电子介体研究进展
2016-03-25丁阿强郑平
丁阿强,郑平,张 萌
(浙江大学环境与资源学院,杭州310058)
电子介体研究进展
丁阿强,郑平*,张 萌
(浙江大学环境与资源学院,杭州310058)
电子介体不仅在胞内生理过程中起着核心作用,也在胞外生态过程中起着重要作用.探明电子介体的生理生态功能,对于微生物电化学过程的研究和污染生物修复技术的研发具有重大的现实意义.电子介体可分为细胞合成的生理性电子介体和非细胞合成的非生理性电子介体.本文综述了几种典型的生理性电子介体和非生理性电子介体的化学结构、氧化还原电位、电子传递机制及其在环境生物技术研发中的应用.
电子传递链;生理性电子介体;非生理性电子介体;生理生态意义
SummaryElectron transport chain(ETC)consists of a series of compounds which is arranged according to electron affinity to transport electron.Respiratory process is the main source of energy in microbe,however,with many blind spots for restriction of in-situ research.It is generally acknowledged that,ETC possesses close relationship with respiratory process,which provides a possible way to further research of ETC and respiratory in microbe.
In general,electron is considered to transfer from extracellular into intracellular through respiratory chain. However,recent studies have demonstrated that electron can also transfer through inverse way,i.e.from intracellular to extracellular.Three main mechanisms were hypothesized for electron transfer:1)through direct contact,2)through nanowire,3)utilizing electron transfer mediator(ETM).Among these researches,the ETM should be paid more attention,for which plays key roles in intracellular physiological as well as in extracellular ecological processes.It is reported that ETMs usually take part in many redox reactions in intracellular and act as a link between these reactions in the respiratory chain.Besides,they can also take electrons to the inside or outside of the cell,expanding cell's metabolic domain.
ETM can be classified into cellular synthesized(physiological ETM,PETM)and extracellular synthesized(non-physiological ETM,nPETM)by the source.PETMs include many small molecules,such as phenazine andflavin.Phenazine is a kind of nitrogenous heterocyclic compound,and four main phenazine,i.e.phenazine-1-carboxylate,pyocyanin,phenazine-1-carboxamide and 1-hydroxyphenazine are common in microbe.Their redox potentials are between-40 to-174 m V,indicating NADH or coenzyme Q is the ideal position for the electron transfer from electron donor.Flavin is a kind of isoalloxazine derivative,and three main compounds are riboflavin,flavin adenine dinucleotide and flavin mononucleotide.They have similar redox potential to phenazine as well as the electron transfer pathway.n PETMs mainly include humus and AQDS.Both of them have benzoquinonyl,indicating that they can transfer electron through variation between benzoquinonyl and phenolic group.Besides,some humus,such as dimethyl sulfone and N-methylaniline,can transfer electron by nitrogen or sulfur containing group.
The utilization of PETM and nPETM has raised more and more attention in environmental pollution control,for their effects in microorganism biodegradation.Recent research focused on microbial fuel cell and microbial electrolysis cell,has proved that the additions of PETM and n PETM can enhance contaminant removal in the control of dye,uranium and other metals pollution.Further research had demonstrated that the additional PETM and nPETM showed positive correlation with pollutant removal.
Clarifying the physiological and ecological functions of ETM would benefit the comprehensive of microbial electrochemical process and the development of bioremediation technology,as well as the development of microbial fuel cell(MFC)and microbial electrolysis cell(MEC).In this paper,a review on the chemical construction,redox potential,electron transfer mechanism and application in environmental biotechnology of typical ETMs was presented.
电子传递链(electron transport chain,ECT)是一系列电子传递体(electron transport complex)按电子亲和力逐渐升高的顺序组成的电子传递系统[1].其中,呼吸链(respiratory chain)是电子传递链的主要存在形态之一.呼吸链位于细胞膜上,是生物能量代谢的主要场所.呼吸链由氢载体(hydrogen carrier)和电子载体(electron carrier)组成.由于氢原子由质子和电子组成,因此氢载体也是电子载体.尽管对于呼吸链的研究存在许多盲点,学者一般认为呼吸链上的电子可向胞内传递(简称胞内电子传递).但近年来研究发现,呼吸链上的电子也可向胞外传递(简称胞外电子传递)[2].胞外电子传递是生物细胞进行内外物质、能量、信息交换的重要方式.
胞外电子传递有3种途径,一是直接传递,细胞直接贴合最终电子受体,通过细胞膜和细胞壁上的蛋白质将电子从胞内传递至胞外;二是纳米导线传递,细胞通过1根从细胞膜伸出的“电线”(蛋白质)连接最终电子受体,将电子从胞内传递至胞外;三是电子介体(electron transfer mediator,ETM)传递,细胞通过电子传递体将电子从胞内传递至胞外[3].
电子介体是一种具有氧化还原功能的小分子物质.在胞外电子传递过程中,电子介体可以先从细胞上接收电子,由氧化态转化成还原态;再将电子传递至电子受体(无机物、有机物或电极),自身从还原态恢复到氧化态.本文拟对电子介体的种类、传电机制和功能等逐一综述,以供参考.
