喹啉氧基乙酰胺的Pr、Nd、Sm配合物的结构、表征和荧光性质
2016-05-03毛盼东吴伟娜王冠杰
毛盼东 徐 君 吴伟娜 贾 磊 王冠杰
(河南理工大学物理化学学院,焦作 454000)
毛盼东徐君*吴伟娜*贾磊王冠杰
(河南理工大学物理化学学院,焦作454000)
摘要:合成并通过单晶衍射表征了3个同构稀土配合物Ln(L)(NO3)3(H2O) (L=N-苯基-2-(5-氯-8-喹啉氧基)乙酰胺,Ln=Pr,1;Nd,2;Sm,3)。在每个配合物中,十配位的稀土离子采取扭曲的双帽四方反棱柱配位构型,分别与来自1个配体L的2个氧原子和1个氮原子,2个双齿配位硝酸根和1个水分子配位。配合物3能够发射Sm离子特征荧光,荧光寿命为11.7 μs。
关键词:酰胺配体;喹啉;稀土配合物;荧光;晶体结构
国家自然科学基金(No.21404033,21401046,21001040)和河南省教育厅自然科学基金(No.12B150011,14B150029)资助项目。*通信联系人。E-mail:xjjl@hpu.edu.cn; wuwn08@hpu.edu.cn;会员登记号:S06N6704M1112。
Owing to their good luminescent properties, such as the large stokes shifts, narrow emission profiles, long lifetimes, the lanthanide(Ln), especially Euand Tbcomplexes are attracting more and more attention and have been widely used in many aspects, such as light-emitting diode (LED), laser materials, optical signal amplification, fluoroimmunoassay[1-2]. Generally, amide open chain type ligands are well suited to act as antenna, because they are flexible in structure and have terminal-group effects, will shield the encapsulated lanthanide ion from interaction with the surroundings effectively, and thus to achieve strong fluorescent emission[3-6].
In fact, our previous work has demonstrated that the Euand/or Smcomplexes with a series of amide open chain type ligands containing quinoline-8-yloxy group exhibit the characteristic emission of the metal ions[7-10]. However, to the best of our knowledge, the investigations on the coordination behavior of such ligands bearing substituted quinoline ring arerelatively scarce[11-12]. As a continuation of our research, we report here the structures of three Lncomplexes (Ln=Pr, Nd and Sm) with a quinoline-8-yloxy acetamide ligand, namely N-phenyl-2-(5-chloro-quinolin-8-yloxl) acetamide, together with the fluorescence property of the Sm complex.
1 Experimental
1.1 Materials and measurements
Solvents and starting materials for synthesis were purchased commercially and used as received. Elemental analysis was carried out on an Elemental Vario EL analyzer. The IR spectra (ν=4 000~400 cm-1) were determined by the KBr pressed disc method on a Bruker V70 FT-IR spectrophotometer.1H NMR spectra of the ligand L was recorded on a Bruker AV400 NMR instrument using CDCl3solvent, with TMS as internal standard. Fluorescent emission spectra were determined on an Edinburgh FLS980 spectrophotometer.
1.2 Preparations of the ligand and complexes 1~3
The ligand L was prepared according to literature methods (Scheme 1)[10], while using 5-chloro-8-hydroxylquinoline instead of 8-hydroxyquinoline. Yield: 0.99 g (60%); m.p. 180~181℃. Elemental analysis Calcd. for C17H13N2O2Cl(%): C: 65.29; H: 4.19; N: 8.96; Found(%): C: 65.07; H: 4.00; N: 9.18.1H NMR (400 MHz, CDCl3):δ 10.31 (1H, s, NH), 9.07 (1H, m, quinoline), 8.61~8.63 (1H, m, quinoline H), 7.11~ 7.69 (8H, m, phenyl and quinoline H), 4.99 (2H, s, CH2). FT-IR (cm-1):ν(C=O) 1 682,ν(C=N) 1 598,ν (Ar-O-C) 241.
