Effectiveness of cerebellar repetitive transcranial magnetic stimulation in essential tremor: study protocol for a randomized, sham-controlled trial
2016-04-10AliShoeibiNahidOlfati
Ali Shoeibi, Nahid Olfati*
Department of Neurology, Ghaem Medical Center, Mashhad University of Medical Sciences, Mashhad, Iran
INTRODUCTION
Essential tremor (ET) is a low-mortality yet truly troublesome disorder that is believed to be the second most common movement disorder worldwide, only next to the restless leg syndrome with an estimated prevalence of 0.9% among all age groups, and 4.6% among those older than 65 years(Louis and Ferreira, 2010). ET is a progressive disorder and a growing body of evidence shows that it might be accompanied by other non-motor symptoms, mostly of neuropsychiatric and cognitive types; and also concurrence with other neurodegenerative disorders has been noticed(Benito-León, 2008, 2014; Louis, 2009, 2010; LaRoia and Louis, 2011; Louis and Okun, 2011).
Studies of ET brains have been suggestive of cerebellar pathology in this disorder at least in a considerable proportion of cases (Jenkins et al., 1993; Deuschl et al., 2000;Louis et al., 2007); this notion led to the development of newer therapeutic options directed to controlling cerebellar overactivity.
Currently, there are several medical therapies available for ET, however in many patients they provide only a modest control over tremor or may cause unacceptable adverse effects or interaction with other necessary medications. There is an undoubted need for treatments that are less harmful,more effective, and more directed to the pathology of the disorder.
Cerebellar low-frequency repetitive transcranial magnetic stimulation (rTMS) has shown preliminary effectiveness in controlling tremor among ET patients (Gironell et al.,2002; Popa et al., 2013). In this study, we plan to assess the effectiveness of low frequency rTMS in reducing the severity of tremor in a larger sample of ET patients with a randomized, sham controlled design and we hope this study would provide a dependable evidence basis for using this therapeutic option for treating essential tremor.
METHODS/DESIGN
Design
The study will be conducted as a prospective, single-center,randomized, triple-blind, sham-controlled, add-on, crossover trial.
Study setting
The study will be performed at our University Speciality Clinic.
Participants
We will recruit at least 30 patients diagnosed with ET based on Movement Disorder Society (MDS) criteria (Deuschl et al., 1998).
Inclusion criteria
- Having a diagnosis of ET based on Movement Disorder Society criteria, involving at least one hand
- Age 18 years or older
- Cognitively eligible to give informed consent of participation in the trial
Exclusion criteria
- Cardiac pace-maker or other implanted magnetic device or having other contraindications of performing a routine non-contrast MRI
- Skin defect at the occipital area preventing placement of a coil
- Currently pregnant or plan for pregnancy in the next 6 months
- History of seizure
- Other comorbid medical conditions capable of producing or enhancing tremor
- Advanced cardiac, renal, hepatic or other disabling conditions making patient physically unable to participate in examination and receiving the intervention
- Use of a recently added medication with potential effect on tremor
Sample size
Based on previous related study (Popa et al., 2013) and assuming type I error as 5%, type II error as 20%, P1 as 20%,and P2 as 65%, n1/n2 as 1, and considering the crossover design of study, the sample size is calculated as 30 with 15 patients in each arm.
Recruitment and randomization
Patients visited at the University Speciality Clinic with a diagnosis of ET on their records would be contacted and those interested in participating in the trial would be randomly assigned into either rTMS or sham stimulation group using block randomization. Patients will be randomized to either: 1) cerebellar rTMS or 2) sham stimulation,with a 1:1 ratio. One researcher who will not be blinded to group assignment will perform rTMS or sham stimulation;however another researcher who will assess the outcome measures will be blinded to group assignment. The third researcher will collect the demographic data and assess adverse events. Blinded data entry and processing will be performed by the fourth researcher as well, performing. All participants will be blinded to the study groups into which they have been placed first, considering sham controlled design of our study.
Interventions
Treatment arm
Patients will be treated with 900 pulses of 1 Hz rTMS on 90% of resting motor threshold (RMT) delivered over each cerebellar hemisphere on a daily basis for 5 consecutive days. Resting motor threshold will be calculated for all patients before starting the therapeutic intervention (we consider the stimulation intensity producing at least 5 visible contractions, and not more than 5 contractions, in abductor policis brevis (APB) muscle of the dominant hand,among a total of 10 stimulations, as the RMT). Magnetic stimulation will be performed using Neuro-MS/D, variant 2 (therapeutic) device (Neurosoft Ltd., Ivanovo, Russia)and a butterfly coil.