1 呼吸链与电子载体
呼吸链是由一系列氢传递反应(hydrogen transfer reactions)和电子传递反应(electron transfer reactions)组成的连续反应体系.它将基质释放的电子传递给氧(或其他电子受体),同时合成ATP.构成呼吸链的电子传递体称为电子载体.电子载体的本质是酶、辅酶或辅基.由于电子载体一般附着于细胞膜上,迁移性较弱,因此可将呼吸链中的电子载体看成固定式电子传递体.在微生物中,典型的呼吸链由4种复合体构成,分别为复合体Ⅰ、复合体Ⅱ、复合体Ⅲ与复合体Ⅳ.
复合体Ⅰ又称为NADH-辅酶Q还原酶,其功能是将还原态辅酶Ⅰ(NADH)中的电子传递给辅酶Q.该复合体可以将糖酵解、三羧酸(TCA)循环或脂肪酸氧化途径连接至呼吸链,贯通细胞膜与细胞体内的电子通路.复合体Ⅰ中的NADH将2个电子依次通过黄素单核苷酸(flavin mononucleotide,FMN)与铁硫蛋白传递给辅酶Q,总反应式为

复合体Ⅱ又称为琥珀酸-辅酶Q还原酶.在TCA循环中,琥珀酸氧化成延胡索酸,释放的电子被黄素腺嘌呤二核苷酸(flavin adenine dinucleotide,FAD)捕获.复合体Ⅱ将琥珀酸释放的2个电子分别通FAD与铁硫蛋白传递给辅酶Q,总反应方程式为
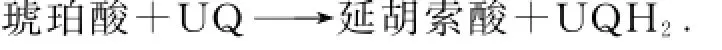
复合体Ⅲ又称为辅酶Q-细胞色素c还原酶.辅酶Q从上游接受电子,通过独特的Q循环传递给细胞色素c,总反应式为
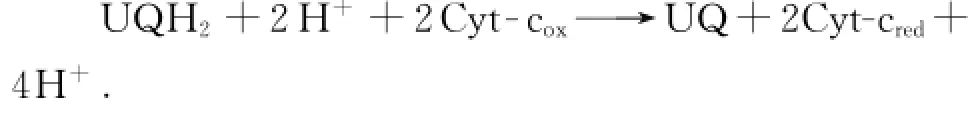
复合体Ⅳ又称为细胞色素c氧化酶.它位于呼吸链末端,将从上游接受的电子传递给最终电子受体氧气.主要电子传递环节有:细胞色素c→CuA中心→血红色a→CuB中心/血红色a3→氧气,总反应方程式为

为保证呼吸链中电子流动的畅通,各类电子载体按氧化还原电势由低到高依次排列,构成的电势塔见图1[4].
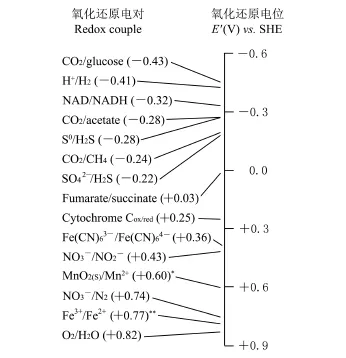
图1 不同物质氧化还原电位组成的电势塔[4]Fig.1 Potential tower composed of different redox potentials[4]
从电势塔可以看出,呼吸链中的几个关键电子载体是NADH、辅酶Q、细胞色素c、细胞色素a和氧气,它们的氧化还原电位分别为-320 m V、+110 m V、+250 m V、+390 m V和+900 m V.从NADH到氧气的电子流动驱动氧化磷酸化合成ATP,这是生物能量代谢的核心.
2 生理性电子介体
除了构成呼吸链的电子载体外,还有许多小分子有机物也具有接受电子和给出电子的能力,充当着细胞内、细胞间、细胞与固体表面之间的电子传递体[56],这些小分子有机物称为电子介体.与呼吸链中的固定式电子传递体相反,电子介体迁移性较强,故可将电子介体看成流动式电子传递体.细胞自身合成的电子介体具有特定的生理功能,称为生理性电子介体(physiological electron transfer mediator,PETM).根据其氧化还原电位高低,生理性电子介体可从呼吸链上的不同电子载体获取电子,并传递给目标电子受体[7].
2.1 吩嗪类PETM
吩嗪是一种含氮杂环化合物,许多微生物能合成吩嗪类物质.迄今为止,文献记载的吩嗪类物质已近100种.吩嗪类PETM主要有4种:1-羧酸吩嗪(phenazine-1-carboxylate,PCA)、绿脓菌素(pyocyanin,PYO)、1-甲酰胺吩嗪(phenazine-1-carboxamide,PCN)和1-羟基吩嗪(1-hydroxyphenazine,1-OHPHZ).其中,PCA是合成其他吩嗪类物质的前体,PYO则在吩嗪类化合物中丰度最高[8].
在铜绿假单胞菌(Pseudomonas aeruginosa)中,吩嗪类物质的合成过程为莽草酸途径→分支酸途径→吩嗪修饰途径[9].莽草酸途径是芳香族氨基酸合成的共同途径[10].糖酵解途径产生的磷酸烯醇式丙酮酸(PEP)和磷酸戊糖途径产生的赤藓糖-4-磷酸(E4P),可合成3-脱氧-阿拉伯庚酮糖酸-7-磷酸(DAHP)进入莽草酸途径.在正常的分支酸途径中,分支酸转化成芳香族氨基酸(酪氨酸、色氨酸和苯丙氨酸).然而在吩嗪类物质合成过程中,分支酸由PhzE酶转化成2-氨基-2-脱氧异分枝酸(ADIC),再依次由PhzD酶、PhzD酶、Phz A酶、PhzB酶、PhzG酶等酶转化成2,3-二氢-3-羟基氨基苯甲酸(DH HA)等中间产物,并最终产生PCA[11].作为吩嗪类物质的前体,PCA进一步由PhzS酶和PhzO酶转化成PYO和1-OHPHZ[12-13].
