Comparisons of phaseolin type and α-amylase inhibitor in common bean (Phaseolus vulgaris L.) in China
2016-04-05YangYaoYiboHuYingyingZhuYueGaoGuixingRen
Yang Yao, Yibo Hu, Yingying Zhu, Yue Gao, Guixing Ren*
Institute of Crop Science, Chinese Academy of Agricultural Sciences, Beijing 100081, China
Comparisons of phaseolin type and α-amylase inhibitor in common bean (Phaseolus vulgaris L.) in China
Yang Yao1, Yibo Hu1, Yingying Zhu, Yue Gao, Guixing Ren*
Institute of Crop Science, Chinese Academy of Agricultural Sciences, Beijing 100081, China
A R T I C L E I N F O
Article history:
Received 5 February 2015
Received in revised form
29 September 2015
Accepted 27 November 2015
Available online 3 December 2015
Keywords:
Common bean
Phaseolin
α-Amylase inhibitor
Phenolic acid
A B S T R A C T
The objective of this study was to characterize the phaseolin type and α-amylase (αAI) level in common bean (Phaseolus vulgaris L.) accessions deposited in the Chinese National Genebank. The 40 accessions sampled were common varieties originating in Asia, North America, South America, Europe, and Africa. No Inca (I-) phaseolin was observed in the accessions. Only four accessions contained Tendergreen (T-) phaseolin and the remaining 36 contained Sanilac (S-) phaseolin.αAI proteins extracted from nine accessions showed higher α-amylase inhibitory activity than the control (Phase 2, IC50= 0.65 μg). These common bean accessions have potential use as nutraceutical ingredients.
©2015 Crop Science Society of China and Institute of Crop Science, CAAS. Production and hosting by Elsevier B.V. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
* Corresponding author.
E-mail address: renguixing@caas.cn (G. Ren)
Peer review under responsibility of Crop Science Society of China and Institute of Crop Science, CAAS.1These authors contributed equally to this work.
http://dx.doi.org/10.1016/j.cj.2015.09.002
2214-5141/©2015 Crop Science Society of China and Institute of Crop Science, CAAS. Production and hosting by Elsevier B.V. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
1. Introduction
Common bean (Phaseolus vulgaris L.) is the most important food legume in the world, accounting for half of grain legumes in direct human consumption [1]. Common bean is rich in protein, unsaturated fatty acids, and dietary fiber in addition to vitamins and minerals [2]. Recently, researchers have focused interest on common bean proteins with specific functions, such as in anti-obesity [3], anti-hypersensitivity, and antioxidant [4] and anti-diabetic [5] activities. Phaseolinisthe major storage protein in common bean seed,accounting for about 50% of total protein. Phase 2 (Pharmachem Laboratories, Kearny, NJ, USA) is a common bean extract product that can reduce human body weight at a daily dose of 500–3000 mg (D. Brady, N. D. CarbXzyme). Clinical studies also showed that Phase 2 has the potential to induce weight loss and reduce spikes in blood sugar caused by carbohydrates through its α-amylase inhibiting activity [6–8].
A proteinaceous inhibitor of α-amylase (αAI) isolated from common bean has been reported to have great potential to treat obesity and diabetes without side effects such as asthma and dermatitis [9]. Several companies have marketed common bean αAI extracts for controlling appetite and energy intake [10]. To date, information on phaseolin type and αAIdeposited in the Chinese National Genebank, Beijing, China is very limited. To contribute to the knowledge in this area, we investigated the phaseolin types and αAI levels of 40 common bean accessions deposited in the Genebank.
2. Materials and methods
2.1. Materials
Forty numbered common bean accessions (Table 1) were obtained from the Chinese National Genebank. The sampled accessionswere themostcommon varieties originating in Asia, North America,South America,Europe,and Africa.Eachsample was milled into fine (60-mesh) powder, cooled immediately, andstoredat−20°C.Porcinepancreaticα-amylase,ammonium sulfate,tert-butanol and bovine serumalbuminwere purchased from Sigma Chemical Co. (St. Louis, MO, USA). Other chemicals used were of analytical grade. Phase 2 extracted from white kidney bean was used as a reference for comparison.
