PMEPA1 guards against TGF-β-mediated prostate cancer bone metastasis
2016-04-27AlexandraM.Blee,HaojieHuang
RESEARCH HIGHLIGHT
PMEPA1 guards against TGF-β-mediated prostate cancer bone metastasis
In advanced-stage prostate cancer,bone metastases are often incurable and cause chronic pain,bone fractures,and decreased quality of life.Researchers have previously identified that transforming growth factor-β(TGF-β) signaling is an important regulator of bone metastases in prostate cancer and that targeting TGF-β signaling is a promising therapeutic strategy[1,2].However,the molecular mechanisms behind TGF-βsignaling and prostate cancer progression remain largely unclear.In a recent study,Fournier et al.[3]confirmed that inhibiting TGF-β signaling is effective in decreasing osteolytic bone metastases of prostate cancer.In addition,this study has identified prostate transmembrane protein androgen induced-1(PMEPA1)as a negative regulator of TGF-β signaling,through a proteasome degradation-independent mechanism.The authors demonstrate that epigenetic silencing of PMEPA1 during prostate cancer progression leads to increased osteolytic metastases and suggest that PMEPA1 expression is a potential biomarker for prostate cancer progression.
Canonical TGF-β signaling is known to play differing roles during cancer progression.In early stages,TGF-β suppresses cell proliferation by activating transcription of cyclin-dependent kinase(CDK)inhibitors and repressing genes such as MYC and CDK4.In later stages of cancer, however,TGF-βpromotes epithelial-to-mesenchymal transition(EMT)by upregulating EMT-associated transcription factors,and downregulating adhesion proteins[4].This secondary malignant outcome of TGF-β signaling is important for prostate cancer metastasis,as Fournier et al.[3] confirmed with the use of SD208,an inhibitor of TGF-β signaling.Preventative treatment with SD208 significantly decreased the area of osteolytic lesions in a mouse xenograft model of prostate cancer and improved overall survival.
During canonical signaling,type 2 TGF-β receptor (TGFBR2)binds TGF-β,and phosphorylates type 1 TGF-β receptor(TGFBR1).This initiates a signaling cascade where phosphorylated receptor-activated SMAD proteins 2 and 3(SMAD2 and SMAD3)interact with SMAD4,translocate to the nucleus,and activate or repress transcription of target genes[4].The authors identified PMEPA1 as a target of SMAD3/4 and specificity protein 1 (SP1)that is transcriptionally activated by TGF-β signaling (Fig.1,left).Based on prostate and breast cancer datasets accessed through Oncomine,the authors found that lower expression of PMEPA1 was linked with poor patient outcomes such as earlier recurrence after treatment, shorter time to metastasis,and decreased overall survival.Their findings are among the first to identify PMEPA1 as a key component of prostate cancer progression and metastasis.
It has previously been demonstrated that PMEPA1 negatively regulates TGF-β signaling[5]as well as androgen receptor(AR)-mediated signaling[6].Further study by Fournier et al.[3]revealed a possible mechanism for PMEPA1-mediated inhibition of TGF-β signaling in prostate cancer,that explains the clinical significance of PMEPA1 loss.The authors demonstrated that only the membranebound isoform of PMEPA1 inhibited TGF-β signaling,and that this activity required both the SMAD interacting motif (SIM)and PPxY domain(for interaction with HECT E3 ubiquitin ligases)of PMEPA1.They proceeded to find that this isoform of PMEPA1 interacted with both SMAD2 and SMAD3,as well as multiple HECT E3 ubiquitin ligases known to negatively regulate TGF-β signaling,including NEDD4-1, NEDD4-2,AIP-4,and SMURF2.In particular,Fournier et al. [3]demonstrated that PMEPA1 was important for association of SMAD2 and SMURF2 and subsequent inhibition of TGF-β signaling,independent of proteasome-mediated protein degradation(Fig.1,left).Their data aligns well with a previously described proteasome-independent mechanism of TGF-β signaling inhibition[7].In this case, SMURF2 mono-ubiquitinates SMAD2 and 3 at several residues and prevents formation of SMAD complexes and localization to the nucleus,effectively inhibiting TGF-β signaling.
Fournier et al.[3]confirmed their findings in the model prostate cancer cell line PC-3.Upon loss of PMEPA1,cells exhibited increased TGF-β signaling marked by increased SMAD2 phosphorylation and nuclear localization.Similarlyin a xenograft mouse model,increased osteolytic metastases were observed upon loss of PMEPA1.
The work by Fournier et al.[3]describes a new mechanism for inhibition of TGF-β signaling by PMEPA1.The authors suggest that in advanced-stage prostate cancer, loss of PMEPA1 expression due to AR-driven DNA methylation[8]leads to uninhibited TGF-β signaling and increased bone metastases(Fig.1,right).Their work is relevant as it provides a new mechanism for regulation of TGF-β signaling in prostate cancer and identifies PMEPA1 expression as a biomarker for prostate cancer progression and metastasis. However,there remains a need for more extensive study of clinical prostate cancer samples to identify how frequently loss of PMEPA1 correlates with bone metastases and decreased patient survival.In addition,further study is needed to better identify the relationship between both TGF-β and AR signaling with regards to PMEPA1 expression. While TGF-β signaling increases PMEPA1 expression,AR-mediated signaling can lead to promoter methylation that decreases PMEPA1 expression.How these two pathways interact during prostate cancer progression remains to be understood.Findings from these and other investigations may lead to both better detection and treatment of metastatic prostate cancer.