4种吩嗪类物质的化学式及氧化还原电位见表1[14].吩嗪类PETM的氧化还原电位大多在-200~0 mV范围,正好介于电子传递链复合体Ⅰ中的NADH(-320 m V)和复合体Ⅰ/Ⅱ/Ⅲ中的辅酶Q(+110 m V)之间.这意味着吩嗪类物质可从呼吸链的NADH获取电子,再将其传递给目标电子受体;也可从其他电子供体获得的电子,再将其传递给辅酶Q.换言之,吩嗪类物质可以中介呼吸链和细胞内的生理过程和细胞外的非生理过程(生态过程).
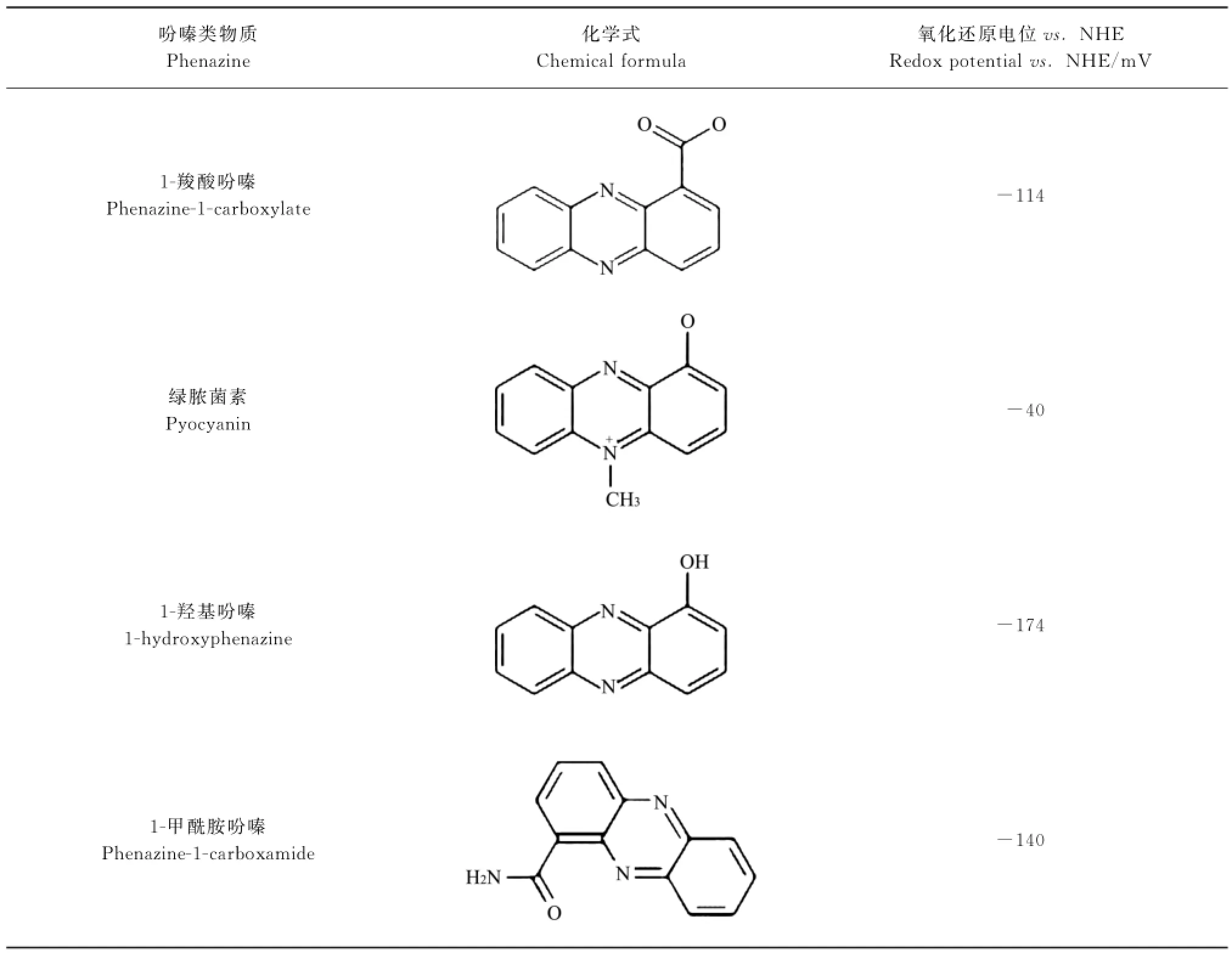
表1 吩嗪类物质化学式及氧化还原电位[14]Table 1_Chemical formula and redox potential of phenazine[14]
吩嗪类物质的电子传递过程见图2[15].其中,R1和R2指吩嗪类物质碳1位和2位上的取代基.氧化态吩嗪(Phz)可获得1个电子产生Phz·-(Step 1);再获得1个电子产生Phz2-(Step 5);接着获得1个质子产生Phz H-(Step 6);也可以获得1个质子产生PhzH·(Step 2),再获得1个电子产生Phz H-(Step 3);最终Phz H-获得1个质子产生Phz H2(Step 4).接受电子时,吩嗪类物质获得2个电子同时吸收2个质子;给出电子时,吩嗪类物质给出2个电子同时释放2个质子.
