养殖氧化塘周边地下水氨氧化细菌分布及环境因子的影响
2016-03-25曾令藻吴劳生施加春
任 娟,张 磊,曾令藻,吴劳生,施加春
(浙江大学环境与资源学院土水资源与环境研究所,杭州310058)
养殖氧化塘周边地下水氨氧化细菌分布及环境因子的影响
任 娟,张 磊,曾令藻,吴劳生,施加春*
(浙江大学环境与资源学院土水资源与环境研究所,杭州310058)
为了研究畜禽养殖场的废水氧化塘对周边地下水的影响,我们通过在海盐县某养猪场氧化塘周边区域布井,采集相应点位的地下水,并采用分子生物学手段实时荧光定量核酸扩增检测(real-time quantitative PCR detecting system,qPCR)和水质理化性质分析手段,研究氧化塘周边相应位点地下水中的氨氧化细菌(ammoniaoxidizing bacteria,AOB)的丰度和水质情况,并结合相关系数,探究AOB丰度与环境因子之间的关系.结果表明:在相应位点的地下水中AOB的丰度与相应地下水中氨氮、硝氮和总氮的质量浓度呈显著正相关,特别是AOB丰度与硝氮呈极显著正相关关系(P<0.01);同时也发现AOB丰度受p H影响,当p H>7.8时,AOB丰度较大,但AOB丰度和p H线性相关性不显著.
地下水;氨氧化细菌(AOB);环境因子;Pearson相关系数
SummaryGroundwater is a precious natural resource,which is used as potable water,industrial water and agricultural water.With the development of breeding industry,lots of waste water and solid wastes were discharged to the environment,which has caused serious groundwater safety problems,especially high nitrogen concentration. Groundwater is a kind of comparatively well living environment for micro-organisms.Because it has abundant nutrition,water,adaptive temperature and p H and all these conditions are beneficial for growth and reproduction of micro-organisams.In water nitrate cycle,micro-organisms play a significant role.The first and rate limiting step of nitrification is ammoxidation,and the functional gene is ammonia monooxygenase,which existed in ammoniaoxidizing bacteria and ammonia-oxidizing archaea.
However,the factors affecting ammonia-oxidizing bacteria distribution pattern in groundwater and the influence mode are still not clear enough.This study attempted to improve the understanding of groundwater ammoniaoxidizing bacteria(_____AOB)abundance distribution and the correlations between the distribution and environmentalfactors around the livestock farm's hog-waste lagoon in Haiyan County,Zhejiang,China.
The amount of AOB and the nitrate concentrations were tested respectively by real-time PCR and continuous flow analyzer.The correlations among environmental factors,AOB abundance and relative abundance of AOB were analyzed by Pearson correlation coefficient.
The results indicated that NH4+-N concentration was ranged from 0.35- 18.98 mg/L,most of which was much higher than 0.5 mg/L in groundwater quality standard(GB/T 14848—93).And NH4+-N,the main pollution in this site,accounted for a large proportion in total nitrogen.All the water samples were weak base with p H ranged from 7.17- 7.87.Due to the shallow buried depth of groundwater in Zhejiang Province,all the water samples temperature were close to air temperature ranged from 22- 25℃.Total nitrogen had a similar distribution pattern with NH4+-N,of which samples beside lagoon No.2>samples beside lagoon No.1 and others.The result of No.2 was built nearly half a year later than No.1 and had a less stable boundary condition.Meanwhile,W18and W24had both high nitrogen concentration and high total organic carbon concentration.This may be caused by the same reason.Principal component analysis(PCA)result showed that the concentrations of NH4+-N and NO3--N are the two principal components of environmental factors with contribution rates are respectively 45.7%and 25.1%.AOB abundance also had a similar distribution pattern with NH4+-N.Besides,in the center of site,W11and W15also had relatively higher AOB abundance.AOB was the dominant bacteria in W11,W15,W24and W25,whose relative abundance was larger than 25%.It was also observed that p H could influence the AOB abundance. AOB abundance of water sample with p H>7.8 was strongly larger than that of water sample with p H<7.3.AOB abundance and AOB relative abundance had a significantly positive correlation with NO3--N(P<0.01). Meanwhile,they had positive correlation with NH4+-N and total nitrogen(P<0.05).
The main findings from this study are that both AOB abundance and AOB relative abundance show positive correlations with nitrogen concentration,including NH4+-N,NO3--N and total nitrogen.The AOB abundance and relative abundance are significantly positive correlated with NO3--N(P<0.01).When p H>7.8,the AOB abundance is significantly larger than the abundance when p H<7.3.However,the AOB abundance is not correlated with p H value.
畜禽养殖业是浙江省海盐县的农业支柱产业之一.然而,长期的畜禽养殖过程中,对养殖场的周围环境,包括水环境、土壤环境、大气环境等都会造成严重的污染[1].大量的养殖废弃物、养殖场淋洗液等随着水分迁徙或生物代谢等物理生物过程,使氨氮污染物进一步通过淋溶、渗滤,转移至地下水环境,对本不易遭受污染的地下水造成污染[23].地下水作为人类重要的饮用水来源,受污染的地下水极有可能经过生物链或者直接摄取的途径长期危害人体健康.
