绿色荧光蛋白转基因大鼠骨髓间充质干细胞培养及鉴定
2016-03-23刘慧娟胡若愚戴王娟刘元果
刘慧娟,胡若愚,戴王娟,刘元果,闵 波,蒋 犁
东南大学附属中大医院 1儿科 2心胸外科,南京 210009
·论著·
绿色荧光蛋白转基因大鼠骨髓间充质干细胞培养及鉴定
刘慧娟1,胡若愚2,戴王娟1,刘元果2,闵波2,蒋犁1
东南大学附属中大医院1儿科2心胸外科,南京 210009
摘要:目的体外分离、培养和鉴定增强型绿色荧光蛋白(EGFP)转基因大鼠骨髓间充质干细胞(BMSCs)。方法取EGFP转基因大鼠胫、股骨骨髓,全骨髓贴壁法分离培养、纯化BMSCs;荧光显微镜观察细胞形态;流式细胞仪分析细胞表型;CCK- 8法绘制细胞生长曲线并与野生型BMSCs增殖情况相比较;分别向成脂、成骨、成软骨诱导分化鉴定;将细胞经鼠尾静脉移植入大鼠体内,观察其在体内定植情况。结果获得稳定表达EGFP的BMSCs,以长梭形为主,融合后呈漩涡状排列;生长曲线示EGFP-BMSCs增殖能力旺盛,与野生型BMSCs相比差异无统计学意义(t=-0.023,P=0.982);细胞CD29、CD90、CD34、CD49d、CD45表达率分别为99.4%、96.4%、0.171%、0.049%、0.038%;成脂、成骨、成软骨诱导后,分别给予油红O、茜素红、甲苯胺蓝染色,结果均呈阳性;在肺组织内可检测到稳定的绿色荧光。结论成功获得高纯度、稳定表达EGFP的BMSCs,干细胞特性不受EGFP影响。细胞定植后,示踪效果良好,可用于后续实验研究。
关键词:骨髓间充质干细胞;绿色荧光蛋白;诱导分化
ActaAcadMedSin,2016,38(1):9-15
间充质干细胞(mesenchymal stem cells,MSCs)是来源于中胚层的一类多能干细胞,主要存在于结缔组织和器官间质中[1- 3]。MSCs有维持组织和器官动态平衡、修复再生等重要作用[3- 9],而来源于骨髓的MSCs(bone marrow mesenchymal stem cells,BMSCs)因其易于分离获取、较强的多向分化潜能、低免疫原性及免疫调节等优点而被重点关注[9- 12]。同时也因为其低免疫原性,缺乏特异分子标志物,使得实验中定位和标记BMSCs成为工作难点[10,13]。目前应用较多标记BMSCs的方法包括超顺磁性氧化铁标记、基因或慢病毒转染荧光标记及荧光转基因技术等[14- 17],其中荧光转基因技术因其直观、敏感、清晰可定量的信号,及低本底优势在生命科学研究中应用愈加广泛[17- 20]。