吩嗪类PETM有着广泛的应用,尤其是在目前研究较热的微生物燃料电池(microbial fuel cell,MFC)或微生物电解池(microbial electrolysis cell,MEC)中.RABAEY,等[16]发现,将吩嗪类物质PYO添加到以P.aeruginosa为生物催化剂的MFC中,输出功率可提高5倍以上;反之,将生物催化剂换成突变株时,输出功率降低到对照的5%;将PYO添加到以突变株为生物催化剂的MFC中,输出功率恢复到对照的50%.ZHANG,等[17]和COURNET,等[18]也发现,以P.aeruginosa为生物催化剂的MFC输出电流和电流密度均远高于对照,通过循环伏安法和气质联用等方法确定,发挥关键作用的是P.aeruginosa分泌的吩嗪类物质.除PYO以外,PCA、PCN等许多吩嗪类物质也已证明对MFC的输出功率具有明显的提升作用[19].但是,各种吩嗪类物质的产生量及其电子传递效率也不尽相同[20].在MFC中,一般PYO浓度比PCA、PCN和1-OHPHZ高20~30倍;添加等量PYO、PCA、PCN和1-OHPHZ,输出电流密度分别较对照提高20、3、8和2倍.P.aeruginosa产生的吩嗪类物质除了可以自己利用,也可供其他微生物(如Brevibacillus)利用,促进产电活性[21].
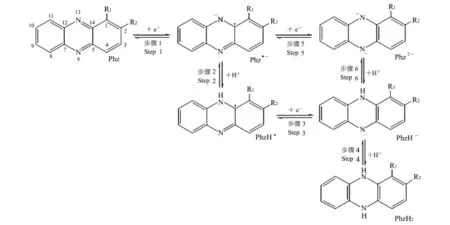
图2 吩嗪类物质的电子传递过程[15]Fig.2 Electron transfer process of phenazine[15]
2.2 黄素类PETM
黄素类物质是一类异咯嗪衍生物.核黄素(riboflavin,RF)是大部分黄素类物质的前体,1879年首次从牛乳中分离获得[22].在细胞电子传递中,FAD和FMN起着重要作用.在p H为7的标准条件下,FAD和FMN的氧化还原电位分别为-219 m V和-205 m V[23].类似吩嗪类物质,黄素类物质的氧化还原电势也介于复合体Ⅰ/Ⅱ/Ⅲ中的NADH与辅酶Q之间,可将其电子传给辅酶Q,也可从FADH/FMNH获得电子.氧化态黄素类物质可通过异咯嗪环获得电子和质子;反之,还原态黄素类物质则可通过异咯嗪环给出电子和质子[2425].
VON CANSTEIN,等[26]和MARSILI,等[27]2个课题组同时证明,Shewanella oneidensis可分泌黄素类物质作为电子传递体.据VON CANSTEIN报道,在厌氧与好氧条件下均可从S.oneidensis培养液中分馏获得RF与FMN.MARSILI发现,将MFC的培养液换成新鲜培养基后,输出电流大幅下降;换回原来的培养液后,输出电流立刻回升.进一步研究发现,添加黄素类物质可促进铁(Ⅲ)氧化物的还原;当铁(Ⅲ)氧化物为唯一电子受体时,还可促进S.oneidensis的生长[2829].上述事例证明,黄素类PETM在电子传递中具有重要作用[30].黄素类PETM也可将电子从胞内向胞外传递给偶氮染料、铀(Ⅵ)和电极,从而强化化学还原和产电过程[3133].据文献报道,添加RF与FMN时,苋菜红降解速率提高3倍,铀(Ⅵ)还原速率提高2倍,MFC输出电流提高4倍.值得注意的是,并不是所有黄素类物质都可充当电子介体.MASUDA,等[34]发现,Lactococcus lactis并不能像S.oneidensis一样利用FAD作为电子介体.
3 非生理性电子介体
除了生理性电子介体外,还有一些电子介体存在于自然界中,但并非由细胞直接合成.这些不是由细胞直接合成的电子介体称为非生理性电子介体(non-physiological electron transfer mediator,nPETM),主要包括腐殖质和2,6-蒽醌二磺酸(anthraquione-2,6-disulfonate,AQDS)等.
3.1 腐殖质
腐殖质是自然界广泛存在的有机物,在土壤和水底沉积物中,腐殖质的质量百分比可达10%[35].腐殖质的碳骨架由烷基和芳香族基团交联而成,主要官能团有羧基、羟基、醇羟基、酮基和含醌基团[3536].TRATNYEK,等[37]和DUNNIVANT,等[38]首次证明,含醌基团是腐殖质充当电子介体的功能基团[3738].此后HERNÁNDEZ-MONTOYA,等[39]、AESCHBACHER,等[40]和RATASUK,等[41]进一步证明,含醌基团数量与电子传递速率/反应速率呈线性相关关系.近年来的研究表明,腐殖质中的非醌类官能团在电子传递中也发挥重要作用,其贡献率可达44%~58%[39].非醌类官能团的电子传递过程可能与一些含氮及含硫基团接受和释放电子有关,这类电子介体有二甲基砜、3-甲硫基丙酸和N-甲基苯胺等[42-43].