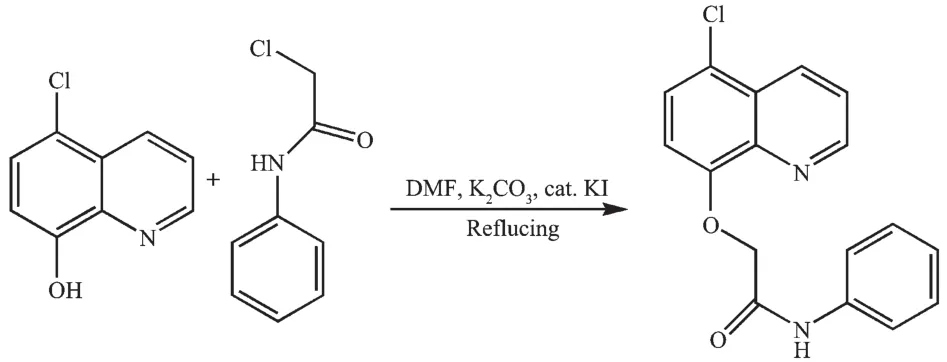
Scheme 1 Reaction scheme for the synthesis of L
1: Colorless block. Anal. Calcd. for C17H15ClN5O12Pr (%): C, 31.05; H, 2.30; N, 10.65. Found (%): C, 31.15; H, 1.92; N, 10.58. FT-IR (cm-1):ν(O-H) 3 416, ν(C=O) 1 654,ν(C=N) 1 569,ν(Ar-O-C) 1 170,ν1(NO3) 1 504,ν4(NO3) 1 289,ρ(O-H) 879.
2: Colorless block. Anal. Calcd. for C17H15ClN5O12Nd (%): C, 30.89; H, 2.29; N, 10.59. Found(%): C, 31.00; H, 2.08; N, 10.48. FT-IR (cm-1):ν(O-H) 3 416, ν(C=O) 1 663,ν(C=N) 1 575,ν(Ar-O-C) 1 168,ν1(NO3) 1 504,ν4(NO3) 1 289,ρ(O-H) 870.
3: Colorless block. Anal. Calcd. for C17H15ClN5O12Sm(%): C, 30.61; H, 2.27; N, 10.50. Found(%): C, 30.82; H, 1.99; N, 10.64. FT-IR (cm-1):ν(O-H) 3 415, ν(C=O) 1 661,ν(C=N) 1 575,ν(Ar-O-C) 1 164,ν1(NO3) 1 508,ν4(NO3) 1 299,ρ(O-H) 885.
1.3 X-ray crystallography
The X-ray diffraction measurement for complexes 1~3 were performed on a Bruker SMART APEXⅡCCD diffractometer equipped with a graphite monochromatized Mo Kα radiation (λ=0.071 073 nm) by using φ-ω scan mode. Semi-empirical absorption correction was applied to the intensity data using the SADABS program[13]. The structures were solved by direct methods and refined by fullmatrixleast-square on F2using the SHELXTL-97 program[14]. All nonhydrogen atoms were refined anisotropically. The H atoms for water molecules are located from difference Fourier map and refined with restraints in bond length and thermal parameters. All the other H atoms were positioned geometrically and refined using a riding model. Details of the crystal parameters, data collection and refinements for complexes 1~3 are summarized in Table 1.
CCDC: 1419222, 1; 1419223, 2; 1419224, 3.
2 Results and discussion
2.1 Crystal structure of the complexes
Selected bond distances, hydrogen bonds information for complexes 1~3 are summarized in Table2 and 3, respectively. As shown in Fig.1a~c, complexes 1~3 are isostructural and crystallize in the monoclinic, space group P21/c. Thus the structure of complex 1 is discussed in detail for an example. The Prion in complex 1 (Fig.1c) is surrounded by one tridentate L with NO2donor set, three bidentate nitrate anions and one water molecule, thus giving bicapped square antiprism coordination geometry (Fig.1d). However, in reported complexes [LnL12(NO3)(H2O)2](NO3)2·nH2O with similar ligand[11], the ratio of the ligand and metal is 2∶1 (L1=N-phenyl-2-(quinolin-8-yloxy)acetamide, Ln=Laor Pr, n=0 or 1). The Pr-N and Pr-O bond lengths (Table 2) are 0.263 5(4) and 0.242 1(4)~ 0.266 4(3) nm, respectively, which are shorter than that of [PrL12(NO3)(H2O)2](NO3)2·H2O[11]. In the crystal, intermolecular O-H…O hydrogen bonds between the coordination water molecules and adjacent nitrate groups link the complexes into one-dimensional chain along b axis. The chains are further connected with each other via intermolecular N -H…O hydrogen bonds, thus forming a 2D supramolecular network (Fig.1e). The Ln-O/N bond length decreases gradually with the increase of the atomic number of Lnions, which is consistent with the effect of lanthanide contraction[5-6,11-12].