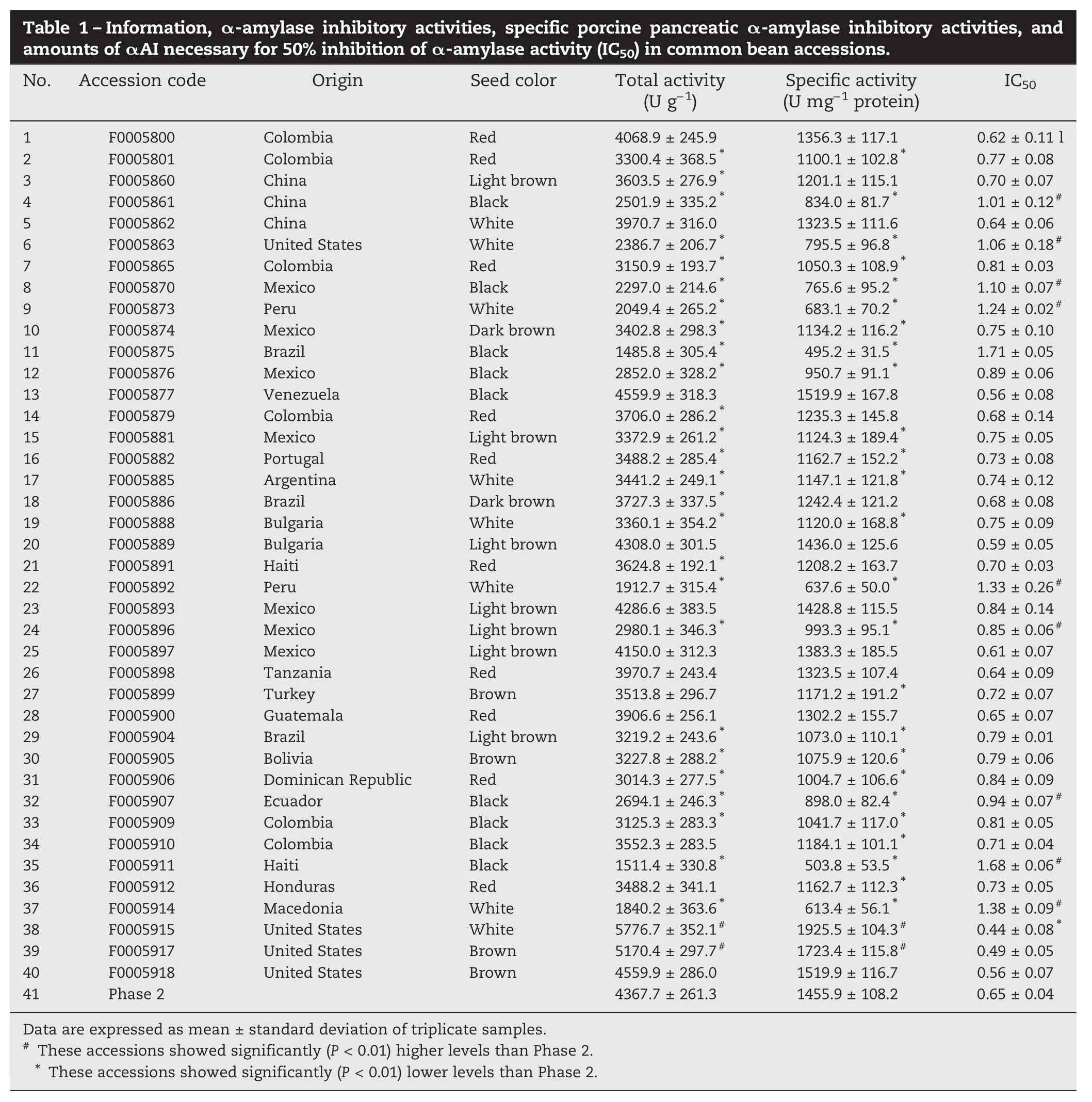
Table 1–Information,α-amylase inhibitory activities, specific porcine pancreatic α-amylase inhibitory activities, and amounts of αAI necessary for 50% inhibition of α-amylase activity (IC50) in common bean accessions. No. Accession code Origin Seed color Total activity (U g−1) 1 Specific activity (U mg−1protein) IC50 F0005800 Colombia Red 4068.9±245.9 1356.3±117.1 0.62±0.11 l 2 F0005801 Colombia Red 3300.4±368.5* 1100.1±102.8* 0.77±0.08 3 F0005860 China Light brown 3603.5±276.9* 1201.1±115.1 0.70±0.07 4 F0005861 China Black 2501.9±335.2* 834.0±81.7* 1.01±0.12#5 F0005862 China White 3970.7±316.0 1323.5±111.6 0.64±0.06 6 F0005863 United States White 2386.7±206.7* 795.5±96.8* 1.06±0.18#7 F0005865 Colombia Red 3150.9±193.7* 1050.3±108.9* 0.81±0.03 8 F0005870 Mexico Black 2297.0±214.6* 765.6±95.2* 1.10±0.07#9 F0005873 Peru White 2049.4±265.2* 683.1±70.2* 1.24±0.02#10 F0005874 Mexico Dark brown 3402.8±298.3* 1134.2±116.2* 0.75±0.10 11 F0005875 Brazil Black 1485.8±305.4* 495.2±31.5* 1.71±0.05 12 F0005876 Mexico Black 2852.0±328.2* 950.7±91.1* 0.89±0.06 13 F0005877 Venezuela Black 4559.9±318.3 1519.9±167.8 0.56±0.08 14 F0005879 Colombia Red 3706.0±286.2* 1235.3±145.8 0.68±0.14 15 F0005881 Mexico Light brown 3372.9±261.2* 1124.3±189.4* 0.75±0.05 16 F0005882 Portugal Red 3488.2±285.4* 1162.7±152.2* 0.73±0.08 17 F0005885 Argentina White 3441.2±249.1* 1147.1±121.8* 0.74±0.12 18 F0005886 Brazil Dark brown 3727.3±337.5* 1242.4±121.2 0.68±0.08 19 F0005888 Bulgaria White 3360.1±354.2* 1120.0±168.8* 0.75±0.09 20 F0005889 Bulgaria Light brown 4308.0±301.5 1436.0±125.6 0.59±0.05 21 F0005891 Haiti Red 3624.8±192.1* 1208.2±163.7 0.70±0.03 22 F0005892 Peru White 1912.7±315.4* 637.6±50.0* 1.33±0.26#23 F0005893 Mexico Light brown 