Sham arm
Sham treatment will be performed with the same protocol(900 daily pulses of 1 Hz sham stimulation for 5 days)using a small device that generates electrical pulses of 2 mA. Electrical pulses will be delivered by surface elec-trodes, placed on the TMS coil (not visible to the patient),to simulate the sensation of a therapeutic stimulation, and a sound similar the rTMS sound will be produced by this device during sham stimulation.
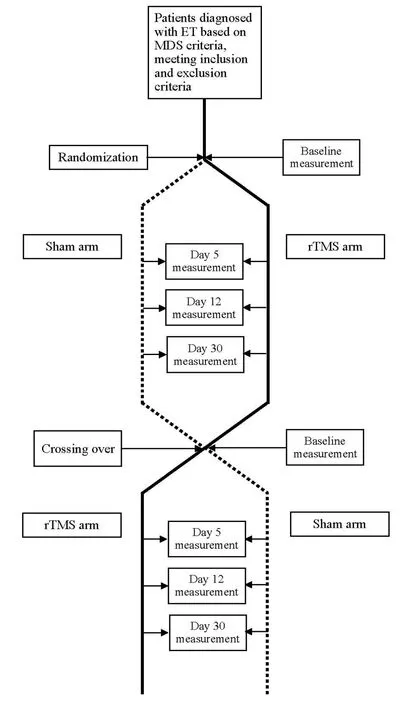
Figure 1: Flow diagram of the study.
The distance of motor and cerebellar cortices from the skin will be measured using MRI techniques and the amount of magnetic stimulation will be adjusted if the measured distance was significantly different between the sites of assessing RMT (motor cortex) and the site receiving intervention (cerebellar cortex) (Stokes et al., 2007). Patients will be provided with ear plugs for sound protection during the time of receiving interventions.
Patients would receive the rTMS or sham stimulation as scheduled for five consecutive days and after a 2-month washout period they would receive the other intervention to follow a crossover design (Figure 1).
Outcomes
Primary outcome
The severity of tremor will be assessed using the Fahn-Tolosa-Marin (FTM) scale (Fahn et al., 1988) at baseline,and reassessed on days 5, 12 and 30 after starting rTMS or sham stimulation (Table 1). Considering worldwide popularity of the FTM scale for assessing tremor severity, use of this scale would have the priority of allowing researchers to compare their results with other studies of the type. FTM scale consists of three subscales: 1) tremor severity based on tremor location (including face, tongue, voice, head, hands,torso, legs and orthostatic tremor; rating from 0 to 4 based on observed tremor amplitude, with score 4 assigned for tremor amplitude of > 4 cm), 2) motor function assessment based on performance of specific motor tasks (including handwriting, drawing and pouring water from one cup into another, scored from 0 to 4), and 3) functional disability in daily living activitiesrated by patients (including functions of: speaking, feeding, hygiene, dressing, writing, working and social performance, scaled from 0 to 4). Total FTM score would be calculated by adding scores assigned for each subscale; the range of scores would be from 0 to 160,in which 0 means no tremor at all and 160 means the highest tremor severity.
Secondary outcome
Adverse events will be assessed as the secondary outcome measures. Adverse events and their severity will be recorded on every visit during the time of receiving intervention, using an adverse event report check list, by asking from the patient and also physical examination performed by researchers. According to the consensus TMS safety guideline (Rossi et al., 2009), the most frequent potential side effect for low-frequency rTMS is headache and local pain, followed by possible transient hearing problems. Seizure and syncope have been rarely reported, however protective effects on seizure have also been reported (Rossi et al., 2009); in our study, TMS will be delivered on the cerebellum which will not confer a risk for seizure and moreover, we will exclude patients with a prior history of seizure to keep the risk of this event as low as possible. Ear plugs, with a protection of 25 decibels,will be used to prevent the possible hearing problems.Medical consult will be available for patients during the study for any possible side effects they experience.The TMS room will be equipped with cardiopulmonary resuscitation unit, and the TMS will be delivered by a physician. Considering stimulation over cerebellum andproximity of the brainstem structures, for preventing any theoretical side effects regarding brainstem stimulation,including syncope, we will perform MRI on all patients and the exact scalp distance of the cerebellar and motor cortices will be measured; the stimulation intensity would then be calculated and adjusted for each patient according to the measured distances to avoid over-stimulation and stimulation of the nearby structures.