腐殖质可促进环境中Fe(Ⅲ)还原.HOBBIE,等[44]和BHUSHAN,等[45]证明,腐殖质可明显提高Fe(Ⅲ)的生物还原速率.不同来源的腐殖质对Fe(Ⅲ)还原的强化作用也不尽相同,来源于自然界的腐殖质电子传递效果显著优于人工合成的腐殖质.这表明,一方面微生物可借助天然电子介体作用拓展微域;另一方面腐殖质在自然界生态过程中可能发挥着重要作用[46].除了促进Fe(Ⅲ)等无机物的生物还原,在生物修复方面,腐殖质可促进五氯苯酚等有机污染物的生物降解[47].在进一步的研究中发现,将腐殖质固化在固相上,通过生物电化学手段可以进一步提高五氯苯酚降解效率[48].
3.2 AQDS
AQDS是醌类物质,它与辅酶Q具有相似的醌类结构,可充当电子介体,在氧化态(醌)、半氧化态(半醌)、还原态(酚)之间变化[49].2,6-蒽醌二磺酸3种状态的氧化还原电势分别为-250 m V、-210 m V和-170 m V,在电势塔中同样介于呼吸链复合体Ⅰ/Ⅱ/Ⅲ中的NADH与辅酶Q之间[50].
AQDS已应用于染料废水脱色、含氮污染物脱氮和难降解有机物降解等方面.VAN DER ZEE,等[51]和DOS SANTOS,等[52]同时证明,在染料活性红2(RR2)生物处理系统中添加AQDS,可明显提高脱色效果和降解速率,染料降解的一级反应速率常数可提高一个数量级以上;常温下添加AQDS的效果远远优于高温下添加.这个结论在单级与二级厌氧反应器中也得到了验证[53].SUN,等[54]采用MFC进行刚果红染料脱色试验,发现添加AQDS后不仅加快了刚果红降解,还提高了MFC的最大能量密度36%以上.AMEZQUITA-GARCIA,等[55]将AQDS固定在活性炭纤维上,用于处理对硝基苯酚废水,添加AQDS可促进其向对氨基苯酚转化.XI,等[56]在反硝化过程中添加抑制剂(鱼藤酮、双香豆素、叠氮化钠)发现,添加AQDS可显著缓解抑制剂的抑制作用;并由此推测,AQDS的作用机制是增强NADH与硝酸盐还原酶之间的电子传递.据报道[50,57],添加AQDS也可提高DDT、四氯化碳等有机污染物的降解效果.同时AQDS可以促进Bacillus sp.对于铬酸盐的降解,在环境生物修复方面有着良好的应用前景[58].
4 电子介体的工作机制与生理生态意义
电子是原子的基本结构成分之一,在电子传递过程中需要各类电子传递体牵线搭桥.对于吩嗪类和黄素类生理性电子介体以及腐殖质和AQDS类非生理性电子介体,参与电子传递的分子结构主要为含氮杂环与苯环,其共同点是具有共轭π键结构.在一般分子中,电子只出现在原子内的轨道上,而在形成共轭π键的分子中,电子可以扩大到整个共轭π键.这样可使分子中相关键的键长趋于平均,提高分子的稳定性,另外也可增大分子中电子活动范围,促进分子之间的电子传递[59].
2个具有共轭π键的分子可以发生边对边或者面对面的π-π堆积.这是一种共轭π键之间的弱相互作用,可发生分子之间的电子传递.由于电子介体的分子尺寸较大,面对面的π-π堆积更利于电子的直接传递.吩嗪类、黄素类、腐殖质和AQDS均可从呼吸链上的NADH获取电子,也可从其他电子供体获取电子,再将其传递给呼吸链上的辅酶Q. NADH和辅酶Q的电子传递结构即具有共轭π键的含氮杂环和苯环,它们是这些电子介体进行高效电子传递的结构基础[60-61].
细胞是生物的结构单元和功能单元,呼吸链是细胞能量代谢的核心.通过生理性电子介体的作用,可将糖酵解、三羧酸循环或脂肪酸氧化途径连接至呼吸链,贯通细胞膜与细胞体内的电子通路;通过生理性和非生理性电子介体的作用,则可将胞外的电子传递过程连接至呼吸链,贯通细胞膜与细胞体外的电子通路.长期以来,生物学研究只限于胞内电子传递所致的生理效应,没有涉及胞外电子传递所致的生态效应.胞外电子传递拓展了细胞功能,对研发生物电化学过程和认识地球物质生物循环具有重大的理论意义和实用价值.