4286.6±383.5 1428.8±115.5 0.84±0.14 24 F0005896 Mexico Light brown 2980.1±346.3* 993.3±95.1* 0.85±0.06#25 F0005897 Mexico Light brown 4150.0±312.3 1383.3±185.5 0.61±0.07 26 F0005898 Tanzania Red 3970.7±243.4 1323.5±107.4 0.64±0.09 27 F0005899 Turkey Brown 3513.8±296.7 1171.2±191.2* 0.72±0.07 28 F0005900 Guatemala Red 3906.6±256.1 1302.2±155.7 0.65±0.07 29 F0005904 Brazil Light brown 3219.2±243.6* 1073.0±110.1* 0.79±0.01 30 F0005905 Bolivia Brown 3227.8±288.2* 1075.9±120.6* 0.79±0.06 31 F0005906 Dominican Republic Red 3014.3±277.5* 1004.7±106.6* 0.84±0.09 32 F0005907 Ecuador Black 2694.1±246.3* 898.0±82.4* 0.94±0.07#33 F0005909 Colombia Black 3125.3±283.3* 1041.7±117.0* 0.81±0.05 34 F0005910 Colombia Black 3552.3±283.5 1184.1±101.1* 0.71±0.04 35 F0005911 Haiti Black 1511.4±330.8* 503.8±53.5* 1.68±0.06#36 F0005912 Honduras Red 3488.2±341.1 1162.7±112.3* 0.73±0.05 37 F0005914 Macedonia White 1840.2±363.6* 613.4±56.1* 1.38±0.09#38 F0005915 United States White 5776.7±352.1# 1925.5±104.3# 0.44±0.08* 39 F0005917 United States Brown 5170.4±297.7# 1723.4±115.8# 0.49±0.05 40 F0005918 United States Brown 4559.9±286.0 1519.9±116.7 0.56±0.07 41 Phase 2 4367.7±261.3 1455.9±108.2 0.65±0.04 Data are expressed as mean±standard deviation of triplicate samples.#These accessions showed significantly (P<0.01) higher levels than Phase 2. * These accessions showed significantly (P<0.01) lower levels than Phase 2.
2.2. Isolation and purification of phaseolin
Phaseolin was purified as described by Carrasco-Castilla et al. [11]. The powder sample was defatted with hexane for 24 h and extracted in NaOH solution (1:10, w/v, pH 9.5), with agitation at 40°C for 30 min. The supernatant from centrifugation at 5000×g for 30 min was adjusted to pH 4.5 with 1 mol L−1HCl.The protein