绿色荧光蛋白(green fluorescent protein,GFP)是在荧光示踪技术中广泛应用标记细胞的一种蛋白,具有高效、稳定、无毒、易于检测等特性[15- 20]。与GFP修饰的质粒或慢病毒转染及超顺磁性氧化铁等标记方法相比,通过GFP转基因标记细胞更加稳定、敏感、损伤小,筛选细胞更加精确、快捷[17- 21]。本研究体外分离、培养和纯化了增强型绿色荧光蛋白(enhanced green fluorescent protein,EGFP)转基因大鼠BMSCs,并对其进行生物学行为研究,以期为后续实验研究提供实验及理论基础。
材料和方法
材料雄性绿色荧光转基因大鼠5只,100~120 g,4~5周龄,由香港科技大学创新药物实验室提供。野生型SD大鼠5只,4周龄,由东南大学动物实验中心提供。胎牛血清(fetal bovine serum,FBS)、胰酶-EDTA(美国Gibco公司),α-MEM培养基、青霉素-链霉素、PBS(美国Hyclone公司),成骨、成脂、成软骨诱导试剂盒(美国Cyagen公司),CD29、CD90、CD34、CD49d、CD45小鼠抗大鼠流式单抗(美国eBioscience公司),流式细胞仪(美国BD公司),CCK8试剂盒(日本同仁),细胞培养箱、离心机(美国Thermo公司),倒置相差荧光显微镜(日本Olympus公司),激光扫描共聚焦显微镜(德国Carl Zeiss公司)。
EGFP转基因大鼠BMSCs体外分离及培养异氟烷吸入麻醉大鼠,75%酒精浸泡5 min。无菌条件下,取双侧股骨、胫骨浸入PBS中。注射器抽取PBS反复冲洗至髓腔发白,冲洗过程中见红色浑浊液体流出,按上述方法冲洗剩余股骨、胫骨。收集混合液,室温条件下,300×g离心7 min,弃上清,用完全培养基(α-MEM培养基+10%FBS+1%双抗)重悬沉淀,细胞计数,按照细胞密度4000/cm2接种于75 cm2培养瓶,置于37℃、5%CO2培养箱中培养。24 h首次半量换液,72 h全量换液,之后每2 d换液1次,待细胞融合70%~80%,0.25%胰酶-EDTA消化,等量完全培养基中和胰酶,收获细胞,300×g离心7 min,完全培养基重悬细胞,1∶3比例接种于新培养瓶。倒置荧光显微镜下观察细胞生长情况。
细胞增殖测定取P3代生长良好的细胞,消化后细胞计数,取8块96孔板,以2×103/孔接种于96孔板中,置于细胞培养箱中培养。每天取1个孔板,加入10 μl/孔CCK8溶液,孵育4 h后,用酶标仪测定其在450 nm处的吸光度并记录。对照组野生型BMSCs测定方法同上。以培养时间为横坐标,对应时间测定的吸光度值为纵坐标,绘制细胞生长曲线。
细胞表型鉴定取P3代细胞,按传代方法收获细胞,调整细胞密度为1×106/ml后,分装各管并标记,分别加入流式单抗CD29、CD90、CD34、CD49d、CD45,室温避光孵育1 h,流式细胞仪检测。
体外诱导多向分化实验
成脂分化:取P3代BMSCs消化后,以2×104/cm2接种于六孔板,常规培养基培养至细胞100%融合,换成诱导液培养3 d,再换成维持液培养1 d,如此3个循环周期后,加上述维持液培养7 d,每3 d换1次液,19 d后,油红O染色检测脂滴。
