere synthesized by solvothermal method using bis(4-(pyridin-4-yl)phenyl)amine (BPPA), 4,4′-(hexafluoroisopropylidenene)bis(benzoic acid) (H2hfipbb), terephthalic acid ( p-H2bdc) and M(NO3)26H2O (M=Co, Zn), respectively. The complexes were characterized by elemental analysis, IR spectra, XRD and TGA, and the crystal structures were determined by single-crystal X-ray diffraction. Crystal structures were determined by single-crystal X-ray diffraction. Structural analyses reveal that complexes 1 and 2 exhibit a two-dimensional (2D) framework structure. Complex 1 represents new 6-connected net with Point Symbol of (4(11).64), and the complex 2 assembles into a 3D supramolecular network. Complex 1 crystallizes in the monoclinic system, space group Pnna, with cell parameter: a=2.640 9(2) nm, b=1.715 78(13) nm, c=1.504 11(11) nm, V=6.815 4(9) nm3, Z=4,μ=0.571 mm(-1), F(000)=2 592, Dc=1.245 g·cm(-3), Mr=1 383.76, R1=0.066 6, and wR2=0.208 9 (I>2σ(I)); Complex 2 crystallizes in the monoclinic system, space group C2/c, with cell parameter: a=2.796 70(9) nm, b=1.814 82(6) nm, c=2.303 13(7) nm,β= 102.136 0(10)°, V=11.428 3(6) nm3, Z=8,μ=0.906 mm(-1), F(000)=4 944, Dc=1.391 g·cm(-3), Mr=1 196.85, R1=0.039 8, and wR2=0.106 4 (I>2σ(I)). Additionally, complexes 1 and 2 display good thermal stability. CCDC: 1434213, 1; 1434214, 2.
Keywords:Cocomplex; Zncomplex; crystal structure; synthesis
收稿日期:2015-11-06。收修改稿日期:2015-12-16。
DOI:10.11862/CJIC.2016.080
中图分类号:O614.81+2;O614.24+1
文献标识码:A
文章编号:1001-4861(2016)04-0706-07