precipitate was retrieved by centrifugation at 10,000×g for 30 min and lyophilized. The lyophilized flour was suspended in the NaCl (0.5 mol L−1) and HCl (0.025 mol L−1) mixture (1:20, w/v, pH 2.0) for 1 h and centrifuged at 13,500×g for30 min.Thesupernatantwascentrifugedagainfor30 minat 4°C and 13,500×g after the addition of five volumes of distilled water at 4°C. The precipitate was washed with distilled water and centrifuged again. The final precipitate was dialyzed against distilled water at 4°C for 24 h and lyophilized.
2.3. Extraction of α-amylase inhibitors
Alpha-amylase inhibitors were extracted as described by Wang et al. [9]. Each common bean powder sample of 10 g was suspended in 100 mL distilled water (pH 6.50), stirred for 2 h at room temperature, and centrifuged at 12,000×g for 60 min. The supernatant (pH 5.25) was heated for 15 min at 70°C to denature heat-labile proteins, which were removed by centrifugation at 12,000×g for 20 min. The remaining supernatants were subjected to 1 h protein partitioning by addition of 30% ammonium sulfate and tert-butanol. The mixture was then centrifuged (2000×g for 10 min) to facilitate the separation of phases. The lower aqueous layer was collected and desalted using a Sephax G-75 column (GE Healthcare, USA) equilibrated with 10 mmol L−1citrate/phosphate buffer (pH 8.0). The products were then subjected to activity testing and protein measurement.
2.4. SDS-PAGE
The protein products were analyzed by SDS-PAGE [12]. Protein loadings were 0.2 and 0.1 mg per well for pure phaseolin and α-amylase inhibitor, respectively. Molecular weight standards (10.0–250.0 kDa; BioRad, USA) were also loaded in a separate well on each gel. The electrophoresis results were visualized by Coomassie brilliant blue staining.