5 小结与展望
近年来,电子介体已成为研究者关注的焦点之一.一方面电子介体在胞内生理过程中起着重要作用,它们是联系各类生物氧化还原反应之间以及这些生物氧化还原反应与呼吸链之间联系的纽带;另一方面电子介体可将胞内电子带到胞外,也可将胞外电子带到胞内,拓展了细胞代谢功能.电子介体所致的胞内外电子传递,不仅有助于生物电化学过程(MFC和MEC)的研发,也有助于土壤和水底沉积物等污染环境的生物修复.今后还可进一步深入研究:1)探明各类电子介体的生理功能,虽然目前多种电子介体均被证明在电子传递方面有着良好的促进作用,但是对于电子介体本身生理功能的研究偏薄弱,电子介体如何从细胞上得到电子又如何将电子传递至细胞外,电子介体的存在对于细胞本身的生长与代谢有何影响仍需探明;2)探寻胞内外传递效率更高的电子介体,目前发现的电子介体仍以自然界中广泛存在或微生物本身分泌的为主,对于新型材料方面的借鉴较少(如碳纳米管),综合各学科领域的成果可能会在寻找高效电子介体方面有所突破;3)探索新的电子介体应用方式,电子介体的应用方式主要集中在污染物的强化去除与提高微生物电化学体系产电性能方面,然而微生物的生长代谢均与电子传递链相关,可以在利用电子介体开发菌种资源、获取目标代谢产物等方面进行探索.
(References):
[1] SOTTOCASA G L,KUYLENSTIERNA B,ERNSTER L,et al.An electron-transport system associated with the outer membrane of liver mitochondria a biochemical and morphological study.Journal of Cell Biology,1967,23(2): 415-438.
[2] LOGAN B E.Exoelectrogenic bacteria that power microbial fuel cells.Nature Reviews Microbiology,2009,7(5):375-381.
[3] GRALNICK J A,NEWMAN D K.Extracellular respiration. Molecular Microbiology,2007,65(1):1-11.
[4] HE Z,LARGUS T A.Application of bacterial biocathode in microbial fuel cells.Electroanalysis,2006,18(19):2009-2015.
[5] STAMS A J M,DE BOK F A M,PLUGGE C M,et al. Exocellular electron transfer in anaerobic microbial communities. Environmental Microbiology,2006,8(3):371-382.
[6] ROSENBAUM M,AULENTA F,VILLANO M,et al. Cathodes as electron donors for microbial metabolism:which extracellular electron transfer mechanisms are involved?. Bioresource Technology,2011,102(1):324-333.
[7] KAZARINOV I A,IGNATOVA A A,NAUMOVA M N. Kinetics of the electrocatalytic oxidation of glucose by Escherichia coli bacterial cells in the presence of exogenous mediators.Russian Journal of Electrochemistry,2014,50(1):97-101.
[8] VAN DER Z F P,CERVANTES F J.Impact and application of electron shuttles on the redox(bio)transformation of contaminants:a review.Biotechnology Advances,2009,27(3):256-277.
[9] 屈丽,刘宏志,胡洪波,等.天然吩嗪化合物的生物合成.中国抗生素杂志,2010(3):168-174.
QU L,LIU H Z,HU H B,et al.The biosynthesis of natural phenazine.Chinese Journal of Antibiotics,2010(3): 168-174.(in Chinese with English abstract)
[10] 汪华,崔志峰.莽草酸生物合成途径的调控.生物技术通报,2009(3):50-53.
WANG H,CUI Z F.Regulation of shikimic acid biosynthesis pathway.Biotechnology Bulletin,2009(3):50-53.(in Chinese with English abstract)
[11] MAVRODI D V,BLANKENFELDT W,THOMASHOW L S.Phenazine compounds in fluorescent Pseudomonas spp. biosynthesis and regulation.Annual Review of Phytopathology,2006,44:417-445.
[12] MAVRODI D V,BONSALL F R,DELANEY S M,et al. Functional analysis of genes for biosynthesis of pyocyanin and phenazine-1-carboxamide from Pseudomonas aeruginosa PAO1.Journal of Bacteriology,2001,183(21):6454-6465.
[13] DELANEY S M,MAVRODI D V,BONSALL R F,et al. phzO,a gene for biosynthesis of 2-hydroxylated phenazine compounds in Pseudomonas aureofaciens.Journal of Bacteriology,2001,183(1):318-327.
[14] WANG Y,KERN S E,NEWMAN D K.Endogenous phenazine antibiotics promote anaerobic survival of Pseudomonas aeruginosa via extracellular electron transfer. Journal of Bacteriology,2009,192(1):365-369.
[15] CHEN J,CHEN W,HE H,et al.Manipulation of microbial extracellular electron transfer by changing molecular structure of phenazine-type redox mediators. Environmental Science&Technology,2013,47(2):1033-1039.
[16] RABAEY K,BOON N,HÖFTE M,et al.Microbial phenazine production enhances electron transfer in biofuel cells.Environmental Science&Technology,2005,39(9): 3401-3408.
[17] ZHANG T,ZH ANG L,SU W,et al.The direct electrocatalysis of phenazine-1-carboxylic acid excreted by Pseudomonas alcaliphila under alkaline condition in microbial fuel cells.Bioresource Technology,2011,102(14):7099-7102.
[18] COURNET A,BERGÉM,ROQUES C,et al. Electrochemical reduction of oxygen catalyzed by Pseudomonas aeruginosa.Electrochimica Acta,2010,55(17):4902-4908.
[19] JAYAPRIYA J,RAMAMURTH Y V.Use of non-native phenazines to improve the performance of Pseudomonas aeruginosa MTCC 2474 catalysed fuel cells.Bioresource Technology,2012,124:23-28.