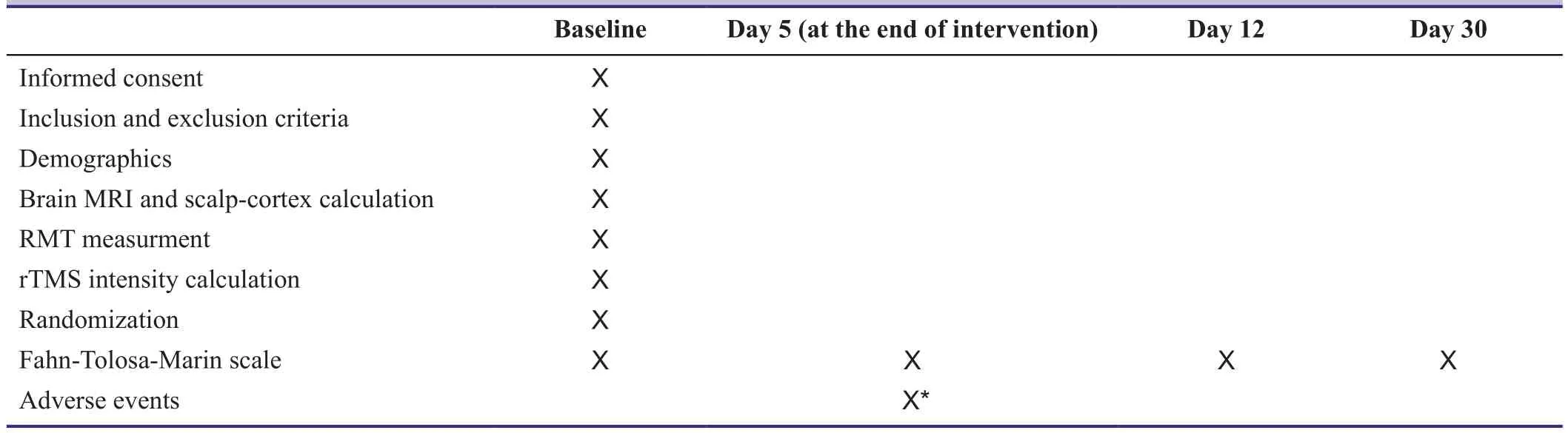
Table 1: Outcome measures and measurement time points
Data analysis
An intention-to-treat analysis regarding the FTM scale scores is assumed; data regarding withdrawal from study or rejection of participation will be collected and reported appropriately.
Baseline data will be collected from all participants at the first visit and reported using descriptive statistics.
Continuous variables will be analyzed using the Mann-Whitney U test or two-sample t-test. Categorical variables will be analyzed using a chi-square test. Pearson’s and Spearman’s correlation coefficients will be used to measure the strength of the linear relationship between primary and secondary outcomes.
Paired t-test and McNemar’s test will be used for intra-group comparisons before and after treatment. A significance level of 0.05 is assumed in this study and SPSS software version 20 will be used for data analysis.
Ethical considerations
Our study has been approved by Research Ethics Committee of our University Institution (approval number: ir.mums.sm.rec.1394.353) and will be performed in accordance with the guidelines of the Declaration of Helsinki. All patients will be asked to sign a written informed consent prior to receiving any intervention.
DISCUSSION
Essential tremor is a disabling condition, affecting people in all age groups with a bimodal overall prevalence that shows tow peaks in around 20 and 60 years. Postural and action tremors, which are the prominent types of tremor in these patients, are truly disabling and are a source of embarrassment and/or social isolation for many of these patients. As well, tremor may interfere with patient’s work or even limit their ability to perform their self-care independently. For many, especially young, patients the adverse effects of the current available medications (e.g., sedation and sexual problems) might be unacceptable and for some older patients, use of these medications might be limited by interference with their other necessary medications. Hence,a non-medical treatment, with a favorable safety profile,could be actually helpful for many of these patients.
Cerebellar rTMS has been used in patients afflicted with ET (Gironell et al., 2002; Popa et al., 2013; Bologna et al.,2015). Although a theta-burst stimulation did not showed any reduction in tremor severity (Bologna et al., 2015),low-frequency stimulation have reported to be beneficial in these patients in two trials (Gironell et al., 2002; Popa et al., 2013). However both trials had the limitation of small sample size and uncontrolled design. In this randomized,sham controlled, add-on and crossover trial, we aim to assess the potential effect of low-frequency rTMS on motor function and the degree of disability among ET patients and to address the shortcomings of previous studies.
TRIAL STATUS
We are recruiting our participants and performing the assessment for inclusion and exclusion criteria, collecting baseline characteristics; and MRI-guided measurement of the motor and cerebellar cortices distance from the skin are being performed at the time of submission.
Declaration of patient consent
The authors certify that they will obtain all appropriate patient consent forms. In the form the patient(s) will give his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Conflicts of interest
We have no conflicts of interest to declare.