2.5. Measurement of α-amylase inhibition activity
Theα-amylaseinhibitionactivitywasdeterminedaspreviously described [13]. Porcine pancreatic α-amylase (40 U mL−1) was dissolved in a sodium succinate buffer (15 mmol L−1NaOH, 20 mmol L−1CaCl2, and 0.5 mol L−1NaCl, pH 5.6). A mixture composed of 100 μL α-amylase solution and 100 μL of extracted α-amylase inhibitor was first incubated in a water bath at 37°C for 30 min. Then, 400 μL of 2% (w/v) soluble starch (dissolved in 20 mmol L−1sodium phosphate buffercontaining6.7 mmol L−1NaCl, pH 6.9) was added and the reaction was stopped after 1 min by an addition of 800 μL of 3,5-dinitrosalicylic acid and heating in a boiling water bath for 10 min. The volume of the mixture was finally increased to 6 mL with double-distilled water. A parallel measurement was performed simultaneously, withthesameamountofenzymebutwithouttheinhibitor.The results of the two measurements were compared to determine the inhibition activity. One inhibitory unit was defined as the amount of α-amylase inhibitor that completely inhibited the activity of one unit of enzyme [13]. The total activity was expressed as inhibitory units per gram of common bean (dry weight). The specific activity was expressed as inhibitory units per milligram of protein. The IC50was defined as the amount of α-amylase inhibitor that inhibited 50% of enzyme activity.

Fig. 1–SDS-PAGE electrophoresis of phaseolins. The numbers from 1–40 correspond to those in Table 1. *These accessions show T-phaseolin patterns.
2.6. Statistical analysis
All results are expressed as mean±standard deviation (SD). The results were subjected to one-way analysis of variance. Tukey's test was performed using SPSS (Statistics for Social Science) version 17.0. The significance of differences was set to P<0.01.
3. Results and discussion
3.1. Phaseolin patterns of the varieties
According to Montoy et al. [14], phaseolin subunits have a molecular weight ranging from 43.1 to 51.5 kDa and can be divided into three types: Tendergreen (T-) phaseolin with three visible subunits and Sanilac (S-) and Inca (I-) phaseolins with two visible subunits. The largest subunit (52 kDa) is present in S- and T-phaseolins but absent in I-phaseolin [15]. We observed 36S-phaseolin patterns showing two major bands in the molecular-weight range of 43–52 kDa (Fig. 1) and 4T-phaseolin patterns (F0005865, F0005873, F0005879, and F0005888) in the 40 common bean accessions. We found no I-phaseolin. Begbie and Rossone [16] have reported lower ileal digestibility of T-phaseolin-containing than of S-phaseolin-containing beans in pigs. Most accessions in this study give evidence of nutritional improvement in common bean breeding programs, given the finding of 36 accessions of S-phaseolin type.
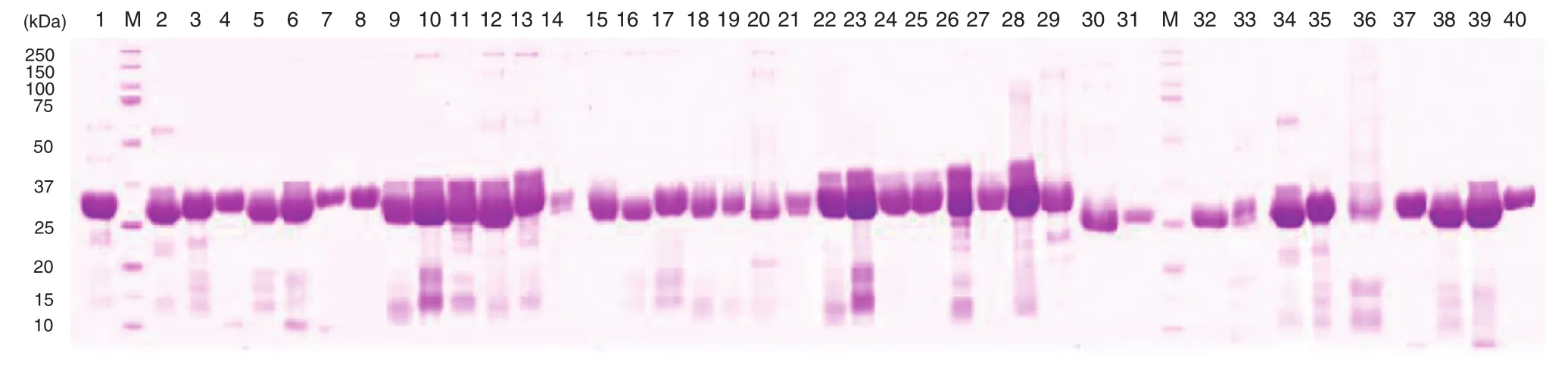
Fig. 2–Polypeptide pattern of α-amylase inhibitors under SDS-PAGE separation. The numbers from 1–40 correspond to those in Table 1.