地下水具备微生物生长繁殖的营养、水分、酸碱度和温度等条件,是微生物良好的生存环境[4].地下水中存在氮相关微生物参与氮循环过程,尤其是当地下水受到氮污染,水中的氮含量升高对微生物有一定驯化作用.
氨氧化是氮循环(图1)中硝化过程的第一步,也是决定硝化反应速率的关键性步骤[5].在氨氧化过程中,功能酶为具有amo A基因编码的氨单加氧酶(ammonia monooxgenase,AMO),其α亚基能催化NH3到NH2OH[6].因此amo A基因是研究氨氧化过程的着力点.具有这种基因的微生物种类包括氨氧化细菌(ammonia-oxidizing bacteria,AOB)以及氨氧化古菌(ammonia-oxidizing archaea,AOA).
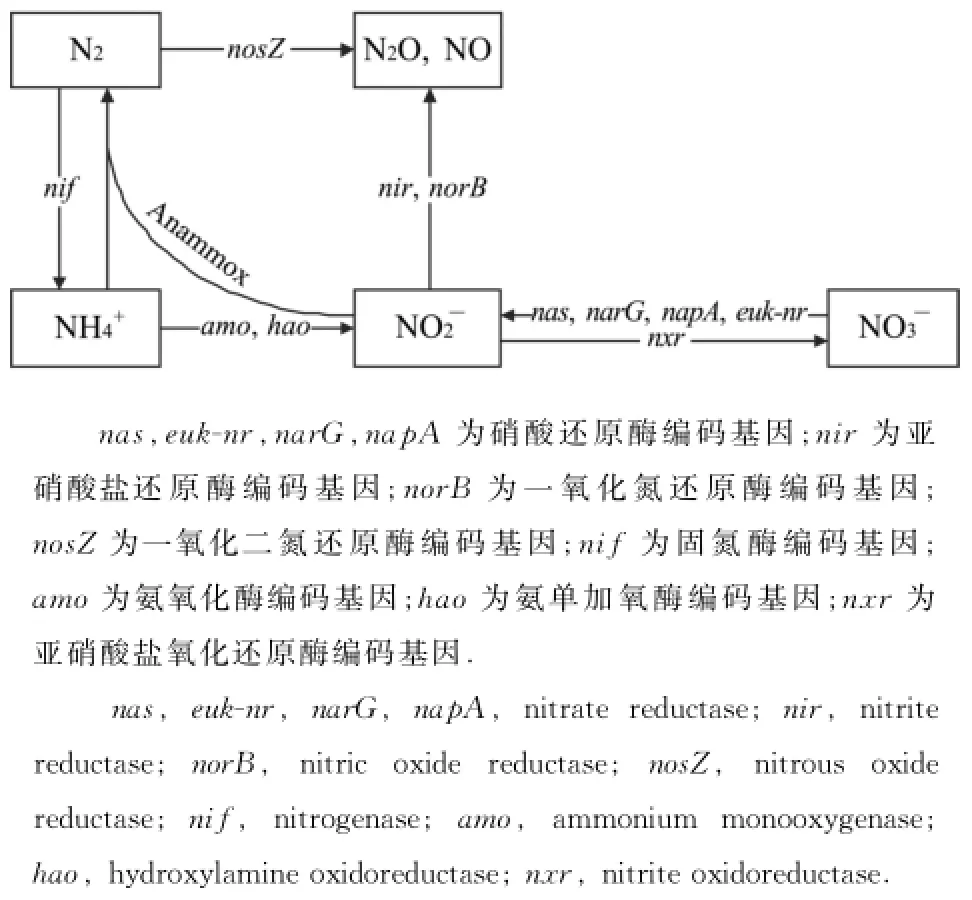
图1 主要生物氮转化途径[7]Fig.1 Major biological nitrogen transformation pathways[7]
目前对于地下水氨氧化细菌分布及其环境因子之间的关系研究尚不清楚.因此本研究主要探索地下水中氮相关微生物AOB的分布规律及其与环境因子的关系.
1 材料与方法
1.1 试验地点与采样点分布
试验地点位于浙江省海盐县某养殖场地周边氧化塘(120°57′33″E,30°35′36″N).经过前期场地水文地质勘查调查得知,土壤类型为水稻土土属黄斑田土种;地下水埋深较浅,实测水位深度为0.3~0.6 m,年变化幅度0.5~1.5 m;由于地下水埋深较浅,地下水温度易受气温影响,随季节变化幅度较大;地下水p H范围为7.0~8.7,水质呈弱碱性.
地下水样全部采自养殖场氧化塘(No.1和No.2)周边的26口监测井,监测井分布见图2.
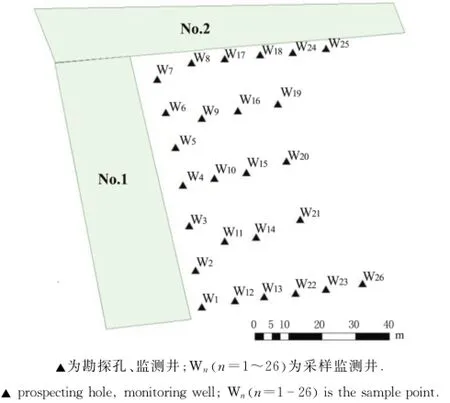
图2 试验地点监测井分布图Fig.2 Distribution of the sample points
共有26口监测井,全部为机械钻探孔,其中孔深10 m的17口监测井为W1,W2,W3,W4,W5,W6,W7,W8,W12,W13,W17,W18,W22,W23,W24,W25,W26;孔深4 m的9口监测井为W9,W10,W11,W14,W15,W16,W19,W20,W21.