[20] VENKATARAMAN A,ROSENBAUM M A,PERKINS SD,et al.Metabolite-based mutualism between Pseudomonas aeruginosa PA14 and Enterobacter aerogenes enhances current generation in bioelectrochemical systems.Energy& Environmental Science,2011(4):4550-4559.
[21] PH AM T H,BOON N,AELTERMAN P,et al. Metabolites produced by Pseudomonas sp.enable a grampositive bacterium to achieve extracellular electron transfer. Applied Microbiology and Biotechnology,2008,77(5): 1119-1129.
[22] WYNTER B A.The composition of cows'milk in health and disease.Journal of the Chemical Society,Faraday Transactions,1879,35:530-538.
[23] WEBER S,SCHLEICHER E.Flavins and Flavoproteins Methods and Protocols.London:Humana Press,2014:18.[24] MASSEY V.The chemical and biological versatility of riboflavin.Biochemical Society,2000,28(4):283-296.
[25] MIURA R.Versatility and specificity in flavoenzymes: control mechanisms of flavin reactivity.The Chemical Record,2001(1):183-194.
[26] VON CANSTEIN H,OGAWA J,SHIMIZU S,et al. Secretion of flavins by Shewanella species and their role in extracellular electron transfer.Applied and Environmental Microbiology,2008,74(3):615-623.
[27] MARSILI E,BARON D B,SHIKH ARE I D,et al. Shewanella secretes flavins that mediate extracellular electron transfer.Proceedings of the National Academy of Sciences,2008,105(10):3968-3973.
[28] ROSS D E,BRANTLEY S L,TIEN M.Kinetic characterization of omc A and mtr C,terminal reductases involved in respiratory electron transfer for dissimilatory iron reduction in Shewanella oneidensis MR-1.Applied and Environmental Microbiology,2009,75(16):5218-5226.
[29] COURSOLLE D,BARON D B,BOND D R,et al.The MTR respiratory pathway is essential for reducing flavins and electrodes in Shewanella oneidensis.Journal of Bacteriology,2009,192(2):467-474.
[30] BRUTINEL E D,GRALNICK J A.Shuttling happens: soluble flavin mediators of extracellular electron transfer in Shewanella.Applied Microbiology and Biotechnology,2012,93(1):41-48.
[31] LE LAZ S,KPEBE A,LORQUIN J,et al.H2-dependent azoreduction by Shewanella oneidensis MR-1:involvement of secreted flavins and both[Ni—Fe]and[Fe—Fe]hydrogenases.Applied Microbiology and Biotechnology,2014,98(6):2699-2707.
[32] SUZUKI Y,KITATSUJI Y,OHNUKI T,et al.Flavin mononucleotide mediated electron pathway for microbial U(Ⅳ)reduction.Physical Chemistry Chemical Physics,2010,12(34):10081.
[33] VELASQUEZ-ORTA S B,HEAD I M,CURTIS T P,et al.The effect of flavin electron shuttles in microbial fuel cells current production.Applied Microbiology and Biotechnology, 2010,85(5):1373-1381.
[34] MASUDA M,FREGUIA S,WANG Y,et al.Flavins contained in yeast extract are exploited for anodic electron transfer by Lactococcus lactis.Bioelectrochemistry,2010,78(2):173-175.
[35] VAN TRUMP J,SUN Y,COATES J D.Microbial interactions with humic substances.Advanced Applied Microbiology,2006,60:55-96.
[36] WATANABE K,MANEFIELD M,LEE M,et al.Electron shuttles in biotechnology.Current Opinion in Biotechnology,2009,20:633-641.
[37] TRATNYEK P G,MACALADY D L.Abiotic reduction of nitro aromatic pesticides in anaerobic laboratory systems. Journal of Agricultural and Food Chemistry,1989,37(1): 248-254.
[38] DUNNIVAN F M,SCHWARRENBACH R P.Reduction of substituted nitrobenzenes in aqueous solutions containing natural organic matter.Environmental Science&Technology,1992,26:2133-2141.
[39] HERNÁNDEZ-MONTOYA V,ALVAREZ L H,MONTESMORÁN M A,et al.Reduction of quinone and non-quinone redox functional groups in different humic acid samples by Geobacter sulfurreducens.Geoderma,2012,183:25-31.
[40] AESCHBACHER M,SANDER M,SCHWARZENBACH R P.Novel electrochemical approach to assess the redox properties of humic substances.Environmental Science& Technology,2010,44(1):87-93.
[41] RATASUK N,NANNY M A.Characterization and quantification of reversible redox sites in humic substances. Environmental Science&Technology,2007,41(22):7844-7850.
[42] FIMMEN R L,CORY R M,CHIN Y,et al.Probing the oxidation-reduction properties of terrestrially and microbially derived dissolved organic matter.Geochimica et Cosmochimica Acta,2007,71(12):3003-3015.
[43] MARTINEZ C M,ALVAREZ L H,CELIS L B,et al. Humus-reducing microorganisms and their valuable contribution in environmental processes.Applied Microbiology and Biotechnology,2013,97(24):10293-10308.