成骨分化:取P3代细胞消化后,以3×104/cm2接种到0.1%明胶包被的六孔板,常规培养基培养24 h后,吸去原培养基,加入成骨诱导分化培养基培养,每3 d换液1次,3周后茜素红染色。
成软骨分化:取P3代细胞消化后,予不完全培养基洗涤细胞1次,以2.5×105/孔接种至六孔板,用成软骨完全培养基诱导,每2 d换1次液,28 d后,将形成的软骨盘进行甲苯胺蓝染色。以上步骤涉及的各种培养基均按照试剂使用说明书配置。
体内示踪检测取P3代EGFP-BMSCs,调整细胞浓度(2×106/ml),经鼠尾静脉注射1 ml细胞悬液于健康野生型大鼠体内,3 d后取大鼠肺组织,常规冰冻切片,DAPI复染胞核,激光扫描共聚焦显微镜下观察细胞定植情况。
统计学处理采用SPSS 19.0统计软件,正态分布计量资料以均数±标准差表示,两组间比较采用独立样本t检验,P<0.05为差异有统计学意义。
结果
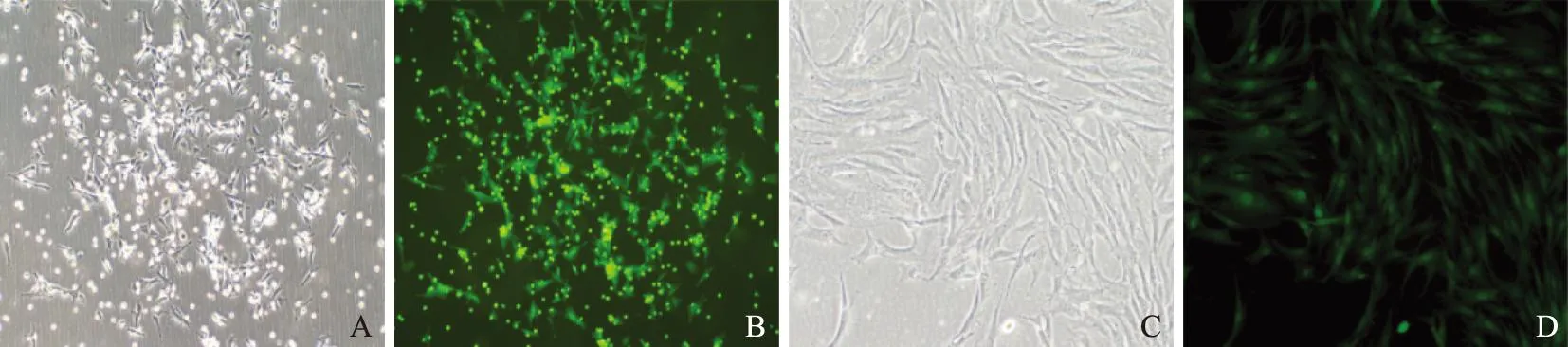
细胞形态和EGFP表达观察细胞接种24 h后,部分细胞即可贴壁,半量换液后镜下观察呈圆形或短梭形。72 h后全量换液,细胞贴壁明显,出现多个葵花样细胞集落,呈克隆样生长(图1A),且EGFP强阳性表达(图1B)。随后细胞生长迅速,逐渐成纺锤形,P3代后,细胞形成趋于均一,呈旋涡状生长(图1C),细胞纯度高并强阳性表达GFP(图1D)。
细胞增殖测定结果P3代EGFP-BMSCs与野生型BMSCs的生长曲线均为S形。在接种第1、2 d为细胞潜伏适应期,增殖较少;第3~6 d细胞密度明显增加;7 d后曲线逐渐变得平缓,细胞增殖明减慢,进入平台期(图2)。两组细胞同一时间点测定的OD值差异无统计学意义(t=-0.023,P=0.982)。
GFP:绿色荧光蛋白
GFP:green fluorescent protein
A. P0代细胞呈葵花样集落生长;B.GFP表达;C. P3代细胞呈梭形、漩涡状生长;D. GFP稳定表达
A. cells at P0 formed the sunflower- like colonies;B. expressed green fluorescent protein gene;C. cells at P3 presented with the fusiform-shaped appearance and the forming of circinate cell colonies;D. stably expressing green fluorescent protein gene
图1倒置显微镜下的细胞形态(×100)