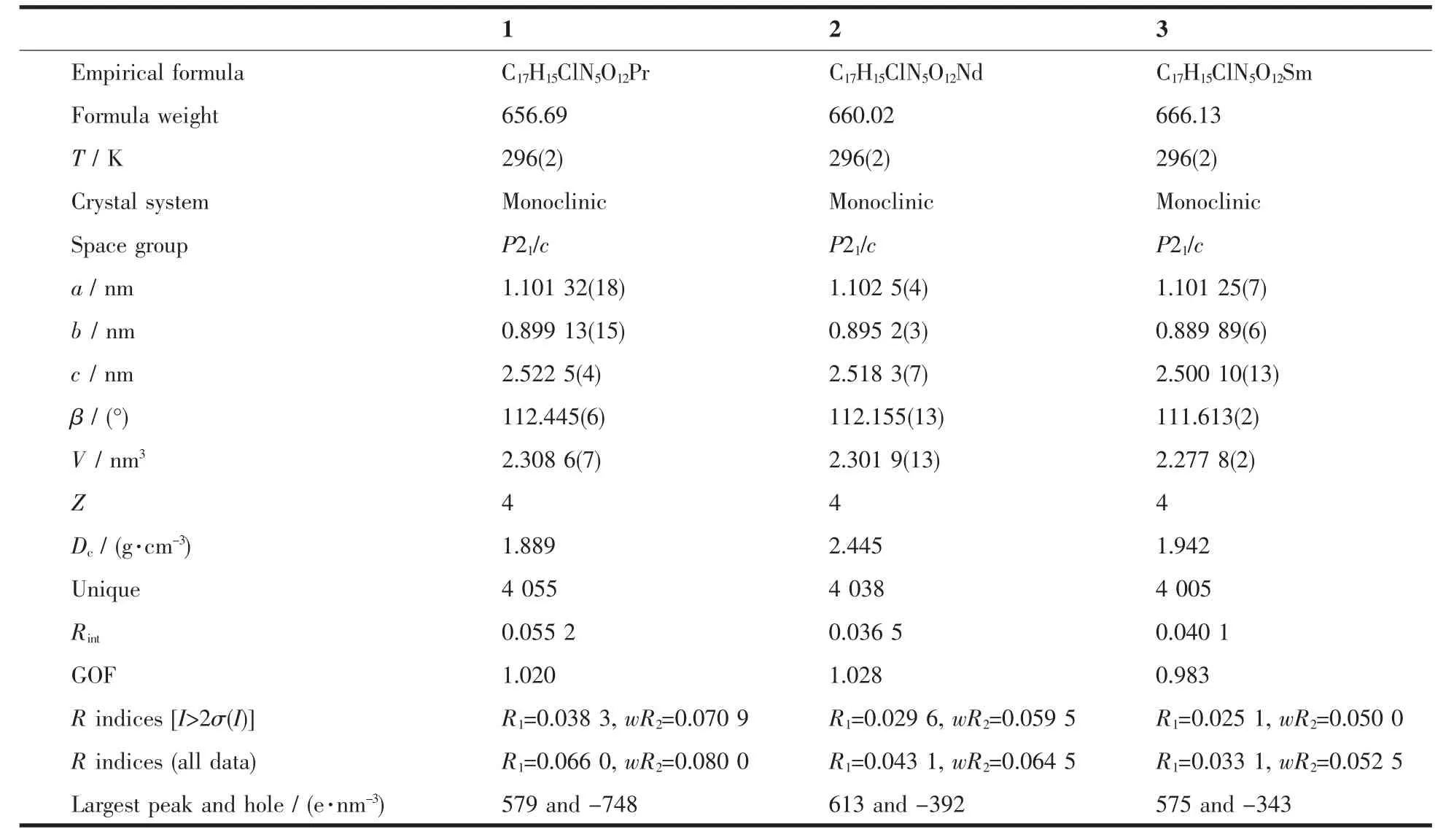
Table1 Selected crystallographic data for complexes 1~3

Table2 Selected bond lengths (nm) in complexes 1~3
2.2 IR spectra
The spectral regions for all the complexes are more or less similar due to the similarity in coordination modes of the ligand with the metal centre. The free ligand L exhibit three absorption bands at 1 682, 1 598 and 1 241 cm-1, assigned to ν(C=O), ν(C=N) and ν(C-O-C), respectively[7-11]. However, in the complexes, such bands shift evidently to lower frequency of 19~28, 23~29 and 70~72 cm-1, respectively, indicating that the oxygen atoms of the carbonyl group, quinoline nitrogen atoms and ethereal oxygen atoms take part in coordination to the central Lnion. Additionally, for all complexes, the two intense absorptions associated with the asymmetric stretching in the range of 1 289~1 299 cm-1(ν4) and 1 504~1 508 cm-1(ν1) established that the NO3-groups (C2v) are coordinated. The differences between the two bands lie in 209~215 cm-1, clearly suggesting that the coordinated nitrate anions are bidentate[6]. Furthermore, the medium intensity bands at about 3 410 and 880 cm-1in each complex are due to the stretching and rocking vibrations of coordinative water molecule, respectively[15]. It is in accordance with the result of the crystal structure study.