2014年9月,采集养殖场氧化塘周边区域的26口监测井中对应位点的地下水样,放于-4℃冰盒中,带回实验室对水样进行水质分析,并提取水样DNA.
1.2 水样项目测定与分析
1.2.1 基本理化性质
参照《水和废水监测分析方法》第四版[8]
温度(T),采用颠倒温度计法;
p H,采用玻璃电极法测定;
总有机碳(total organic carbon,TOC),采用燃烧氧化-非分散红外吸收法测定;
电导率(specific conductance,SPC),采用实验室电导率仪法测定
1.2.2 无机氮测定方法
根据地下水取样规范要求(HJ495—2009)取样后,在实验室用双圈定性滤纸过滤水样,如滤液仍浑浊则重复过滤至滤液澄清无悬浮物,取适量滤液用连续流动分析仪(SAN++,SKALAR,The Netherlands)法测定[9],分析过程所用试剂均为优级纯,所用水均为超纯水.
1.2.3 水样AOB及总菌丰度测量方法
1.2.3.1 水样DNA提取
水样DNA用Fast DNA®SPIN kit for soil(MP Biomedicals LLC)试剂盒提取.提取方法参考该试剂盒给出的土壤样品提取方法,具体如下:
①用注射器取水样10 m L,用0.22μm有机滤膜过滤,取滤膜,晾干后用剪刀尽可能剪碎,称取5 g剪碎待测样品加入裂解管中,加入978μL磷酸钠缓冲溶液和122μL MT缓冲溶液.
②用Fast Prep核酸提取仪以6 m/s的速率裂解细胞40 s.
③裂解结束后,将离心管置于高速冷冻离心机中,4℃14 000 g离心15 min,将上清液转移到2 m L离心管中.
④将离心管置于离心机,4℃14 000 g冷冻离心10 min,沉淀残渣.
⑤转移上清液至2-m L离心管,加入250μL聚苯硫醚(polyphenylene sulfide,PPS),正反摇晃10次,摇匀,继续4℃14 000 g离心5 min,再次沉淀.
⑥转移上清液至15-m L离心管中,摇匀Binding Matrix并取1 m L加入15-m L离心管中,涡旋振荡2 min,使DNA与其充分结合,静置3 min.
⑦小心移除500μL上清液后混匀管中剩余物,取600μL到过滤柱收集管中,14 000 g离心1 min,将所收集滤液弃置,再取600μL离心管中混合物至过滤柱收集管中,再次离心(重复操作至离心管中混合物全部转移为止).
⑧加入500μLSEWS-M(提前用100mL 100%乙醇稀释),14000g离心1min,弃置滤液,14000g再次离心2min,完全去除SEWS-M,然后保留过滤柱,换上新的收集管.
⑨室温下风干5min.
⑩14000g离心1min,将DNA洗脱至干净收集管,弃置过滤柱,-20℃保存.
1.2.3.2 荧光定量PCR
提取DNA所用试剂盒为大连宝生物工程有限公司的SYBR®PremixExTaqTMkit,提取纯化后-20℃保存.然后以amoA-1F,amoA-2R为引物(表1),扩增AOB的amoA基因.
根据XIE,等[10]的方法,得到AOBamoA基因的重组质粒,根据已知质粒和阿伏伽德罗常数(6.02×1023分子数/mol)分别计算各自基因拷贝数.分别以10倍梯度稀释各重组质粒,获得各自的标准曲线,然后根据标准曲线计算出样品中的基因拷贝数,每个样品3次重复.实时荧光定量PCR的反应体系为20μL,包含10μLSYBR®PremixEx TaqTMMix,前后引物各0.4μL,7.2μLPCR-H2O和2μLDNA模板.于CFX96Real-TimePCR System扩增仪上进行定量分析.
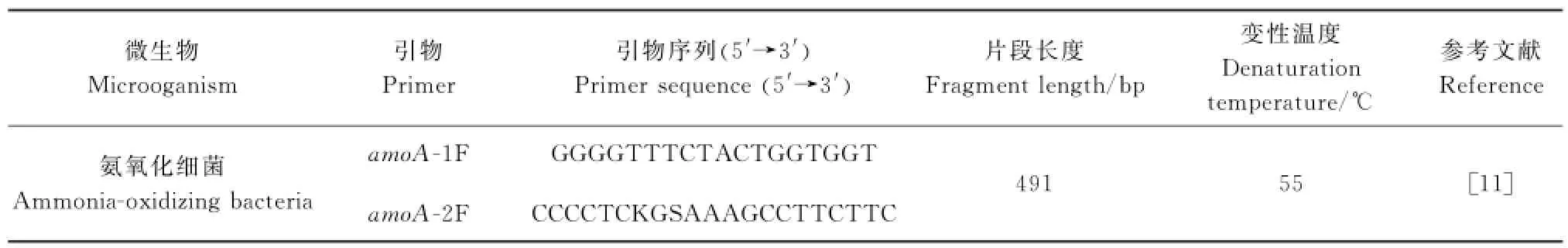
表1 实时荧光定量所用引物和反应条件Table1 Primersandreactionconditionsforreal-timePCR
1.3 数据分析
应用软件SPSS16.0进行Pearson相关性分析,评估了AOB丰度和相对丰度与不同环境因子间的相关关系.AOB丰度进行了以10为底的对数转换.