Fig1Cell morphology under inverted microscope(×100)
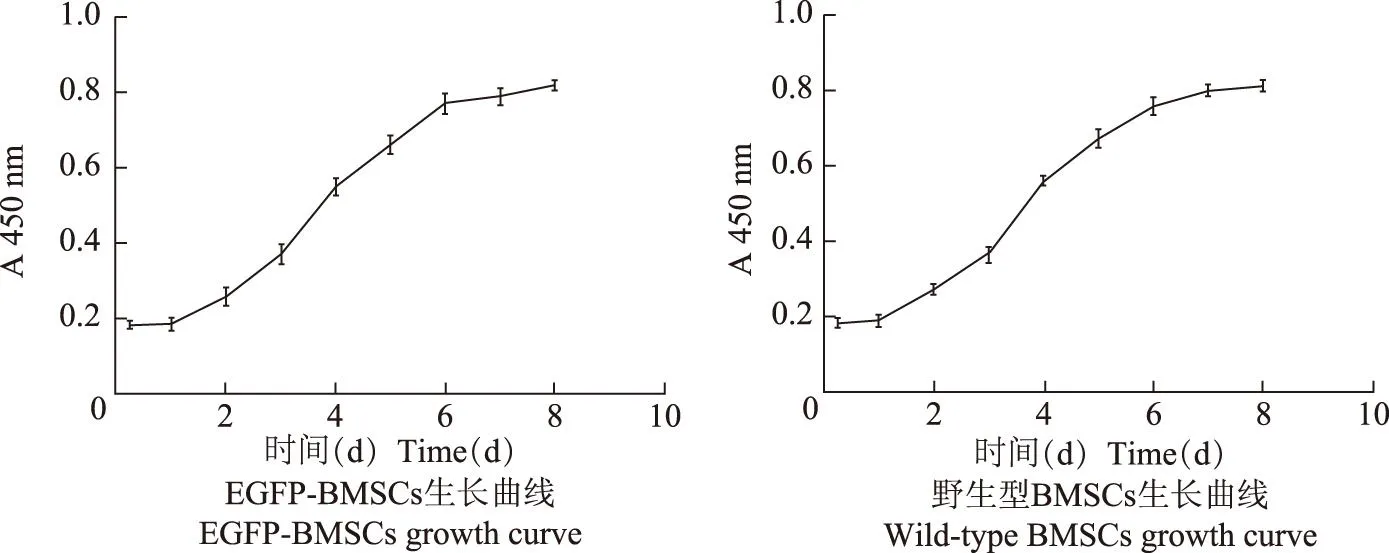
EGFP:增强型绿色荧光蛋白;BMSCs:骨髓间充质干细胞
EGFP:enhanced green fluorescent protein;BMSCs:bone marrow mesenchymal stem cells
图2P3代EGFP-BMSCs与野生型BMSCs生长曲线
Fig2The growth curve of EGFP-BMSCs and wild-type BMSCs at P3
细胞表型鉴定结果流式细胞仪检测结果显示,P3代BMSCs阳性表达CD29、CD90,表达率分别为99.4%、96.4%;CD34、CD49d、CD45阴性表达,表达率分别为0.171%、0.049%、0.038%(图3)。
体外诱导多向分化结果
成脂分化:经成脂诱导后,细胞增殖减慢,胞形态变为椭圆形或多边形,胞浆内脂滴聚集,油红O染色后呈鲜红色(图4A)。
成骨分化:经分化培养基成骨诱导3周后,细胞形态由纺锤形变成多角形,排列密集,经茜素红染色见红色钙盐结节(图4B)。
成软骨分化:成软骨诱导分化28 d后,六孔板中出现数个细胞盘状物(图4C),经甲苯胺蓝染色后呈阳性(图4D)。
体内示踪检测结果经静脉将EGFP-BMSCs植入大鼠体内3 d后,可在大鼠肺组织内检测到EGFP-BMSCs定植,绿色荧光蛋白表达较强(图5)。
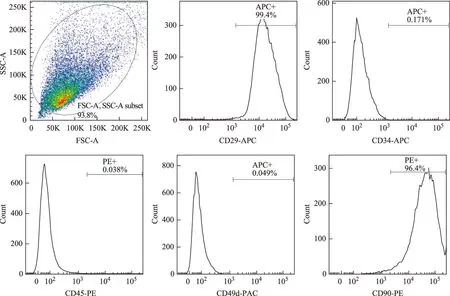
图3绿色荧光蛋白转基因大鼠BMSCs表面标志物
Fig3Surface markers expression of EGFP-transgenic rat BMSCs
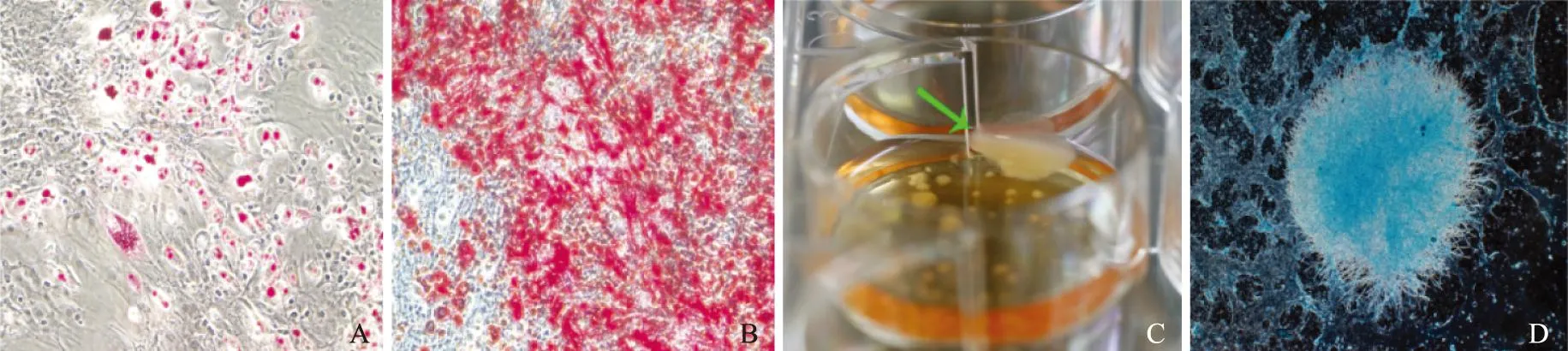
A. 成脂诱导后,油红O染色(×200);B.成骨诱导后,茜素红染色(×200);C. 成软骨诱导后,形成软骨盘;D. 甲苯胺蓝染色(×40)
A. adipogenic induction of cells at P3 by oil red O staining(×200);B.osteogenic induction by alizarin red staining(×200);C. after chondrogenic induction,cells formed some cartilage discs;D. stained by toluidine blue(×40)
图4体外诱导BMSCs向脂肪、骨、软骨转分化
Fig4The transdifferentiation of bone marrow stem cells into fat,bone,and cartilage tissueinvitro