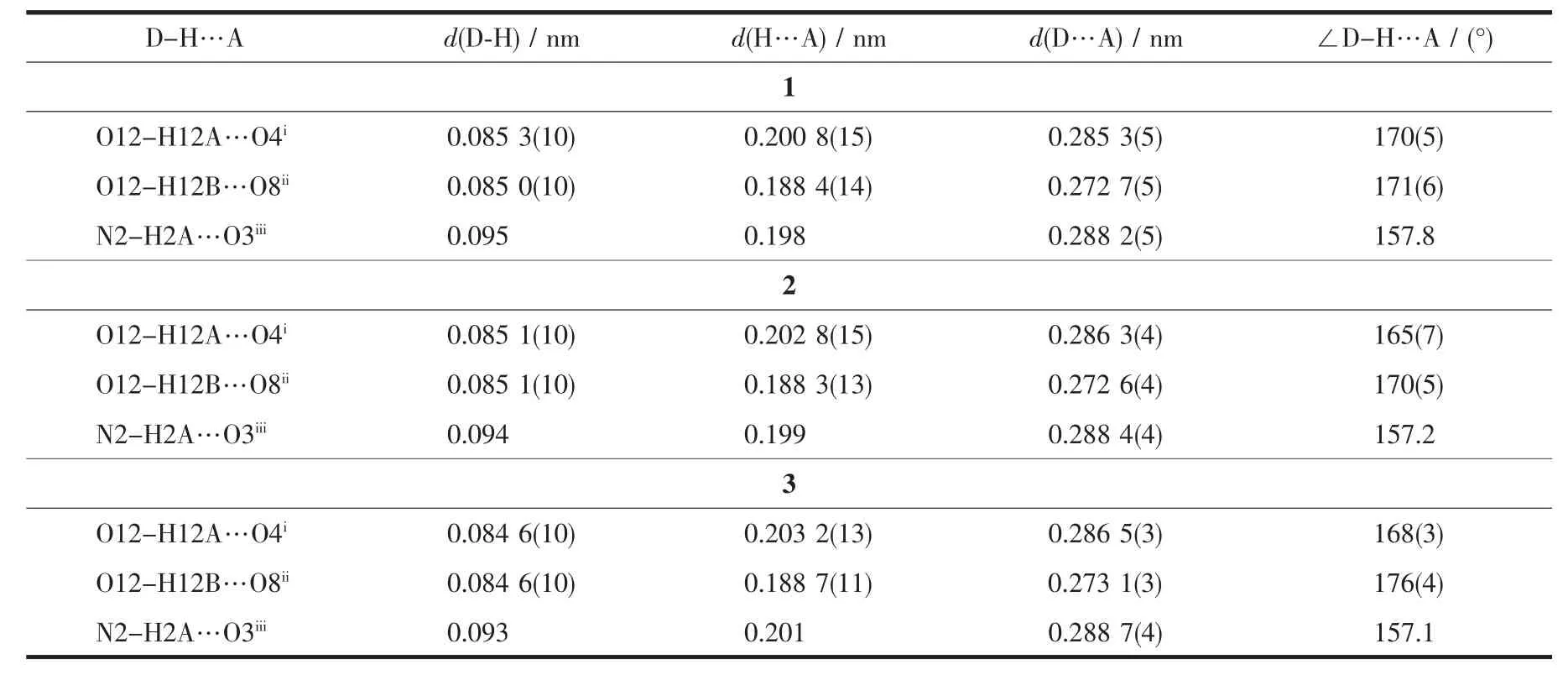
Table3 Hydrogen bonds information in complexes 1~3
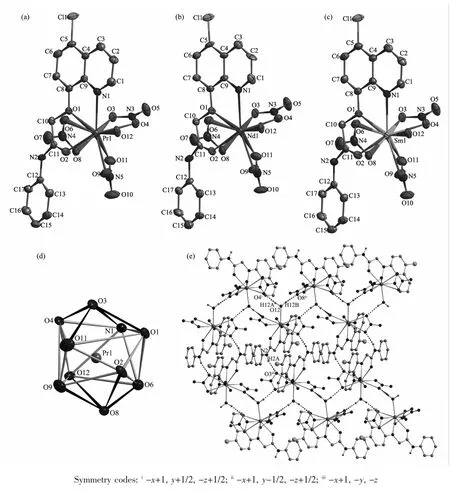
Fig.1 Molecular structures of complexes 1~3 (a~c) shown with 30% probability displacement ellipsoids; (d) Coordination geometry of the center Prion in complex 1, coordination atoms shown with 30% probability displacement ellipsoids; (e) Extended 2D supramolecular structure in complex 1 formed by intermolecular hydrogen bonds between chains
2.3 Fluorescence spectra
When excited by ultraviolet light (310 nm) at room temperature, the free ligand L shows a broad emission at 409 nm, while complexes 3 could exhibit intense red color by naked eyes at the excitation of 338 nm (Fig.2). The characteristic narrow band emissions (λex=338 nm) of complex 3 at 562, 595 and 642 nm are corresponding to the4G5/2→6HJ(J=5/2, 7/2, 9/2) transitions of Smion, respectively[16]. By contrast, reported compound SmL12(NO3)3[10](excited at 350 nm) has similar fluorescence emission spectra as that of complex 3. The luminescence decay of 3 and SmL12(NO3)3were measured in solid state at room temperature (Fig.3). The results indicate that the average lifetime observed for complexes 3 (τ=11.7μs) are relatively shorter than that of SmL12(NO3)3(τ=34.5 μs).
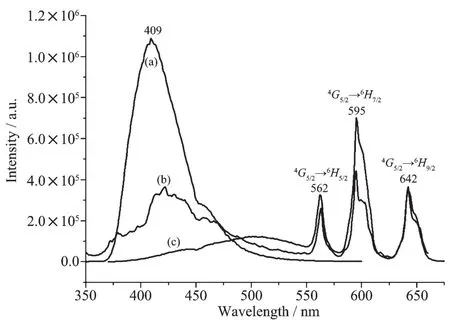