2 结果与分析
对水样进行检测后发现部分水样检测指标明显异常.经过对现场监测井进行仔细检查,结果表明,部分监测井由于场地管道改建、自然天气灾害等原因已受到不同程度的损坏,造成井水来源复杂,不再是单纯地下水,所以在后续结果分析中不再对受损监测井水样进行分析.
2.1 试验场地水样的基本理化性质
场地的环境因子分布见表2,氨氮质量浓度范围在0.35~18.98mg/L之间,大部分样品的氨氮远高于地下水质量标准(GB/T14848—93)中V类水所规定的0.50mg/L;pH变化范围小,呈弱碱性,较适合AOB类微生物表现硝化活性[12];由总氮(totalnitrogen,TN)和氨氮值可以看出来,整个场地中,氮污染物主要以氨氮的形式存在,总氮与氨氮的分布规律相似;不同采样井的TOC/TN值相差较远,范围从0.63~43.76,最大可相差2个数量级;电导率较高的点分别为W18、W24和W25,都达到了2ms/cm以上;温度范围为22~25℃,推断是由于江浙地区地下水埋深较浅,地下水受地表影响大,所以温度接近地表温度.
氨氮质量浓度较高的采样井为W15、W18和W24;硝氮质量浓度较高的采样井为W15和W24;TOC和TN质量浓度较高的采样井均为W18和W24,由图2可知,除采样井W15外,W18和W24都分布在No.2氧化塘边.分析其原因可能是由于No.2氧化塘的修建时间晚于No.1氧化塘近半年,所以塘边界条件较No.1更不稳定,所以有大量的污染物经由No.2氧化塘堤坝渗漏到地表和地下水,导致周边地下水污染.
对所得到的全部环境因子数据进行主成分分析(PCA),提取到2个主因子氨氮和硝氮,贡献率分别是45.7%和25.1%(图3),主因子累积贡献率为70.8%,由此认为这2个主成分可以表征地下水中主要环境因子.因此结合考虑2个主因子,对采样点进行3个Cluster聚类分组.ClusterⅠ为W1,W2,W3,W4,W5;ClusterⅡ为W9,W10,W12,W13,W22,W23;ClusterⅢ为W18和W24.
