Cognitive function and biomarkers after traumatic brain injury:protocol for a prospective inception cohort study
2016-04-10WeiLinLikunYangSangCaiJieZhuYiFengLixiangYangZhizhongFengPeipeiLiJunhuiChenYuhaiWang
Wei Lin, Li-kun Yang, Sang Cai, Jie Zhu, Yi Feng, Li-xiang Yang, Zhi-zhong Feng, Pei-pei Li, Jun-hui Chen,Yu-hai Wang*
Department of Neurosurgery, the 101st Hospital of PLA, Wuxi, Jiangsu Province, China
INTRODUCTION
Traumatic brain injury (TBI) occurs when external forces,such as a blow, collision, or penetration, traumatically damage the brain, resulting in mechanical deformations in the skull, meninges, blood vessels and tissues. TBI is a major cause of death and disability worldwide, especially in children and young people, and can cause considerable suffering and financial strain to patients and those close to them (D'Ambrosio and Perucca, 2004; Maas et al., 2008;Kochanek et al., 2012; Almeida et al., 2015; Levin and Diaz-Arrastia, 2015; Lind et al., 2016). Moreover, the number of patients with TBI is increasing owing to the growing number of vehicles on the road (Maas et al., 2008).

Table 1: Studies of biomarkers and imaging analysis in patients with traumatic brain injury (TBI) published in 2014–2016 in the MEDLINE database
TBI comprises primary and secondary injuries. Primary intracranial injuries mostly occur when the tissues and blood vessels are damaged directly, leading to cell death or neurological damage. Secondary injuries are varied and complex, involving damage to the blood-brain barrier,cytokine secretion, free radical overload, excessive release of neurotransmitters, calcium and sodium ion influx in neurons, mitochondrial dysfunction, cerebral blood flow changes, substantial changes in intracranial pressure and movement dysfunction, traumatic epilepsy, and personality changes (Parikh et al., 2007; Park et al., 2008; Kochanek et al., 2012; Strathmann et al., 2014; Helmick et al., 2015;Levin and Diaz-Arrastia, 2015). Patients with TBI develop long-term physiological, cognitive, social, emotional and behavioral impairments. Current tools for post-TBI cognitive function assessment are simple, real-time scales (Yang et al., 2012) that cannot provide effective evidence for prognosis or treatment (Michael et al., 2015). Therefore, there is an urgent need for an objective means of assessing TBI.
Functional magnetic resonance imaging (fMRI) is an established neuroimaging detection tool that measures hemodynamic variation caused by neuronal activity. Its spatiotemporal resolution is relatively high, and the technique is non-invasive and non-radiative. As such, fMRI has been widely used in the assessment of brain function since the 1990s (Belliveau et al., 1990), including measuring changes in brain function and network connectivity at different stages of TBI (Sharp et al., 2014; Altmeyer et al., 2016;Banks et al., 2016; Herrera et al., 2016; Li et al. , 2016).Tau proteins are microtubule-associated proteins mainly found in neuronal axons and cytoplasm, which promote tubulin polymerization into microtubules, stabilize microtubules and maintain neurological effects (Buée et al., 2000).It was recently observed that tau was released from the neurons into the cerebrospinal fluid during nerve cell necrosis and apoptosis (Tapiola et al., 2009; Skillbäck et al., 2015).Tau protein is therefore considered a marker of nerve cell damage. Existing evidence from patients with TBI suggests that there is an excess of tau protein in the brain during longterm follow-up and at autopsy. This may be the main cause of continued cognitive, movement, behavioral and mental disorders after TBI (Mitsis et al., 2014; Laskowski et al.,2015). Combining fMRI and cerebrospinal fluid biomarker analysis will help us to understand the changes in cognitive function that occur after TBI.
Our study will determine the relationship between changes in brain imaging, level of tau in the cerebrospinal fluid, and cognitive function in patients with TBI, thereby providing a clinical basis for the early diagnosis, prevention and treatment of cognitive disorders in TBI patients.
By retrieving articles in Medline and ClinicalTrial.gov from 2014 to 2016, we found that relevant studies focused only on fMRI and cerebrospinal fluid biomarker analysis in patients with TBI (Tables 1, 2), without investigating the relationships of these measures to cognitive function.