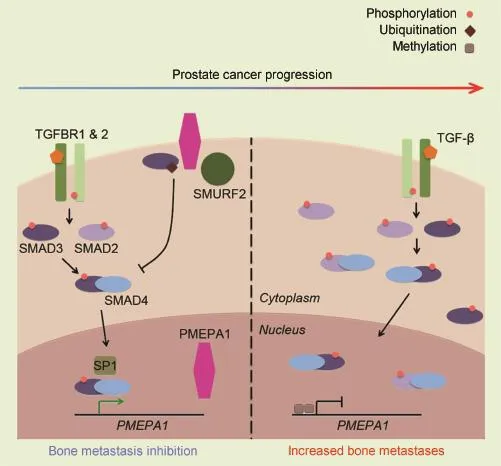
Figure 1PMEPA1 antagonizes TGF-β signaling-mediated prostate cancer cell metastases.Left,membrane-bound PMEPA1 acts as a negative regulator of TGF-β signaling by binding HECT E3 ligases and SMAD proteins,ultimately resulting in decreased bone metastases.Right,during prostate cancer progression,the promoter of the PMEPA1 gene is methylated.As a result,TGF-β signaling proceeds without inhibition,ultimately leading to increased bone metastases.PMEPA1,prostate transmembrane protein androgen induced-1;TGF-β,transforming growth factor-β;TGFBR1,type 1 TGF-β receptor;TGFBR2,type 2 TGF-β receptor.
Conflicts of interest
The authors declare no conflict of interest.
[1]Hu Z,Gupta J,Zhang Z,Gerseny H,Berg A,Chen Y,et al. Systemic delivery of oncolytic adenoviruses targeting transforming growth factor-beta inhibits established bone metastasis in a prostate cancer mouse model.Hum Gene Ther 2012; 23:871-82.
[2]Wan X,Li ZG,Yingling JM,Yang J,Starbuck MW,Ravoori MK, et al.Effect of transforming growth factor beta(TGF-beta) receptor I kinase inhibitor on prostate cancer bone growth. Bone 2012;50:695-703.
[3]Fournier PG,Juarez P,Jiang G,Clines GA,Niewolna M,Kim HS, et al.The TGF-beta signaling regulator PMEPA1 suppresses prostate cancer metastases to bone.Cancer Cell 2015;27: 809-21.
[4]Pickup M,Novitskiy S,Moses HL.The roles of TGFbeta in the tumour microenvironment.Nat Rev Cancer 2013;13: 788-99.
[5]Watanabe Y,Itoh S,Goto T,Ohnishi E,Inamitsu M,Itoh F,et al. TMEPAI,a transmembrane TGF-beta-inducible protein,sequesters Smad proteins from active participation in TGF-beta signaling.Mol Cell 2010;37:123-34.
[6]Xu LL,Shi Y,Petrovics G,Sun C,Makarem M,Zhang W,et al. PMEPA1,an androgen-regulated NEDD4-binding protein,exhibits cell growth inhibitory function and decreased expression during prostate cancer progression.Cancer Res 2003;63: 4299-304.
[7]Tang LY,Yamashita M,Coussens NP,Tang Y,Wang X,Li C,et al. Ablation of Smurf2 reveals an inhibition in TGF-beta signalling through multiple mono-ubiquitination of Smad3.EMBO J 2011; 30:4777-89.
[8]Sharad S,Ravindranath L,Haffner MC,Li H,Yan W, Sesterhenn IA,et al.Methylation of the PMEPA1 gene,a negative regulator of the androgen receptor in prostate cancer. Epigenetics 2014;9:918-27.
Alexandra M.Blee
Haojie Huang*
Department of Biochemistry and Molecular Biology, Mayo Graduate School,Mayo Clinic College of Medicine, Mayo Clinic,Rochester,MN,USA
*Corresponding author. E
-mail address:huang.haojie@mayo.edu(H.Huang)
8 November 2015
Available online 26 November 2015
http://dx.doi.org/10.1016/j.ajur.2015.11.002
2214-3882/ⓒ2016 Editorial Office of Asian Journal of Urology.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
杂志排行
Asian Journal of Urology的其它文章
- Unusual case of nephrocutaneous fistula-Our experience
- A case of giant prostatic hyperplasia
- Tumour lysis syndrome:A rare acute presentation of locally advanced testicular cancer-Case report and review of literature
- Who needs further evaluations to diagnose upper urinary tract urothelial cancers among patients with abnormal findings by enhanced CT?
- Can intravesical prostatic protrusion predict bladder outlet obstruction even in men with good flow?
- Zoledronic acid combined with androgendeprivation therapy may prolong time to castration-resistant prostate cancer in hormone-naïve metastatic prostate cancer patients-A propensity scoring approach