对比监测井分布可得各分组的分布特点: ClusterⅠ分布于No.1氧化塘边;ClusterⅡ分布于远离氧化塘的区域,ClusterⅢ分布于No.2氧化塘边(图4).整体氮素污染物质量浓度ClusterⅢ>ClusterⅡ>ClusterⅠ.
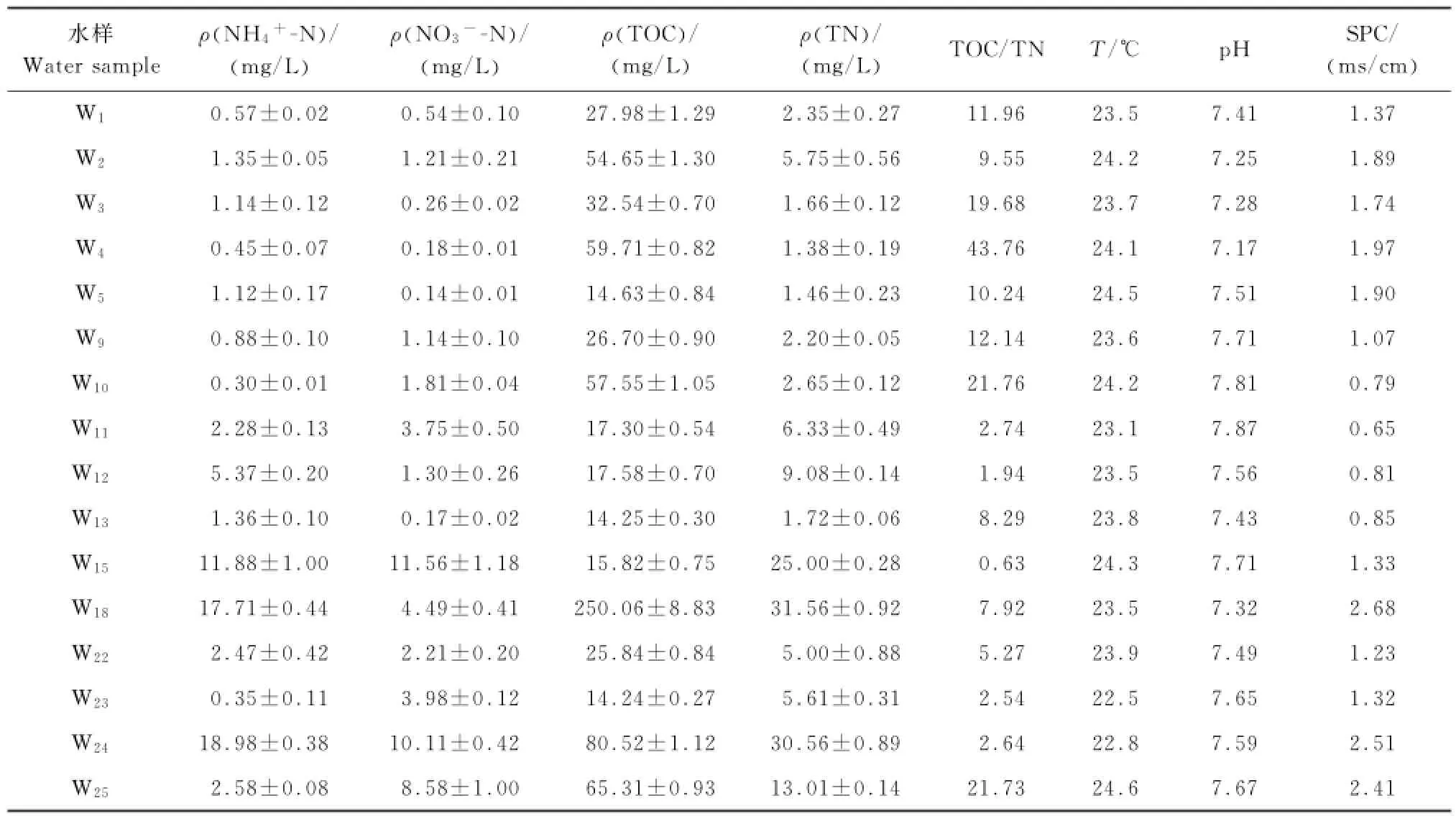
表2 地下水样理化性质Table2 Chemical properties of the groundwater samples
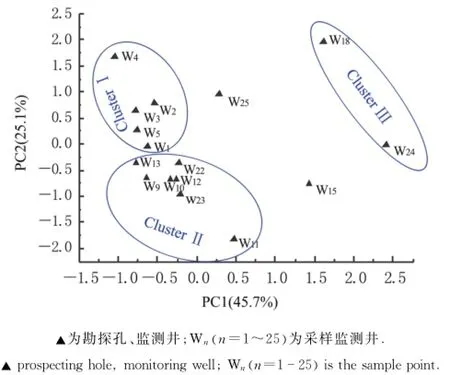
图3 环境因子分布PCA分析结果Fig.3 PCA result of environmental factors distribution
2.2 AOB丰度
各采样井水样中的amo A功能基因拷贝数见图5.结合环境因子分布来看,amoA功能基因拷贝数的数量分布类似于环境因子中碳氮污染的分布规律,靠近No.2氧化塘的W18,W24和W25水样中AOB数量较多.同时,W11和W15水样的amo A基因拷贝数也较多,查看了蔬菜大棚的施肥记录发现,采样前14 d,大棚内曾在W15的位置施肥1次,肥料种类为复合型氮肥,可能对周围区域地下水的氨氮含量及amoA基因数量影响较大.
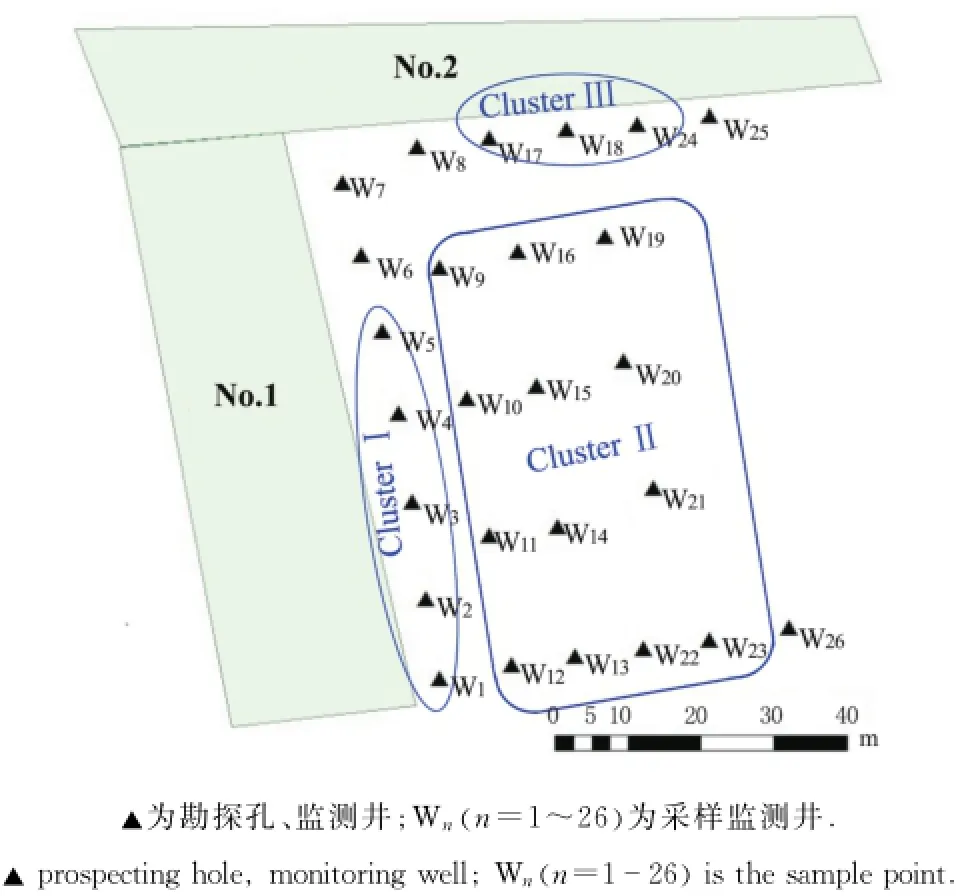
图4 试验地点监测井分组Fig.4 Sampling points grouping
利用Arc-GIS做出AOB amo A基因拷贝数分布图见图6,可得到与PCA主因子聚类分组相似,AOB amo A基因的分布也可以分为3个Clusters. ClusterⅢ区域基因拷贝数最高,其次是ClusterⅡ区域,ClusterⅠ区域amo A基因拷贝数最少.