METHODS/DESIGN
Study design
A single-group prospective inception cohort study.
Study setting
This trial will be carried out at the 101stHospital of PLA,Wuxi, China.
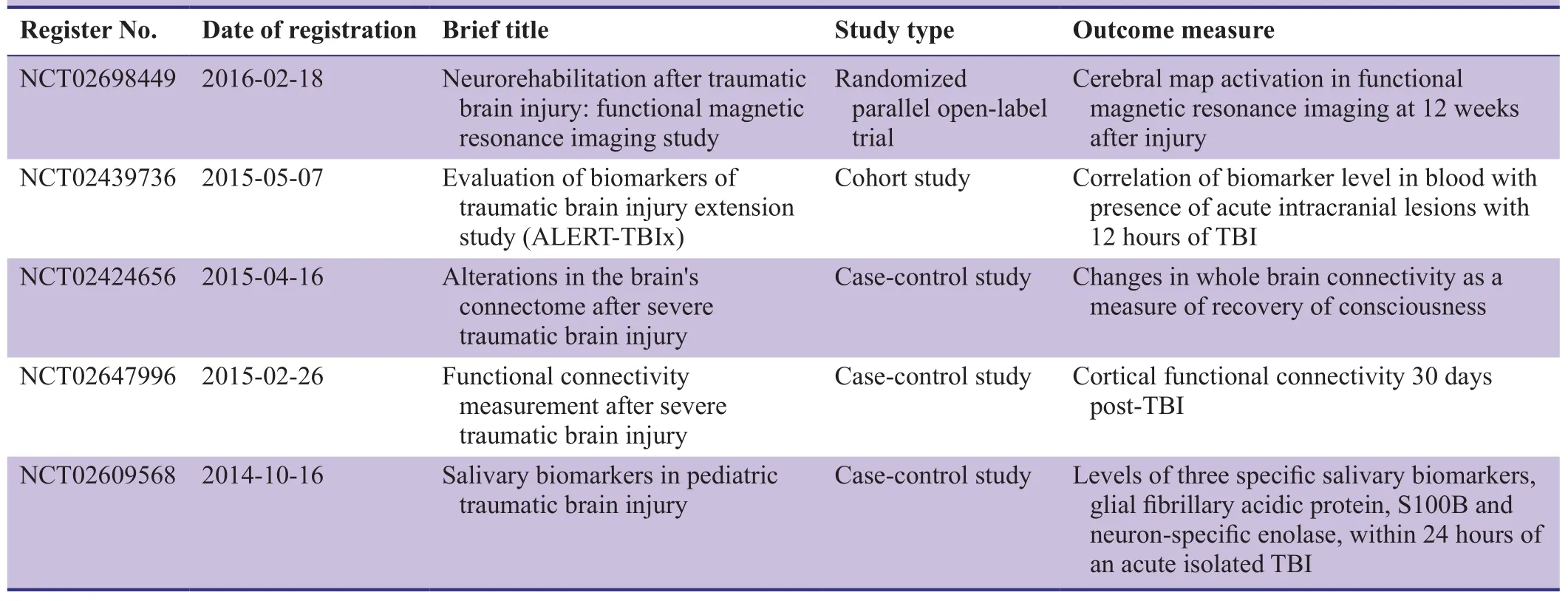
Table 2: Clinical trials registered at ClinicalTrials.gov addressing biomarker and imaging analysis in patients with traumatic brain injury (TBI)

Figure 1: Flow chart of the trial protocol.
Study procedures
We will recruit 100 patients admitted to the 101stHospital of PLA with TBI, who will be screened through inclusion and exclusion criteria and baseline assessment. All patients’legal representatives will be invited to give informed consent prior to the trial. Each patient will be assessed using the Mini-Mental State Examination, Montreal Cognitive Assessment, Hamilton Depression Scale, fMRI, and tau protein measurement in the cerebrospinal fluid within 24 hours of admission and 0.5, 1, and 2 years after TBI. We will analyze the correlation between cognitive function, fMRI results, and tau levels. The flow chart of the study protocol is shown in Figure 1.
Study participants
Patients with TBI will be recruited from the inpatients or outpatients admitted to the 101stHospital of PLA.