[44] HOBBIE S N,LI X,BASEN M,et al.Humic substancemediated Fe(Ⅲ)reduction by a fermenting Bacillus strain from the alkaline gut of a humus-feeding scarab beetle larva. Systematic and Applied Microbiology,2012,35(4):226-232.
[45] BHUSHAN B,HALASZ A,HAWARI J.Effect of iron(Ⅲ),humic acids and anthraquinone-2,6-disulfonate on biodegradation of cyclic nitramines by Clostridium sp. EDB2.Journal of Applied Microbiology,2006,100(3): 555-563.
[46] XIE L,SHANG C,ZHOU Q.Effect of Fe(Ⅲ)on the bromate reduction by humic substances in aqueous solution.Journal Environment Science(China),2008,20(3):257-261.
[47] ZHANG C,KATAYAMA A.Humin as an electron mediator for microbial reductive dehalogenation.Environmental Science& Technology,2012,46(12):6575-6583.
[48] ZHANG D D,ZHANG C F,LI Z L,et al.Electrochemical stimulation of microbial reductive dechlorination of pentachlorophenol using solid-state redox mediator(humin)immobilization.Bioresource Technology,2014,164:232-240.
[49] ROSSO K M,SMITH D M A,WANG Z,et al.Selfexchange electron transfer kinetics and reduction potentials for anthraquinone disulfonate.The Journal of Physical Chemistry A,2004,108(16):3292-3303.
[50] DOONG R,CHIANG H.Transformation of carbon tetrachloride by thiol reductants in the presence of quinone compounds.Environmental Science&Technology,2005,39(19):7460-7468.
[51] VAN DER ZEE F P,BOUWMAN R H M,STRIK D P B T,et al.Application of redox mediators to accelerate the transformation of reactive azo dyes in anaerobic bioreactors. Biotechnology and Bioengineering,2001,75(6):691-701.
[52] DOSSANTOS A B,TRAVERSE J,CERVANTES F J,et al.Enhancing the electron transfer capacity and subsequent color removal in bioreactors by applying thermophilic anaerobic treatment and redox mediators.Biotechnology and Bioengineering,2005,89(1):42-52.
[53] RODRIGUES DA SILVA M E,FIRMINO P I M,DOS SANTOS A B.Impact of the redox mediator sodium anthraquinone-2,6-disulphonate(AQDS)on the reductive decolourisation of the azo dye reactive red 2(RR2)in oneand two-stage anaerobic systems.Bioresource Technology,2012,121:1-7.
[54] SUN J,LI W,LI Y,et al.Redox mediator enhanced simultaneous decolorization of azo dye and bioelectricity generation in air-cathode microbial fuel cell.Bioresource Technology,2013,142:407-414.
[55] AMEZQUITA-GARCIA H J,RAZO-FLORES E,CERVANTES F J,et al.Anchorage of anthraquinone molecules onto activated carbon fibers to enhance the reduction of 4-nitrophenol.Journal of Chemical Technology &Biotechnology,2015,90(9):1685-1691.
[56] XI Z,GUO J,LIAN J,et al.Study the catalyzing mechanism of dissolved redox mediators on bio-denitrification by metabolic inhibitors.Bioresource Technology,2013,140: 22-27.
[57] LIU C,XU X,FAN J.Accelerated anaerobic dechlorination of DDT in slurry with hydragric acrisols using citric acid and anthraquinone-2,6-disulfonate(AQDS).Journal of Environmental Sciences,2015,38:87-94.
[58] HONG Y G,WU P,LI W R,et al.Humic analog AQDS and AQS as an electron mediator can enhance chromate reduction by Bacillus sp.strain 3C3.Environmental Biotechnology,2012,93:2661-2668.
[59] INGOLD C K.Structure and Mechanism in Organic Chemistry.Ithaca:Cornell University Press,1696:660.
[60] PADHI S K,KOBAYASHI K,MASUNO S,et al.Protoninduced dynamic equilibrium between cyclometalated ruthenium r NHC(remote N-heterocyclic carbene)tautomers with an NAD+/NADH function.Inorganic Chemistry,2011,50(12):5321-5323.
[61] LENAZ G,GENOVA M L.Mobility and function of coenzyme Q(ubiquinone)in the mitochondrial respiratory chain.Biochimica et Biophysica Acta(BBA)-Bioenergetics,2009,1787(6):563-573.
Progress in research of electron transfer mediator(ETM).Journal of Zhejiang University(Agric.& Life Sci.),2016,42(5):573- 581
DING Aqiang,ZHENG Ping*,ZHANG Meng
(College of Environmental and Resource Sciences,Zhejiang University,Hangzhou 310058,China)
electron transport chain;physiological ETM;non-physiological ETM;physiological and ecological significance
X 172
A
10.3785/j.issn.1008-9209.2016.05.251
浙江省自然科学基金(Z15E080001);浙江省创新团队项目(2013 TD12).
*通信作者(Corresponding author):郑平(http://orcid.org/0000-0002-7686-3019),Tel:+86- 571- 88982339,E-mail:pzheng@zju. edu.cn
联系方式:丁阿强(http://orcid.org/0000-0001-6151-4775),E-mail:lsylbrian@163.com
(Received):2016- 02- 25;接受日期(Accepted):2016- 06- 13;
日期(Published online):2016- 09- 18
URL:http://www.cnki.net/kcms/detail/33.1247.S.20160918.1543.016.html