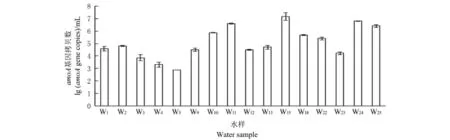
图5 各水样中的氨氧化细菌的丰度Fig.5 Abundance of AOB in different water samples
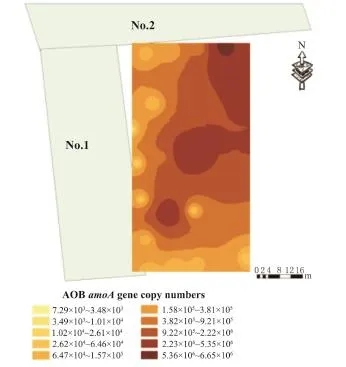
图6 AOB amoA基因拷贝数位置分布Fig.6 Biogeography of AOB amo A gene copy numbers
AOB的相对丰度即含amo A基因的细菌量与总菌量的比值,总菌量及相对丰度见图7和图8.由图可以得到,总菌量≥107的采样点基本集中在No.2氧化塘边和W15施肥点周围.同时,W11、W15、W24和W25的4个采样井中,AOB占据了主导地位,大于等于总菌量的25%.
2.3 Pearson相关系数
AOB丰度及相对丰度和环境因子的相关关系见表3.AOB丰度和相对丰度都和硝氮质量浓度呈极显著正相关(P<0.01);AOB丰度与氨氮、总氮质量浓度及AOB相对丰度与氨氮质量浓度也均呈显著正相关(P<0.05).说明AOB丰度以及相对丰度都受到水中氮污染物的影响,氮污染物质量浓度越高,则AOB的丰度及相对丰度更大;尤其是硝氮质量浓度和AOB相对丰度呈极显著正相关(P<0.01).

图7 各水样中细菌的总量Fig.7 Total bacteria amount in different water samples

图8 各水样中氨氧化细菌/细菌总量的相对丰度Fig.8 Relative abundance of AOB/total amount of bacteria in different water samples
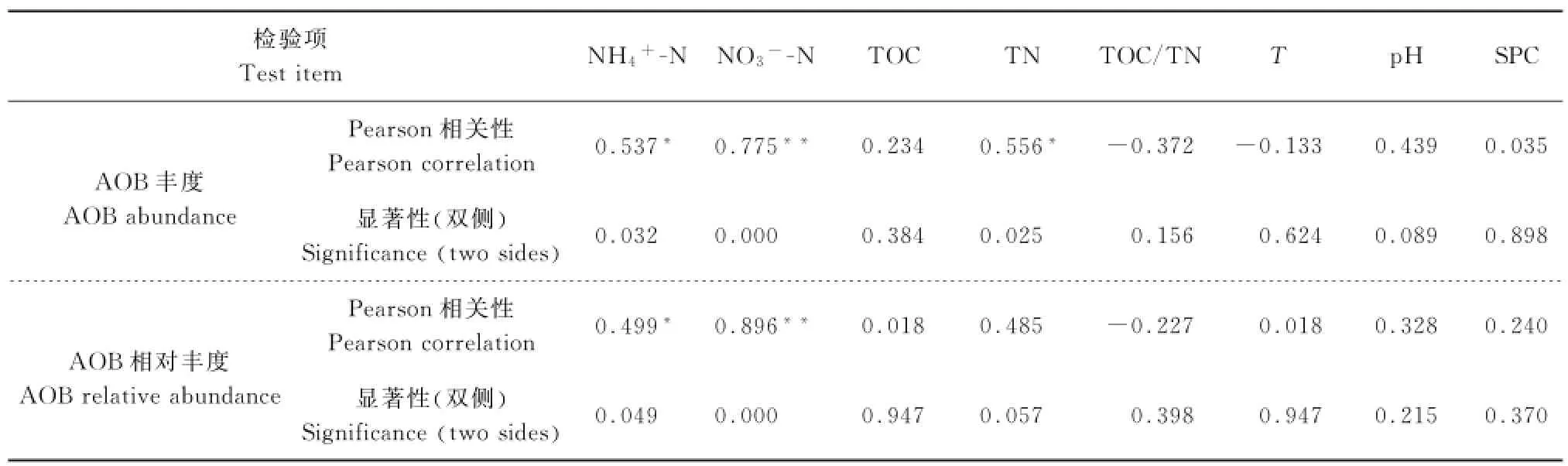
表3 AOB丰度和相对丰度与环境因子的相关关系Table3 Correlations between AOB abundance,relative abundance and environmental factors
3 讨论
氨氧化是地球生物循环的一个重要步骤[13],在氨氧化过程中,amo A基因是核心基因.作为氨氧化的第一步,amo A基因的数量直接关系到硝化过程的速率,进而影响到整个氮循环过程的速率.