Inclusion criteria
Patients who meet all the following criteria will be eligible for this trial:
· Brain damage caused by external force
· Cerebral edema/intracranial hematoma shown by brain CT or X-ray
· Short-term (or no) decrease in consciousness, or showing cognitive, behavioral or mental impairment
· 18–80 years of age
Exclusion criteria
Patients who meet one of the following criteria will be excluded from the trial:
· Severe chronic diseases, such as cancer, chronic renal failure, severe respiratory insufficiency
· Severe heart failure, central nervous system disorders and systemic infections
· Crush syndrome
· Other organic damages
· Hemoglobin and albumin loss resulting from active bleeding from other body parts
· Onset > 6 hours before admission, or death within 72 hours of admission
· Pregnant
Withdrawal criteria
Patients will be withdrawn from the trial if one of the following conditions occurs:
· Complications affecting efficacy and safety of judgment
· Unable to complete a 2-year follow-up visit
Sample size
As in previous comparable studies (Vos et al., 2010; Wu et al., 2011; Lei et al., 2015), the proposed sample size of 100 patients takes into account the available research funds. The sample size will be calculated in line with the intention-to-treat principle.
Baseline data
Data collected at baseline will include demographic data(gender, age, body weight, level of education), causes of brain damage, and general medical history (Table 3).
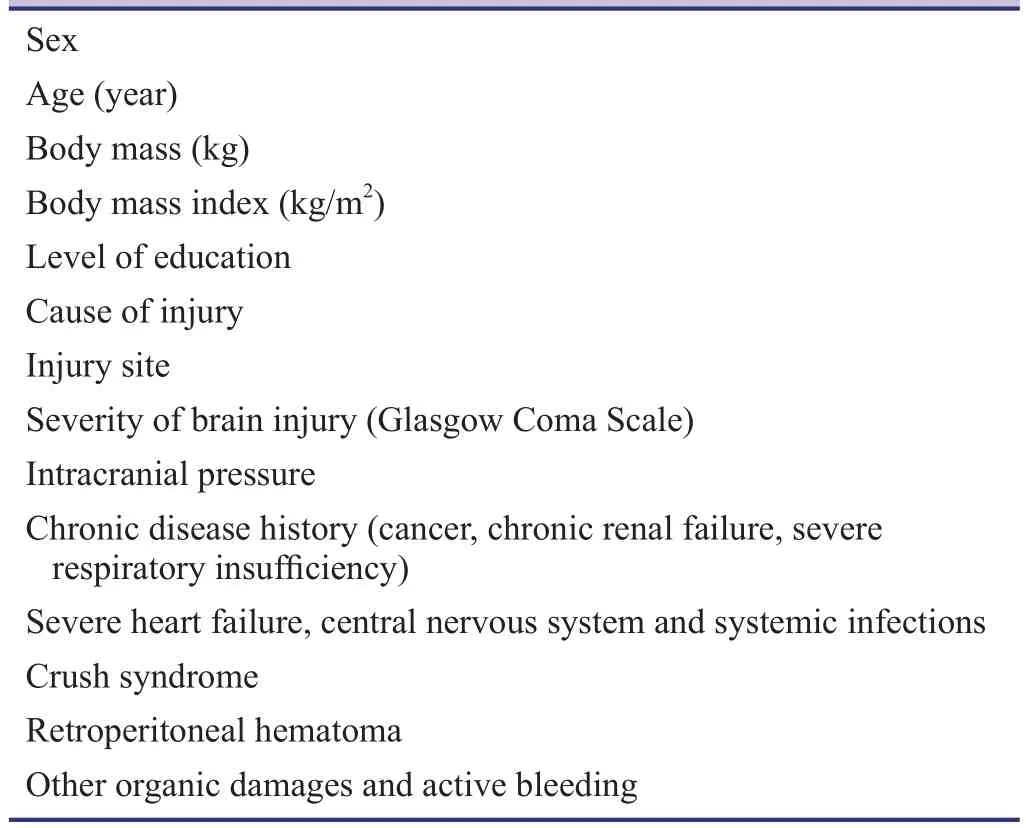
Table 3: Baseline assessment of patients
Recruitment
Ongoing recruitment of outpatients or inpatients will be carried out by neurological physicians. Patients interested in participating in the trial will contact the lead investigator by telephone, e-mail or WeChat.