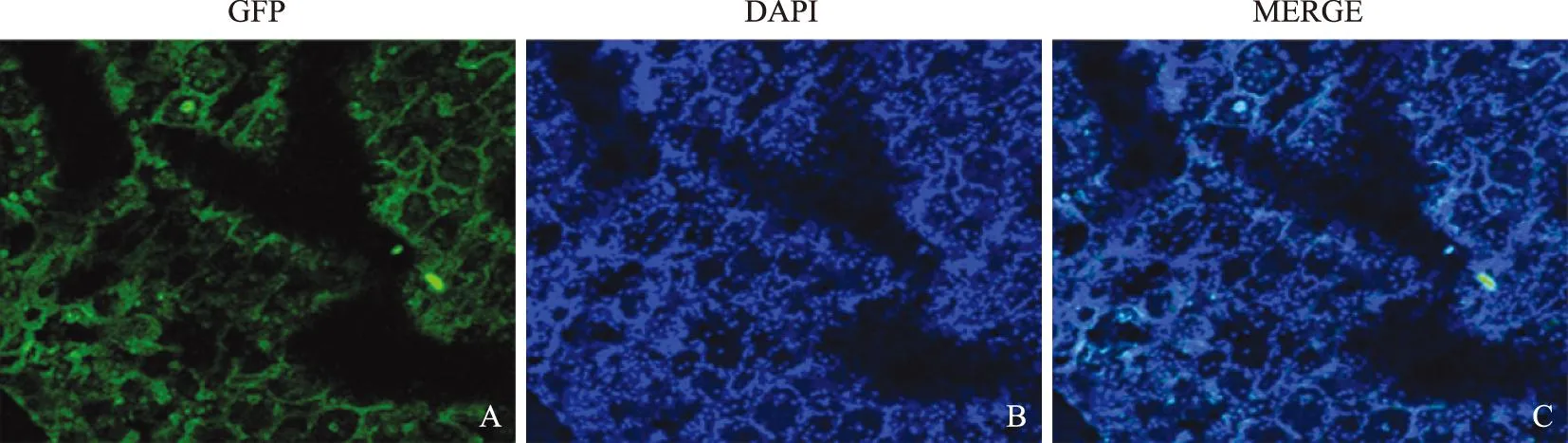
A.绿色荧光蛋白表达;B.DAPI复染细胞核;C.合并图
A.greenfluorescentproteinexpression;B.stainingnucleiwithDAPI;C.merginggraph
图5EGFP-BMSCs在野生型大鼠的肺组织定植 (×200)
Fig5EGFP-BMSCs engrafted in the lung tissue of wild-type rats (×200)
讨论
由于胚胎干细胞的应用受到了严格的法律和伦理限制,广泛存在于成体组织器官中的间充质干细胞越来越受到关注。作为最常见的MSCs之一,BMSCs最早被发现并应用于实验及临床[1- 3]。在实验中,BMSCs分离最常采用密度梯度离心法和全骨髓贴壁法。密度梯度离心法获取的细胞纯度较高,但量少,且步骤较为繁杂,污染机率较高;骨髓贴壁法分离BMSCs虽混有红细胞,但骨髓液中的滋养细胞和营养因子可提供细胞生长所需的微环境,有助于细胞存活和功能维持,因此贴壁迅速,状态良好,经多次传代纯化后,可获得大量的BMSCs。
当前MSCs鉴定尚无统一标准,主要通过观察细胞形态学特征、分析增殖能力、检测表面标志物、鉴定多向分化潜能等方法予以鉴定。本实验获取的EGFP转基因大鼠BMSCs经换液、传代纯化后呈长梭形或多角形等,融合后呈漩涡状细胞排列;增殖能力旺盛,与野生型BMSCs无明显差异;经流式细胞仪检测,分离纯化的细胞高表达CD29、CD90,而低表达骨髓造血干细胞/祖细胞的重要标志CD34、CD45和CD49d,提示分离获取的细胞纯度高,具有MSCs表型特征,且很少混有造血干细胞/祖细胞。油红O和茜素红染色进一步证实,在不同的诱导条件下,其分别具有向脂肪细胞、骨细胞分化的潜能。笔者在诱导BMSCs成软骨分化过程中,采用的方法与传统的细胞三维微球法不同,将细胞接种于六孔板中进行成软骨诱导分化,细胞能更充分地与诱导培养基接触,且有效避免换液时的细胞丢失,提高成软骨诱导分化成功率。甲苯胺蓝染色阳性证实了此法在诱导成软骨分化中行之有效。以上结果均证实本研究提取的EGFP-MSCs符合动物来源的MSCs生物学标准[22]。
MSCs缺少有效特异性标记,传统示踪方法,如通过慢病毒、质粒转染荧光染料标记等均需在离体状态下鉴定,而且转染效率难以控制,且对MSCs活力、归巢及分泌等功能有影响[17- 18]。以超顺磁性氧化铁标记为代表的的放射标记法通过非特异性吸收标记物进入胞内,细胞的标记率与标记物浓度、孵育时间有关,胞内标记物浓度过高对细胞的活力、增殖、分化、功能以及其他生物活性均有不利影响,难以同时兼顾标记率与示踪效果。且因其具有可降解性,随着细胞传代,胞内浓度会相应降低,标记率和时间均难以满足实验中长期检测观察细胞的需求[21]。