Author contributions
Both authors contributed equally to concept and design of study,manuscript drafting and manuscript review, and approved the final manuscript for publication.
Plagiarism check
This paper was screened twice using CrossCheck to verify originality before publication.
Peer review
This paper was double-blinded and stringently reviewed by international expert reviewers.
Benito-León J (2008) Essential tremor: from a monosymptomatic disorder to a more complex entity. Neuroepidemiology 31:191-192.
Benito-León J (2014) Essential Tremor: A Neurodegenerative Disease? Tremor Other Hyperkinet Mov 4:252.
Bologna M, Rocchi L, Leodori G, Paparella G, Conte A (2015)Cerebellar continuous theta burst stimulation in essential tremor.Cerebellum 14:133-141.
Deuschl G, Bain P, Brin M (1998) Consensus statement of the Movement Disorder Society on Tremor. Ad Hoc Scientific Committee. Mov Disord 13 Suppl 3:2-23.
Deuschl G, Wenzelburger R, Löffler K, Raethjen J, Stolze H (2000)Essential tremor and cerebellar dysfunction clinical and kinematic analysis of intention tremor. Brain 123(Pt 8):1568-1580.
Fahn S, Tolosa E, Marin C (1988) Clinical rating scale for tremor.In: Parkinson’s Disease and Movement Disorders (Jankovic J,Tolosa E, ed), pp225-234. Baltimore (MD): Urban & Schwarzenberg.
Gironell A, Kulisevsky J, Lorenzo J, Barbanoj M, Pascual-Sedano B, Otermin P (2002) Transcranial magnetic stimulation of the cerebellum in essential tremor: a controlled study. Arch Neurol 59:413-417.
Jenkins IH, Bain PG, Colebatch JG, Thompson PD, Findley LJ,Frackowiak R, Marsden CD, Brooks DJ (1993) A positron emission tomography study of essential tremor: evidence for overactivity of cerebellar connections. Ann Neurol 34:82-90.
LaRoia H, Louis ED (2011) Association between essential tremor and other neurodegenerative diseases: what is the epidemiological evidence. Neuroepidemiology 37:1-10.
Louis ED (2009) Factor analysis of motor and nonmotor signs in essential tremor: are these signs all part of the same underlying pathogenic process? Neuroepidemiology 33:41-46.
Louis ED (2010) Essential tremor as a neuropsychiatric disorder. J Neurol Sci 289:144-148.
Louis ED, Ferreira JJ (2010) How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord 25:534-541.
Louis ED, Okun MS (2011) It is time to remove the “benign” from the essential tremor label. Parkinsonism Relat Disord 17:516-520.
Louis ED, Faust PL, Vonsattel JP, Honig LS, Rajput A, Robinson CA, Rajput A, Pahwa R, Lyons KE, Ross GW, Borden S, Moskowitz CB, Lawton A, Hernandez N (2007) Neuropathological changes in essential tremor: 33 cases compared with 21 controls.Brain 130:3297-3307.
Popa T, Russo M, Vidailhet M, Roze E, Lehéricy S, Bonnet C, Apartis E, Legrand AP, Marais L, Meunier S, Gallea C (2013) Cerebellar rTMS stimulation may induce prolonged clinical benefits in essential tremor, and subjacent changes in functional connectivity: An open label trial. Brain Stimul 6:175-179.
Rossi S, Hallett M, Rossini P, Pascual-Leone A, The Safety of TMS Consensus Group (2009) Safety, ethical considerations,and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 120:2008-2039.
Stokes M, Chambers C, Gould I, English T, McNaught E, McDonald O, Mattingley J (2007) Distance-adjusted motor threshold for transcranial magnetic stimulation. Clin Neurophysiol 118:1617-1625.
杂志排行
Asia Pacific Journal of Clinical Trials:Nervous System Diseases的其它文章
- Utilization patterns of benzodiazepines in psychiatric patients in a tertiary care teaching hospital
- Homeopathic prophylaxis for recurrent urinary tract infections following spinal cord injury: study protocol for a randomized controlled trial
- Bispectral index-guided fast track anesthesia by sevoflurane infusion combined with dexmedetomidine for intracranial aneurysm embolization: study protocol for a multi-center parallel randomized controlled trial
- Cognitive function and biomarkers after traumatic brain injury:protocol for a prospective inception cohort study
- Electroencephalographic changes following trigeminal nerve stimulation for major depressive disorder: study protocol for a randomized sham-controlled trial
- Effect of intravenous acetaminophen on post-operative opioidrelated complications: study protocol for a randomized,placebo-controlled trial