Fig.2 Fluorescence emission spectra of the ligand L (a) (excited at 310 nm), complex 3 (b) (excited at 338 nm) and SmL12(NO3)3(c) (excited at 350 nm) in solid state at room temperature
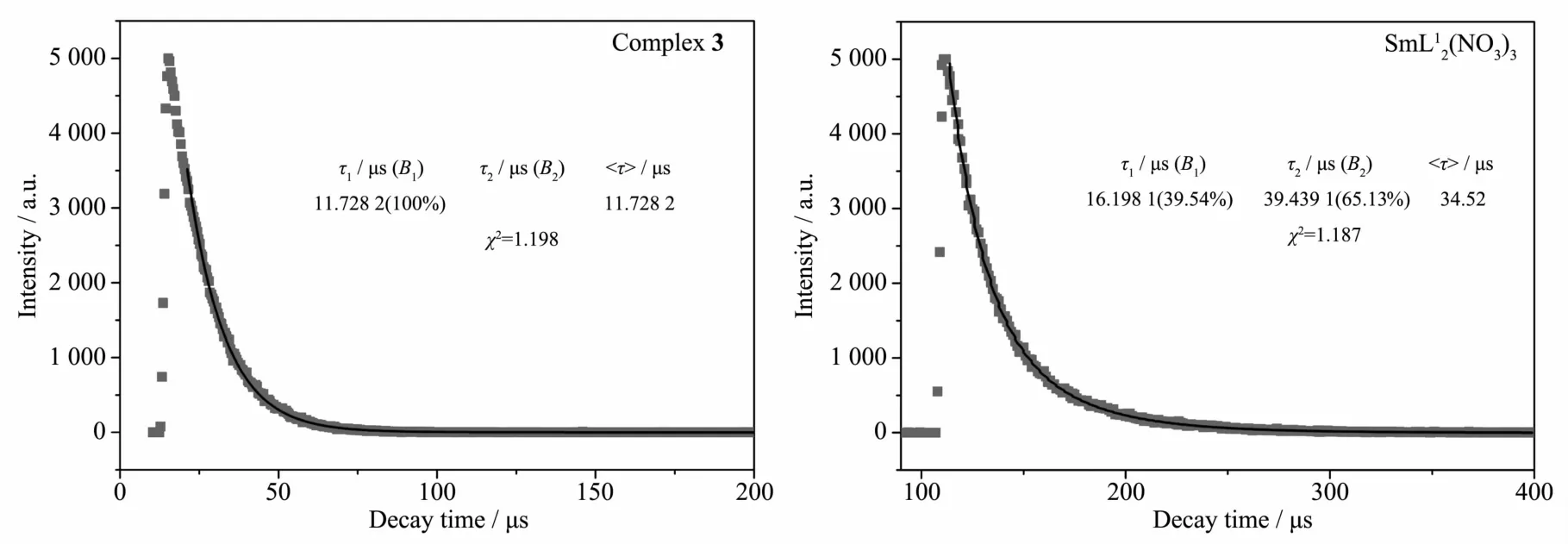
Fig.3 Fluorescence decay dots and fitted curves of complex 3 (a) and SmL12(NO3)3(b)
References:
[1] Binnemans K. Coord. Chem. Rev., 2015,295:1-45
[2] Bünzli J C G. Coord. Chem. Rev., 2015,293-294:19-47
[3] Yan Z Z, Hou N, Wang C M. Spectrochim. Acta A, 2015, 137:1265-1269
[4] Song X Q, Xing D Y, Lei Y K, et al. Inorg. Chim. Acta, 2013,404:113-122
[5] Song X Q, Wang Y W, Zheng J R, et al. Spectrochim. Acta A, 2007,68:701-704
[6] Song X Q, Yu Y, Liu W S, et al. J. Solid State Chem., 2007, 180:2616-2624
[7] Wu W N, Tang N, Yan L. Spectrochim. Acta A, 2008,71: 1461-1465
[8] Wu W N, Cheng F X, Yan L, et al. J. Coord. Chem., 2008, 61:2207-2215
[9] Wu W N, Tang N, Yan L. J. Fluoresc., 2008,18:101-107
[10]Wu W N, Yuan W B, Tang N, et al. Spectrochim. Acta A, 2006,65:912-918
[11]CAI Hong-Xin(蔡红新), YE Xing-Pei(叶行培), WU Wei-Na(吴伟娜), et al. Chinese J. Inorg. Chem.(无机化学学报), 2014,30:2134-2140
[12]ZHANG Zhao-Po (张照坡), WANG Yuan(王元), WU Wei-Na(吴伟娜), et al. Chinese J. Inorg. Chem.(无机化学学报), 2013,29:2239-2244
[13]Sheldrick G M. SADABS, University of Göttingen, Germany, 1996.