Outcome measures
Recovery of neurological function after TBI usually takes more than 2 years, although it can be achieved within 6 months (Ponsford et al., 2008). All indicators will therefore be measured within 24 hours, and 0.5, 1, and 2 years after TBI.
Primary outcomes
· Montreal Cognitive Assessment: modified from the Mini-Mental State Examination, this is a widely used scale for detecting cognitive impairment. This scale is easy to use and correlates cognitive impairment with different brain injury sites. The scale assesses the following domains:orientation, attention, naming, visuospatial and implementation capacity, memory, abstraction and language skills. The maximum score is 30; patients with scores ≤26 are considered to have cognitive impairment. Patients who have over 12 years of education will have 1 point subtracted to their test score to correct for the deviation of education (Julayanont et al., 2015).
· fMRI indicators: 3T MRI scanners (GE, Boston, MA,USA) will be used for fMRI detection. The patient will lie flat on his/her back, keeping still and quiet, and T1,T2, DTI and resting state functional sequences will be collected.
· Tau protein level in the cerebrospinal fluid: cerebrospinal fluid samples from patients with TBI will be collected during subarachnoid puncture or postoperative drainage within 24 hours of injury, and at 0.5, 1, and 2 years after injury. Tau protein level in the cerebrospinal fluid will be measured using an ELISA kit (Abcam Trading(Shanghai) Company Ltd., Shanghai, China).
Secondary outcomes
· Mini-Mental State Examination Scale (Folstein et al.,1975): This scale is one of the most influential standardized tools for intelligence assessment, and involves orientation, memory, attention/calculation, recall, and language skills (maximum score of 30). It can be used to detect cognitive impairment.
· Hamilton Depression Scale (Hamilton, 1960): This is the most commonly used scale for clinical assessment of depression. It has 24 domains; higher scores indicate more severe depression.
The schedule for outcome assessments is shown in Table 4.
Data collection, management, analysis, and open access
Data collection
Clinical researchers will be responsible for the accurate, full,and timely completion of case report forms. All data will be electronically input by two independent data entry clerks.
Data management
After database confirmation, the database will be locked by the lead investigator. The locked data will not be altered and will be preserved by the 101stHospital of PLA.
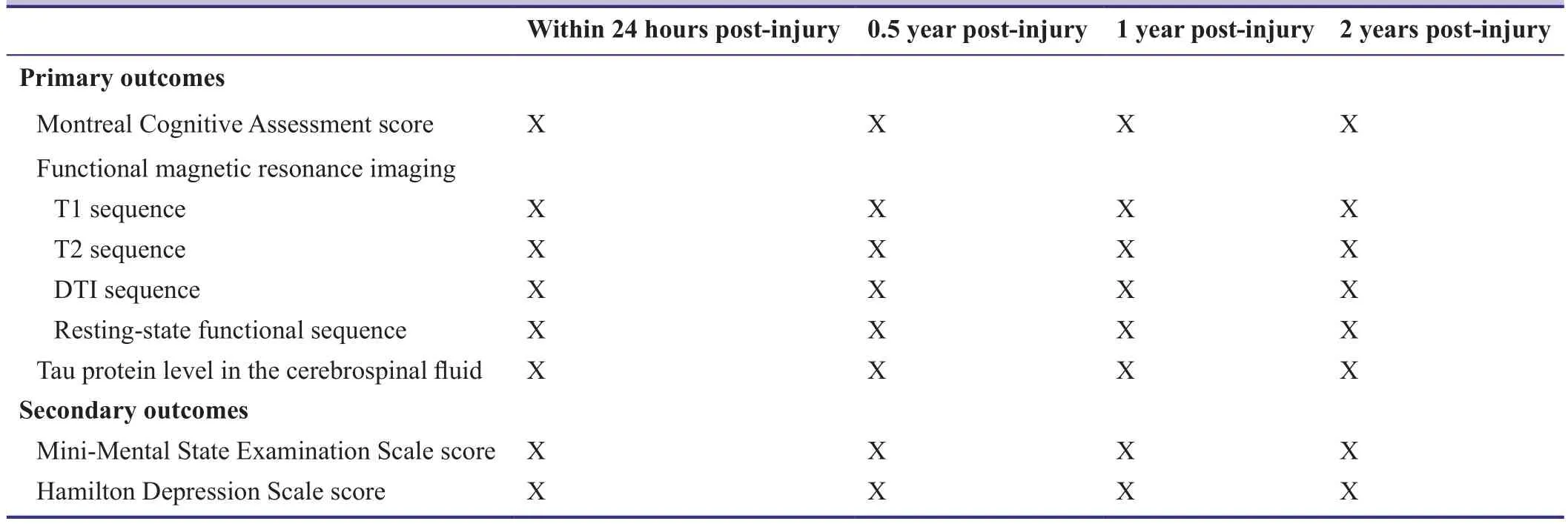
Table 4: Timing of outcome assessment
Data analysis
All data will be analyzed by professional statisticians.