3.2. Alpha-amylase inhibition activity
The 40 common bean accessions showed different αAI protein banding patterns (Fig. 2). Large variation in total and specific activitiesofαAIwasobservedamong these accessions(Table1). The αAI total activity ranged from 1485.83 U g−1dry weight for F0005875 to 5776.75 for F0005915. The αAI specific activity ranged from 613.40 U g−1protein for F0005914 to 1925.58 for F0005915. F0005915 and F0005917 also showed significantly higher levels of total and specific activities, respectively, than the control. Wang et al. [9] observed higher αAI specific activity in black than in white-colored varieties of common bean. However, Frels and Rupnow [17] reported the contrary result that white common bean showed significantly higher αAI specific activity than black common bean. In this study, we found no clear correlation between αAI specific activity and seed color. Such differences may be due to the purification procedure of αAI, in which phenolic compoundswere removed.
A low IC50value indicates potent porcine pancreatic α-amylase inhibitory activity of a given protein. Nine αAI proteins extracted from the common bean accessions F0005915, F0005917, F0005918, F0005889, F0005877, F0005891, F0005800, F0005862, and F0005898 had lower IC50than that of the control (IC50= 0.65 μg), with the αAI protein from F0005915 showing the strongest activity (IC50= 0.44 μg). The IC50of the tested accessions ranged from 0.44 to 1.71 μg. This result is similar to that of Wang et al. [9] who reported an IC50interval of 0.40–1.60 μg.
In conclusion, the 40 common bean accessions showed considerable variation in phaseolin type and α-amylase inhibition activity. Most were of the S-phaseolin type, with only four being of the T-phaseolin type. No accessions of the I-phaseolin type were found. Nine accessions had excellent α-amylase inhibitors and show potential use as sources of nutraceutical ingredients.
Acknowledgments
This study was supported by the Program of Science and Technology Cooperation with Hong Kong, Macao, and Taiwan, China (2013DFH30050), the special fund for Agro-scientific Research in the Public Interest (201403063), the earmarked fund for China Agriculture Research System (CYTX-014) and Agricultural Science and Technology Innovation Program.
R E F E R E N C E S
[1] P. McClean, J. Kami, P. Gepts, Genomics and genetic diversity in common bean, in: R.F. Wilson, H.T. Stalker, E.C. Brummer (Eds.), Legume Crop Genomics, AOCS Press, Champaign, IL 2004, pp. 60–82.
[2] T. Kutos, T. Golob, M. Kac, A. Plestenjak, Dietary fibre content of dry and processed beans, Food Chem. 80 (2003) 231–235.
[3] X. Wu, X. Xu, J. Shen, N. Perricone, H. Preuss, Enhanced weight loss from a dietary supplement containing standardized Phaseolus vulgaris extract in overweight men and women, J. Appl. Res. 10 (2010) 73–79.
[4] K. Sandeep, K.V. Alok, S. Akanksha, R. Ruchi, K. Dinesh, B.H. Giridhar, T. Anurag, P.C. Bhushan, S.K.J. Mukul, D.D. Premendra, Phaseolin: a 47.5 kDa protein of red kidney bean (Phaseolus vulgaris L.) plays a pivotal role in hypersensitivity induction, Int. Immunopharmacol. 19 (2014) 78–90.