EGFP转基因纯合子大鼠的所有体细胞均有稳定绿色荧光表达,在研究MSCs的生物学行为及移植体内动态观察其在体内增殖分化、归巢等方面有着天然优势,EGFP对明确外源性MSCs在实验中的作用和机制具有重要意义。本研究结果显示,从EGFP转基因大鼠骨髓提取的BMSCs能稳定表达EGFP,体外扩增传代和分化诱导没有导致EGFP的表达衰减、失活,细胞传至第15代时仍具有强绿色荧光表达。细胞经静脉移植到大鼠体内3 d后,可在肺组织检测到强绿色荧光表达,示踪效果良好。此外,与其他传统方法相比,此法对细胞活力、增殖、分化潜能以及其他生物活性没有影响,使得细胞示踪更为简便、直观、稳定。
关于MSCs应用于动物实验病理模型中已十分普遍[23- 25],也有临床试验报道主要集中在利用其分化潜能促进组织修复与重建,如肌细胞、神经元、退变椎间盘,以及旁分泌功能减轻炎症反应、抑制肿瘤生长等方面[26- 28]。EGFP转基因BMSCs应用于细胞疗法,可用于细胞定位,动态观察BMSCs生物学行为变化,以探究BMSCs治疗疾病的机制。本研究采用全骨髓贴壁法成功体外分离培养出稳定表达EGFP的转基因大鼠BMSCs,并经流式细胞术及成骨、成脂、成软骨分化鉴定符合BMSCs的特性,有较强增殖、分化能力,可为后续实验研究提供大量状态良好、以EGFP为示踪因子的种子细胞。
参考文献
[1]Kagami H,Agata H,Inoue M,et al. The use of bone marrow stromal cells (bone marrow-derived multipotent mesenchymal stromal cells) for alveolar bone tissue engineering:basic science to clinical translation[J]. Tissue Eng Part B Rev,2014,20(3):229- 232.
[2]Yu KR,Kang KS. Aging-related genes in mesenchymal stem cells:a mini-review[J]. Gerontology,2013,59(6):557- 563.
[3]Kagami H,Agata H,Tojo A. Bone marrow stromal cells (bone marrow-derived multipotent mesenchymal stromal cells) for bone tissue engineering:basic science to clinical translation[J]. Int J Biochem Cell Biol,2011,43(3):286- 289.