Open data
Published data will be released at http://www.figshare.com.
Statistical analysis
Measurement data will be expressed as the mean ± SD, and count data as percentages. All data will be analyzed statistically using SPSS 19.0 (IBM Corp., Armonk, NY, USA).Repeated measures analysis of variance will be used for intragroup comparisons between time points, and Spesarman correlation analysis for calculating the relationship of fMRI indicators and tau level in the cerebrospinal fluid with cognitive function in patients. A value of P < 0.05 will be considered statistically significant.
Auditing
Trial progress will be reported to the ethics committee of the 101stHospital of PLA every 3 months, and relevant data will be updated in the Chinese Clinical Trial Registry.
Confidentiality
Electronic data will be stored on a dedicated computer, and the password will be set and managed by a data administrator. Paper reports will be preserved in a secure and locked place by the data administrator and lead investigator.
TRIAL STATUS
Recruitment is ongoing at the time of submission.
DISCUSSION
Previous studies have attempted to monitor cognitive function in patients with TBI using a variety of markers for brain damage, all of which were confirmed unsuitable for clinical use because of tissue-specific issues (Strathmann et al., 2014). In the present trial, we will conduct a follow-up assessment of participants’ cognitive function,and analyze the relationship between changes in brain imaging, cerebrospinal fluid biomarkers, and cognitive function after TBI.
The 101stHospital of PLA is the military TBI treatment center, and as such is the largest center for brain trauma in Wuxi, China. The hospital sees a sufficient number of patients with TBI who will meet the inclusion criteria and whose rights will be guaranteed as written informed consent will be obtained prior to participation. The follow-up time will be up to 2 years, to cover the period of recovery from TBI (Maas et al., 2008).
A limitation of the study is that tau level in the cerebrospinal fluid will only be detected after TBI, and its baseline will not be known. This might have an impact on subsequent analysis, so future case-control studies will be warranted.The aim of this trial is to offer an effective biomarker for predicting the development of and recovery from cognitive impairment in patients with TBI, thereby providing an objective basis for the clinical prevention and treatment of TBI.
Declaration of patient consent
The authors certify that they will obtain all appropriate patient consent forms. In the form the patient(s) will give his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Conflicts of interest
None declared.
Author contributions
YHW is responsible for the trial; WL and LKY are the main implementers; others are project-specific operators. All authors have approved the final version of the manuscript.
Plagiarism check
This paper was screened twice using CrossCheck to verify originality before publication.
Peer review
This paper was double-blinded and stringently reviewed by international expert reviewers.
Almeida OP, Hankey GJ, Yeap BB, Golledge J, Flicker L (2015)Prevalence, associated factors, mood and cognitive outcomes of traumatic brain injury in later life: the health in men study(HIMS). Int J Geriatr Psychiatry 30:1215-1223.
Altmeyer W, Steven A, Gutierrez J (2016) Use of magnetic resonance in the evaluation of cranial trauma. Magn Reson Imaging Clin N Am 24:305-323.
Banks SD, Coronado RA, Clemons LR, Abraham CM, Pruthi S,Conrad BN, Morgan VL, Guillamondegui OD, Archer KR (2016)Thalamic functional connectivity in mild traumatic brain injury:longitudinal associations with patient-reported outcomes and neuropsychological tests. Arch Phys Med Rehabil doi: 10.1016/j.apmr.2016.03.013.