amo A基因受多种环境因子的影响[14]. KOWCALCHUK,等[15]的研究表明p H是AOB的amo A基因的重要影响因素之一[15],AOB种群结构在不同p H环境下,优势种群不同.p H显著上升时,amo A基因拷贝数会明显增加[16].刘彪,等[17]研究发现,当p H为7.5~8.0时,AOB的amo A基因丰度较高,p H为8.0时,amo A基因丰度最高.在本研究中,所有水样p H在7.0~8.0范围内,p H>7.8的水样W10、W11的amo A基因丰度显著大于p H<7.3的水样W2、W3和W4.AOB绝对丰度与p H间相关系数为0.439(P>0.05),因此不存在显著的相关性.
氨氧化细菌丰度和相对丰度与氮污染物质量浓度呈显著正相关,氮污染物质量浓度越高,AOB绝对丰度越大.SAUDER,等[18]的研究也表明,当氨氮质量浓度大于0.1 mg/L时,AOB在氨氧化微生物中占据主导地位,并且AOB的生物量和氨氮质量浓度呈显著正相关.而陈国元,等[19]对西湖沉积物间隙水的研究发现,AOB丰度与氨氮质量浓度呈显著相关性,这与本研究的结果一致.WHITBY,等[20]对淡水湖的研究发现,AOB丰度与氨氮质量浓度无显著性差异.因此推测在不同环境中,环境因子对AOB影响方式不同.JIA,等[21]和DI,等[22]通过对土壤环境研究发现,AOB是富氨氮土壤环境中氨氧化过程的主导者.推测在富氨氮水环境中,AOB也是氨氧化过程的主导者.大量研究表明,AOB适合在高氮的环境下生长,而AOA适合在氮源相对贫乏的环境中生长[10].
环境因子的PCA聚类分组结果表明,环境因子按照主成分1氨氮质量浓度和主成分2硝氮质量浓度结合考虑,可以分为3个区域,分别是靠近No.1氧化塘边的ClusterⅠ、靠近No.2氧化塘边的ClusterⅢ和远离氧化塘的区域ClusterⅡ,而且AOB amo A基因拷贝数的分布也可以划分为与其位置基本吻合的3个区域,同时氮素污染物呈现: ClusterⅢ>ClusterⅡ>ClusterⅠ,AOB中amoA基因拷贝数:ClusterⅢ>ClusterⅡ>ClusterⅠ.因此推测地下水和氧化塘之间的位置关系会影响地下水的环境因子和AOB数量,靠近No.2氧化塘边的地下水污染质量浓度最高,AOB数量最多;靠近No.1氧化塘边的地下水污染质量浓度最低,AOB数量最少.
TOC与TOC/TN和AOB丰度无任何相关性,说明在此场地内,TOC和TOC/TN不是氨氧化细菌繁殖的限制性因素,这可能是由于场地内有足够的碳源供氨氧化细菌吸收利用.
4 结论
4.1 虽然p H值和AOB绝对丰度无相关性,但是p H值会影响AOB绝对丰度的大小:当p H>7.8时,AOB绝对丰度较大;但当p H<7.3时,AOB绝对丰度显著减小.
4.2 AOB绝对丰度和相对丰度与氮污染物呈显著正相关,尤其是硝氮质量浓度,呈极显著正相关(P<0.01).
(References):
[1] JIAN M W,KAI W,XUE L C,et al.Evaluation on feed factors affecting the dung pollutants of livestock and poultry.Animal Husbandry and Feed Science,2009,1(8/10):14-16,19.
[2] LEE S.Geochemistry and partitioning of trace metals in paddy soils affected by metal mine tailings in Korea. GEODERMA,2006,135:26-37.
[3] RODRÍGUEZ L,RUIZ E,ALONSO-AZCÁRATE J,et al. Heavy metal distribution and chemical speciation in tailings and soils around a Pb-Zn mine in Spain.Journal of Environmental Management,2009,90(2):1106-1116.
[4] 李政红,张翠云,张胜,等.地下水微生物学研究进展综述.南水北调与水利科技,2007,5(5):60-63.
LI Z H,ZHANG C Y,ZHANG S,et al.Groundwater microbiology research progress summary.South to North Water Transfers and Water Science&Technology,2007,5(5):60-63.(in Chinese with English abstract)
[5] KÖNNEKE M,BERNHARD A E,JOSÉR,et al.Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature,2005,437(7058):543-546.
[6] PROSSER J I,NICOL G W.Relative contributions of archaea and bacteria to aerobic ammonia oxidation in the environment.Environmental Microbiology,2008,10(11): 2931-2941.
[7] CANFIELD D E,GLAZER A N,FALKOWSKI P G.The evolution and future of Earth's nitrogen cycle.Science,2010,330(6001):192-196.