[4]Dmitrieva RI,Revittser AV,Klukina MA,et al. Functional properties of bone marrow derived multipotent mesenchymal stromal cells are altered in heart failure patients,and could be corrected by adjustment of expansion strategies[J]. Aging (Albany NY),2015,7(1):14- 25.
[5]Dai LJ,Moniri MR,Zeng ZR,et al. Potential implications of mesenchymal stem cells in cancer therapy[J]. Cancer Lett,2011,305(1):8- 20.
[6]Wang YT,Wu XT,Wang F. Regeneration potential and mechanism of bone marrow mesenchymal stem cell transplantation for treating intervertebral disc degeneration[J]. J Orthop Sci,2010,15(6):707- 719.
[7]Ahn SY,Chang YS,Park WS. Mesenchymal stem cells transplantation for neuroprotection in preterm infants with severe intraventricular hemorrhage[J]. Korean J Pediatr,2014,57(6):251- 256.
[8]Donega V,Nijboer CH,van Tilborg G,et al. Intranasally administered mesenchymal stem cells promote a regenerative niche for repair of neonatal ischemic brain injury[J]. Exp Neurol,2014,261(12):53- 64.
[9]Maltman DJ,Hardy SA,Przyborski SA. Role of mesenchymal stem cells in neurogenesis and nervous system repair[J]. Neurochem Int,2011,59(3):347- 356.
[10]Sindberg GM,Lindborg BA,Wang Q,et al. Comparisons of phenotype and immunomodulatory capacity among rhesus bone-marrow-derived mesenchymal stem/stromal cells,multipotent adult progenitor cells,and dermal fibroblasts[J]. J Med Primatol,2014,43(4):231- 241.
[11]Comite P,Cobianchi L,Avanzini MA,et al. Immunomodulatory properties of porcine,bone marrow-derived multipotent mesenchymal stromal cells and comparison with their human counterpart[J]. Cell Mol Biol (Noisy-le-grand),2011,57(Suppl):L1600- L1605.
[12]Grove JE,Bruscia E,Krause DS. Plasticity of bone marrow-derived stem cells[J]. Stem Cells,2004,22(4):487- 500.
[13]Dominici M,Le Blanc K,Mueller I,et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement[J]. Cytotherapy,2006,8(4):315- 317.
[14]Odintsov B,Chun JL,Berry SE. Whole body MRI and fluorescent microscopy for detection of stem cells labeled with superparamagnetic iron oxide (SPIO) nanoparticles and DiI following intramuscular and systemic delivery[J]. Methods Mol Biol,2013,1052(13):177- 193.
[15]Guo Y,Su L,Wu J,et al. Assessment of the green florescence protein labeling method for tracking implanted mesenchymal stem cells[J]. Cytotechnology,2012,64(4):391- 401.
[16]Bauer G,Dao MA,Case SS,et al.Invivobiosafety model to assess the risk of adverse events from retroviral and lentiviral vectors[J]. Mol Ther,2008,16(7):1308- 1315.
[17]Remy S,Tesson L,Usal C,et al. New lines of GFP transgenic rats relevant for regenerative medicine and gene therapy[J]. Transgenic Res,2010,19(5):745- 763.
[18]Livet J,Weissman TA,Kang H,et al. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system[J]. Nature,2007,450(7166):56- 62.
[19]Ripoll CB,Bunnell BA. Comparative characterization of mesenchymal stem cells from eGFP transgenic and non-transgenic mice[J]. BMC Cell Biol,2009,10:3. DOI:10.1186/1471- 2121- 10- 3.
[20]Zheng YM,Zheng YL,He XY,et al. Multipotent differentiation of the EGFP gene transgenic stem cells derived from amniotic fluid of goat at terminal gestational age[J]. Cell Biol Int,2011,35(12):1243- 1246.
[21]Rosenberg JT,Sellgren KL,Sachi-Kocher A,et al. Magnetic resonance contrast and biological effects of intracellular superparamagnetic iron oxides on human mesenchymal stem cells with long-term culture and hypoxic exposure[J]. Cytotherapy,2013,15(3):307- 322.
[22]Dominici M,Le Blanc K,Mueller I,et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement[J]. Cytotherapy,2006,8(4):315- 317.