Belliveau JW, Rosen BR, Kantor HL, Rzedzian RR, Kennedy DN,McKinstry RC, Vevea JM, Cohen MS, Pykett IL, Brady TJ (1990)Functional cerebral imaging by susceptibility-contrast NMR.Magn Reson Med 14:538-546.
Buée L, Bussière T, Buée-Scherrer V, Delacourte A, Hof PR (2000)Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Rev 33:95-130.
D'Ambrosio R, Perucca E (2004) Epilepsy after head injury. Curr Opin Neurol 17:731-735.
Folstein MF, Folstein SE, McHugh PR (1975) "Mini-mental state".A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189-198.
Hamilton M (1960) A rating scale for depression. J Neurol Neurosurg Psychiatry 23:56-62.
Helmick KM, Spells CA, Malik SZ, Davies CA, Marion DW, Hinds SR (2015) Traumatic brain injury in the US military: epidemiology and key clinical and research programs. Brain Imaging Behav 9:358-366.
Herrera JJ, Bockhorst K, Kondraganti S, Stertz L, Quevedo J, Narayana PA (2016) Acute white matter tract damage after frontal mild traumatic brain injury. J Neurotrauma doi:10.1089/neu.2016.4407.
Julayanont P, Tangwongchai S, Hemrungrojn S, Tunvirachaisakul C, Phanthumchinda K, Hongsawat J, Suwichanarakul P, Thanasirorat S, Nasreddine ZS (2015) The Montreal Cognitive Assessment—Basic: a screening tool for mild cognitive impairment in illiterate and low-educated elderly adults. J Am Geriatr Soc 63:2550-2554.
Kochanek PM, Carney N, Adelson PD, Ashwal S, Bell MJ, Bratton S, Carson S, Chesnut RM, Ghajar J, Goldstein B, Grant GA, Kissoon N, Peterson K, Selden NR, Tasker RC, Tong KA, Vavilala MS, Wainwright MS, Warden CR, American Academy of Pediatrics-Section on Neurological Surgery, et al. (2012) Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents--second edition. Pediatr Crit Care Med 13 Suppl 1:S1-82.
Laskowski RA, Creed JA, Raghupathi R (2015) Pathophysiology of mild TBI: implications for altered signaling pathways. In: Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects (Kobeissy FH, ed). Boca Raton, FL, USA: CRC Press/Taylor & Franci.
Lei J, Gao G, Feng J, Jin Y, Wang C, Mao Q, Jiang J (2015) Glial fibrillary acidic protein as a biomarker in severe traumatic brain injury patients: a prospective cohort study. Crit Care 19:362.
Levin HS, Diaz-Arrastia RR (2015) Diagnosis, prognosis, and clinical management of mild traumatic brain injury. Lancet Neurol 14:506-517.
Li L, Sun G, Liu K, Li M, Li B, Qian SW, Yu LL (2016) White matter changes in posttraumatic stress disorder following mild traumatic brain injury: a prospective longitudinal diffusion tensor imaging study. Chin Med J 129:1091-1099.
Lind K, Toure H, Brugel D, Meyer P, Laurent-Vannier A, Chevignard M (2016) Extended follow-up of neurological, cognitive,behavioral and academic outcomes after severe abusive head trauma. Child Abuse Negl 51:358-367.
Liu CL, Chen CC, Lee HC, Cho DY (2014) Matrix metalloproteinase-9 in the ventricular cerebrospinal fluid correlated with the prognosis of traumatic brain injury. Turk Neurosurg 24:363-368.Maas AI, Stocchetti N, Bullock R (2008) Moderate and severe traumatic brain injury in adults. Lancet Neurol 7:728-741.
Manevich Y, Hutchens S, Halushka PV, Tew KD, Townsend DM,Jauch EC, Borg K (2014) Peroxiredoxin VI oxidation in cerebrospinal fluid correlates with traumatic brain injury outcome. Free Radical Biol Med 72:210-221.
Mayer AR, Hanlon FM, Dodd AB, Ling JM, Klimaj SD, Meier TB(2015) A functional magnetic resonance imaging study of cognitive control and neurosensory deficits in mild traumatic brain injury. Hum Brain Mapp 36:4394-4406.
Michael AP, Stout J, Roskos PT, Bolzenius J, Gfeller J, Mogul D,Bucholz R (2015) Evaluation of cortical thickness after traumatic brain injury in military veterans. J Neurotrauma 32:1751-1758.