[14]Sheldrick G M. SHELX-97, Program for the Solution and the Refinement of Crystal Structures, University of Göttingen, Germany, 1997.
[15]Wang Y, Yang Z Y. Trans. Met. Chem., 2005,30:902-906
[16]Li J, Li H, Yan P, et al. Inorg. Chem., 2012,51:5050-5057
MAO Pan-Dong XU Jun*WU Wei-Na*JIA Lei WANG Guan-Jie
(Department of Physics and Chemistry, Henan Polytechnic University, Jiaozuo, Henan 454000, China)
Abstract:Three lanthanidecomplexes Ln(L)(NO3)3(H2O) [Ln=Pr (1), Nd (2) and Sm (3)] based on L (L=N-phenyl-2-(5-chloro-quinolin-8-yloxy) acetamide) have been synthesized and characterized by elemental analyses, IR spectra and X-ray diffraction analyses. The results reveal that the Lnion in each complex is surrounded by one tridentate L with NO2donor set, three bidentate nitrate anions and one water molecule, thus giving bicapped square antiprism coordination geometry. In addition, complexes 3 could exhibit characteristic fluorescent emissions of the Smion in the visible region with the average lifetime being 11.7μs. CCDC: 1419222, 1; 1419223, 2; 1419224, 3.
Keywords:amide type ligand; quinolone; lanthanide complex; fluorescence; crystal structure
收稿日期:2015-09-14。收修改稿日期:2016-01-25。
DOI:10.11862/CJIC.2016.079
中图分类号:O614.33+4;O614.33+5;O614.33+7
文献标识码:A
文章编号:1001-4861(2016)04-0677-06