[5] H.G. Preuss, Bean amylase inhibitor and other carbohydrate absorption blockers: effects on diabesity and general health, J. Am. Coll. Nutr. 28 (2009) 266–276.
[6] M. Barrett, J. Udani, A proprietary alpha-amylase inhibitor from white bean (Phaseolus vulgaris): a review of clinical studies on weight loss and glycemic control, J. Nutr. 10 (2011) 1–10.
[7] L. Celleno, N.V. Perricone, H.G. Preuss, Effect of a dietary supplement containing standardized Phaseolus vulgaris extract on the body composition of overweight men and women, Int. J. Med. Sci. 4 (2007) 45–52.
[8] J. Udani, M. Hardy, D.C. Madsen, Blocking carbohydrate absorption and weight loss: a clinical trial using Phase 2 brand proprietary fractionated white bean extract, Altern. Med. Rev. 9 (2004) 63–69.
[9] H.H. Wang, C.L. Chen, T.L. Jeng, J.M. Sung, Comparisons of α-amylase inhibitors from seeds of common bean mutants extracted through three phase partitioning, Food Chem. 128 (2011) 1066–1071.
[10] D. Chokshi, Toxicity studies of blackal, a dietary supplement containing phase 2 starch neutralizer (Phase 2), a standardized extract of the common white kidney bean (Phaseolus vulgaris), Int. J. Toxicol. 25 (2006) 361–371.
[11] J. Carrasco-Castilla, A.J. Hernandez-Alvarez, C. Jimenez-Martinez, C. Jacinto-Hernandez, M. Alaiz, J. Giron-Calle, J. Vioque, G. Davila-Ortiz, Antioxidant and metal chelating activities of Phaseolus vulgaris L. var. Jamapa protein isolates, phaseolin and lectin hydrolysates, Food Chem. 131 (2012) 1157–1164.
[12] P. Salgado, L. Montagne, J.P.B. Freire, R. Ferreira, A. Teixeira, O. Bento, M. Abreu, R. Toullec, J.P. Lalle's, Legume grainsenhance ileal losses of specific endogenous serine-protease proteins in weaned pigs, J. Nutr. 132 (2002) 1913–1920.
[13] J.J. Pueyo, D.C. Hunt, M.J. Chrispeels, Activation of bean (Phaseolus vulgaris)α-amylase inhibitor requires proteolytic processingoftheproprotein,PlantPhysiol.101(1993)1341–1348.
[14] C.A. Montoy, P. Leterme, S. Beebe, W.B. Souffran, D. Molles, J.P. Lalles, Phaseolin type and heat treatment influence the biochemistry of protein digestion in the rat intestine, Brit. J. Nutr. 99 (2008) 531–539.
[15] S. Beebe, J. Rengifo, E. Gaitan, M.C. Duque, J. Tohme, Diversity and origin of Andean Landraces of common bean, Crop Sci. 41 (2001) 854–862.
[16] R. Begbie, A.W. Ross, Resistance of the kidney bean reserve protein, phaseolin, to proteolysis in the porcine digestive tract, J. Sci. Food Agric. 61 (1993) 301–307.
[17] J.M. Frels, J.H. Rupnow, Purification and partial characterization of two a-amylase inhibitors from black bean (Phaseolus vulgaris), J. Food Biochem. 8 (1984) 281–301.
杂志排行
The Crop Journal的其它文章
- 7th International Crop Science Congress Announcement
- Editorial Board of The Crop Journal
- Genotypic variation for seed protein and mineral content among post-rainy season-grown sorghum genotypes
- Fosmid library construction and screening for the maize mutant gene Vestigial glume 1
- Intra-population genetic variance for grain iron and zinc contents and agronomic traits in pearl millet
- Analysis of simple sequence repeats in rice bean (Vigna umbellata) using an SSR-enriched library