Mitsis EM, Riggio S, Kostakoglu L, Dickstein DL, Machac J, Delman B, Goldstein M, Jennings D, D'Antonio E, Martin J, Naidich TP, Aloysi A, Fernandez C, Seibyl J, DeKosky ST, Elder GA,Marek K, Gordon W, Hof PR, Sano M, et al. (2014) Tauopathy PET and amyloid PET in the diagnosis of chronic traumatic encephalopathies: studies of a retired NFL player and of a man with FTD and a severe head injury. Transl Psychiatry 4:e441.
Parikh S, Koch M, Narayan RK (2007) Traumatic brain injury. Int Anesthesiol Clin 45:119-135.
Park E, Bell JD, Baker AJ (2008) Traumatic brain injury: can the consequences be stopped? CMAJ 178:1163-1170.
Ponsford J, Draper K, Schönberger M (2008) Functional outcome 10 years after traumatic brain injury: its relationship with demographic, injury severity, and cognitive and emotional status. J Int Neuropsychol Soc 14:233-242.
Sharp DJ, Scott G, Leech R (2014) Network dysfunction after traumatic brain injury. Nat Rev Neurol 10:156-166.
Skillbäck T, Farahmand BY, Rosén C, Mattsson N, Nägga K,Kilander L, Religa D, Wimo A, Winblad B, Schott JM, Blennow K, Eriksdotter M, Zetterberg H (2015) Cerebrospinal fluid tau and amyloid-β1-42 in patients with dementia. Brain 138:2716-2731.Strathmann FG, Schulte S, Goerl K, Petron DJ (2014) Blood-based biomarkers for traumatic brain injury: Evaluation of research approaches, available methods and potential utility from the clinician and clinical laboratory perspectives. Clin Biochem 47:876-888.
Tapiola T, Alafuzoff I, Herukka SK, Parkkinen L, Hartikainen P,Soininen H, Pirttilä T (2009) Cerebrospinal fluid {beta}-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol 66:382-389.
Vergara VM, Damaraju E, Mayer AB, Miller R, Cetin MS, Calhoun V (2015) The impact of data preprocessing in traumatic brain injury detection using functional magnetic resonance imaging.Conf Proc IEEE Eng Med Biol Soc 2015:5432-5435.
Vos PE, Jacobs B, Andriessen TM, Lamers KJ, Borm GF, Beems T,Edwards M, Rosmalen CF, Vissers JL (2010) GFAP and S100B are biomarkers of traumatic brain injury: an observational cohort study. Neurology 75:1786-1793.
Wu X, Sha H, Sun Y, Gao L, Liu H, Yuan Q, Zhang T, Zhu J, Zhou L, Hu J (2011) N-terminal pro-B-type natriuretic peptide in patients with isolated traumatic brain injury: a prospective cohort study. J Trauma 71:820-825.
Yang XH, Liu J, Liu WH (2012) Research progress on cognitive function rating scale in cerebral trauma patients. Huli Yanjiu 26:1827-1829.
Yen HC, Chen TW, Yang TC, Wei HJ, Hsu JC, Lin CL (2015) Levels of F2-isoprostanes, F4-neuroprostanes, and total nitrate/nitrite in plasma and cerebrospinal fluid of patients with traumatic brain injury. Free Radic Res 49:1419-1430.
杂志排行
Asia Pacific Journal of Clinical Trials:Nervous System Diseases的其它文章
- Utilization patterns of benzodiazepines in psychiatric patients in a tertiary care teaching hospital
- Homeopathic prophylaxis for recurrent urinary tract infections following spinal cord injury: study protocol for a randomized controlled trial
- Effectiveness of cerebellar repetitive transcranial magnetic stimulation in essential tremor: study protocol for a randomized, sham-controlled trial
- Bispectral index-guided fast track anesthesia by sevoflurane infusion combined with dexmedetomidine for intracranial aneurysm embolization: study protocol for a multi-center parallel randomized controlled trial
- Electroencephalographic changes following trigeminal nerve stimulation for major depressive disorder: study protocol for a randomized sham-controlled trial
- Effect of intravenous acetaminophen on post-operative opioidrelated complications: study protocol for a randomized,placebo-controlled trial