[23]Li Z,Hu X,Mao J,et al. Optimization of mesenchymal stem cells (MSCs) delivery dose and route in mice with acute liver injury by bioluminescence imaging[J]. Mol Imaging Biol,2015,17(2):185- 194.
[24]Zhu LH,Bai X,Zhang N,et al. Improvement of human umbilical cord mesenchymal stem cell transplantation on glial cell and behavioral function in a neonatal model of periventricular white matter damage[J]. Brain Res,2014,1563(5):13- 21.
[25]Pereira CL,Goncalves RM,Peroglio M,et al. The effect of hyaluronan-based delivery of stromal cell-derived factor- 1 on the recruitment of MSCs in degenerating intervertebral discs[J]. Biomaterials,2014,35(28):8144- 8153.
[26]Mendicino M,Bailey AM,Wonnacott K,et al. MSC-based product characterization for clinical trials:an FDA perspective[J]. Cell Stem Cell,2014,14(2):141- 145.
[27]Pacini S. Deterministic and stochastic approaches in the clinical application of mesenchymal stromal cells (MSCs)[J]. Front Cell Dev Biol,2014,2(2):1- 13.
[28]Raynaud CM,Rafii A. The Necessity of a systematic approach for the use of MSCs in the clinical setting[J]. Stem Cells Int,2013,2013:892340.DOI:10.1155/2013/892340.
Culture and Identification of Bone Marrow Mesenchymal Stem Cells from Enhanced Green Fluorescent Protein-transgenic Rats
LIU Hui-juan1,HU Ruo-yu2,DAI Wang-juan1,LIU Yuan-guo2,MIN Bo2,JIANG Li11Department of Pediatrics,2Department of Cardiothoracic Surgery,Zhongda Hospital,
Southeast University,Nanjing 210009,China Corresponding author:JIANG LiTel:025- 83272182,E-mail:jiangli77777@126.com
ABSTRACT:ObjectiveTo isolate,culture,and identify bone marrow mesenchymal stem cells(BMSCs) from enhanced green fluorescent protein (EGFP)-transgenic rats in vitro. MethodsBone marrows were isolated from tibia and femur of healthy EGFP-transgenic rats of specific pathogen free (SPF) grade. Then,the whole bone marrow adherent method was used for isolation,culture,and purification of BMSCs. The morphological change was noted by continuous observation under inverted fluorescence microscope. The growth curve of cells was drawn through the method of CCK- 8 and the proliferation compared with wild type BMSCs. The surface markers of BMSCs were detected by flow cytometry. The BMSCs were induced to differentiate into osteoblasts,adipocytes,and chondrocytes lineages. The EGFP-BMSCs were transplanted into the rats intravenously,and the expression of GFP was detected. ResultsBMSCs stably expressing EGFP gene were obtained successfully,with the fusiform-shaped appearance and the forming of circinate cell colonies. The growth curve of EGFP-MSCs showed the characteristic of active proliferation,showing no significant difference compared with the wild-type BMSCs. The expression rates of the surface markers of BMSCs CD29,CD90,CD34,CD49d,and CD45 were 99.4%,96.4%,0.171%,0.049%,and 0.038%. The GFP were detected in lung 3 days after transplantation. After osteogenic,adipogenic,and chondrogenic induction,oil red-O and alizarin red positive signals and toluidine blue positive cells were detected. ConclusionsHigh-purity BMSCs stably expressing green fluorescent protein gene can be cultured using the whole bone marrow adherent method. EGFP does not affect the stem cell properties and expresses stably after transplantation. The cells can be used as seed cells for subsequent research.
Key words:bone marrow mesenchymal stem cells;green fluorescent protein;induction differentiation
(收稿日期:2015- 04- 15)
DOI:10.3881/j.issn.1000- 503X.2016.01.002
中图分类号:R394.2
文献标志码:A
文章编号:1000- 503X(2016)01- 0009- 07
通信作者:蒋犁电话:025- 83272182,电子邮件:jiangli77777@126.com
基金项目:国家自然科学基金(81370739)和江苏省自然科学基金(BK20131303)Supported by the National Natural Sciences Foundation of China(81370739) and the Natural Science Foundation of Jiangsu Province(BK20131303)