[8] 国家环境保护总局水和废水监测分析方法编委会.水和废水监测分析方法:第四版.北京:中国环境科学出版社,2002: 236-261.
State Environmental Protection Administration,Water and Waste Water Monitoring Analysis Method:4th ed.Beijing:China Environmental Science Press,2002:236-261.(in Chinese)
[9] ZHOU J B,CHEN Z J,LIU X J,et al.Nitrate accumulation in soil profiles under seasonally open‘sunlight greenhouses'in northwest China and potential for leaching loss during summer fallow.Soil Use and Management,2010,26(3):332-339.
[10] XIE Z,LE ROUX X,WANG C,et al.Identifying response groups of soil nitrifiers and denitrifiers to grazing and associated soil environmental drivers in Tibetan alpine meadows.Soil Biology and Biochemistry,2014,77:89-99.
[11] ROTTH AUWE J,WITZEL K,LIESACK W.The ammonia monooxygenase structural gene amo A as a functional marker: molecular fine-scale analysis of natural ammonia-oxidizing populations.Applied and Environmental Microbiology,1997,63(12):4704-4712.
[12] 陆诗敏.淡水养殖池塘环境中氨氧化微生物的研究.武汉:华中农业大学,2014:54-69.
LU S M.Study on the ammonia-oxidizing microorganisms in the freshwater aquaculture pond environment in China. Wuhan:Huazhong Agricultural University,2014:54-69.(in Chinese with English abstract)
[13] KOOPS H P,PURKHOLD U,POMMERENING-RÖSER A,et al.The Prokaryotes:An Evolving Electronic Resource for the Microbiological Community.New York:Springer-Verlag,2003.
[14] HE J,SHEN J,ZH ANG L,et al.Quantitative analyses of the abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea of a Chinese upland red soil under long-term fertilization practices.Environmental Microbiology,2007,9(9):2364-2374.
[15] KOWALCHUK G A,STEPHEN J R,DE BOER W,et al. Analysis of ammonia-oxidizing bacteria of the beta subdivision of the class Proteobacteria in coastal sand dunes by denaturing gradient gel electrophoresis and sequencing of PCR-amplified 16S ribosomal DNA fragments.Applied andEnvironmental Microbiology,1997,63(4):1489-1497.
[16] MENDUM T A,HIRSCH P R.Changes in the population structure ofβ-group autotrophic ammonia oxidizing bacteria in arable soils in response to agricultural practice.Soil Biology and Biochemistry,2002,34(10):1479-1485.
[17] 刘彪.仿生植物附着氨氧化微生物群落结构及其对环境因子的响应研究.苏州,江苏:江苏大学,2014:54-100.
LIU B.Research on community structure of ammoniaoxidizing microorganisms in the biofilm attached bionic plants and its responses to environmental factor.Suzhou,Jiangsu: Jiangsu University,2014:54-100.(in Chinese with English abstract)
[18] SAUDER L A,ENGEL K,STEARNSJ C,et al.Aquarium nitrification revisited:Thaumarchaeota are the dominant ammonia oxidizers in freshwater aquarium biofilters.PLoS ONE,2011,6(8):e23281.
[19] 陈国元,黄晓鸣.泉州西湖沉积物中硝化细菌的分布及其作用.微生物学通报,2011,38(11):1632-1638.
CHEN G Y,HUANG X M.Distribution and role of nitrifying bacteria in the sediments of Xihu Lake in Quanzhou.Microbiology,2011,38(11):1632-1638.(in Chinese with English abstract)
[20] WHITBY C B,SAUNDERS J R,PICKUP R W,et al.A comparison of ammonia-oxidizer populations in eutrophic and oligotrophic basins of a large freshwater lake.Antonie van Leeuwenhoek,2001,79(2):179-188.
[21] JIA Z,CONRAD R.Bacteria rather than archaea dominate microbial ammonia oxidation in an agricultural soil. Environmental Microbiology,2009,11(7):1658-1671.
[22] DI H J,CAMERON K C,SHEN J P,et al.Nitrification driven by bacteria and not archaea in nitrogen-rich grassland soils.Nature Geoscience,2009,2(9):621-624.
Groundwater ammonia-oxidizing bacteria distribution and the correlations between the distribution and environmental factors around livestock lagoons.Journal of Zhejiang University(Agric.&Life Sci.),2016,42(5):589- 597
REN Juan,ZHANG Lei,ZENG Lingzao,WU Laosheng,SHI Jiachun*
(Institute of Soil and Water Resource and Environmental Science,College of Environmental and Resource Sciences,Zhejiang University,Hangzhou 310058,China)
groundwater;ammonia-oxidizing bacteria(AOB);environmental factors;Pearson correlation coefficient
X 523
A
10.3785/j.issn.1008-9209.2015.11.182
国家高技术研究发展计划(863)项目(2012AA062603).
*通信作者(Corresponding author):施加春(http://orcid.org/0000-0002-4279-4908),E-mail:jcshi@zju.edu.cn
联系方式:任娟(http://orcid.org/0000-0002-4906-8896),E-mail:renjuan518@gmail.com
(Received):2015 11 18;接受日期(Accepted):2016 04 18;
日期(Published online):2016 09 18 URL:http://www.cnki.net/kcms/detail/33.1247.S.20160918.1535.012.html
