Intrinsic Kinetic Modeling of Thermal Dimerization of C5Fraction
2016-03-22GuoLiangWangTiefengLiDongfengWangJinfu
Guo Liang; Wang Tiefeng; Li Dongfeng; Wang Jinfu
(1. Beijing Key Laboratory of Green Reaction Engineering and Technology, Department of Chemical Engineering, Tsinghua University, Beijing 100084; 2. Beijing Research Institute of Chemical Industry, Beijing 100013)
Intrinsic Kinetic Modeling of Thermal Dimerization of C5Fraction
Guo Liang1,2; Wang Tiefeng1; Li Dongfeng2; Wang Jinfu1
(1. Beijing Key Laboratory of Green Reaction Engineering and Technology, Department of Chemical Engineering, Tsinghua University, Beijing 100084; 2. Beijing Research Institute of Chemical Industry, Beijing 100013)
This work aims to investigate the intrinsic kinetics of thermal dimerization of C5fraction in the reactive distillation process. Experiments are conducted in an 1000-mL stainless steel autoclave under some selected design conditions. By means of the weighted least squares method, the intrinsic kinetics of thermal dimerization of C5fraction is established, and the corresponding pre-exponential factor as well as the activation energy are determined. For example, the pre-exponential factor A is equal to 4.39×105and the activation energy Eais equal to 6.58×104J/mol for the cyclopentadiene dimerization reaction. The comparison between the experimental and calculated results shows that the kinetics model derived in this work is accurate and reliable, which can be used in the design of reactive distillation columns.
steam cracking C5fraction, thermal dimerization, intrinsic kinetics
1 Introduction
At present, the C5fraction is normally obtained from the thermal cracking of naphtha or other heavy hydrocarbons in ethylene production processes, accounting for about ten to twenty percent of ethylene production. With the rapid development of the ethylene production industry, effective utilization of the cracking C5fraction is becoming more and more important. The C5fraction contains more than 20 kinds of components, in which isoprene (IP), cyclopentadiene (CPD) and 1,3-pentadiene (1,3-PD) are dominant and account for about forty to sixty percent of the total C5fraction[1-11]. These dienes are important raw materials because of their high activity in chemistry.
Generally, it is difficult to separate IP and CPD by the simple distillation process because the boiling point of IP (34 ℃) is very close to that of CPD (41.5 ℃). Instead, the thermal dimerization method is used widely to get a better separation of C5fraction thanks to the high dimerization activity of both IP and CPD. CPD is more susceptible to conversion to dicyclopentadiene (DCPD)[12−17]via thermal dimerization reaction. The boiling point of DCPD (166.6 ℃) is signi fi cantly higher than that of IP, so it is easy to separate IP and DCPD via distillative separation. Besides the self-dimerization of CPD in the dimerization reactor, however, the self-dimerization of IP and the codimerization of CPD with IP (or 1,3-butadiene (1,3-BD)) would also occur at an appropriate temperature to produce (IP+IP)dimerand (CPD+IP)dimer(or (CPD+BD)dimer), respectively. In addition, a long reaction time is also bene fi cial to the dimerization of C5fraction. For example, when the temperature exceeds 110 ℃, the loss rate of IP due to its dimerization reaches 10% after having been subject to reaction for about 1 hour. These by-products, such as (IP+IP)dimerand (CPD+IP)dimer, not only reduce the purity of DCPD, but also reduce the yield of IP. Therefore, it is important to limit the reaction temperature and time in a rational extent to improve the conversion rate of CPD and reduce the loss of IP simultaneously.
Hu Jingmin, et al.[18-19]found out that reactive distillation processes were very effective to separate CPD from C5fraction formed in the steam cracking process. They have pointed out that reactive distillation has the following advantages:
1) Improve the conversion rate and selectivity of the reaction equilibrium. In the reactive distillation column, IP accumulates at the top, whereas PD and CPD flow towards the bottom because of their difference in the boil-ing points. The concentration of CPD on the feed plate is the highest and decreases rapidly both upward and downward, because CPD has the highest dimerization activity and hence is largely converted to DCPD on the feed plate. DCPD moves downward and is separated from other single hydrocarbon molecules continually, which improves the self-dimerization of CPD. Moreover, the lower concentration of CPD reduces the copolymerization of CPD with IP and as a consequence can reduce the loss rate of IP;
2) Reduce the energy consumption. In the reactive distillation column, the bottom and the top temperature is about 90 ℃ and 50 ℃, respectively, both of which is lower than the conventional reaction temperature (110 ℃); 3) Reduce the number of equipment pieces. The dimerization reaction occurs in the column and an independent dimerization reactor is no longer needed.
The kinetic data for the dimerization of C5fraction have been reported by some researchers[20−22]. However, most of these papers focused on the dimerization of cyclopentadiene (CPD)[16]and only a few concerned the dimerization of IP. In addition, the applicable temperature range for those reported kinetic data is generally related to 80—130 ℃[20-21], which go beyond the range of the reactive distillation process[18-19]. In this work, the intrinsic kinetics of the thermal dimerization of C5fraction at a lower temperature was studied and an intrinsic kinetic model was derived to promote the design of reactive distillation columns for treating C5fraction.
2 Experimental
2.1 Reagents
The C5fraction used in this work came from the byproduct of the naphtha cracking plant at Beijing Yansan Petrochemical Company, Sinopec. The analysis of C5fraction is listed in Table 1.
2.2 Apparatus
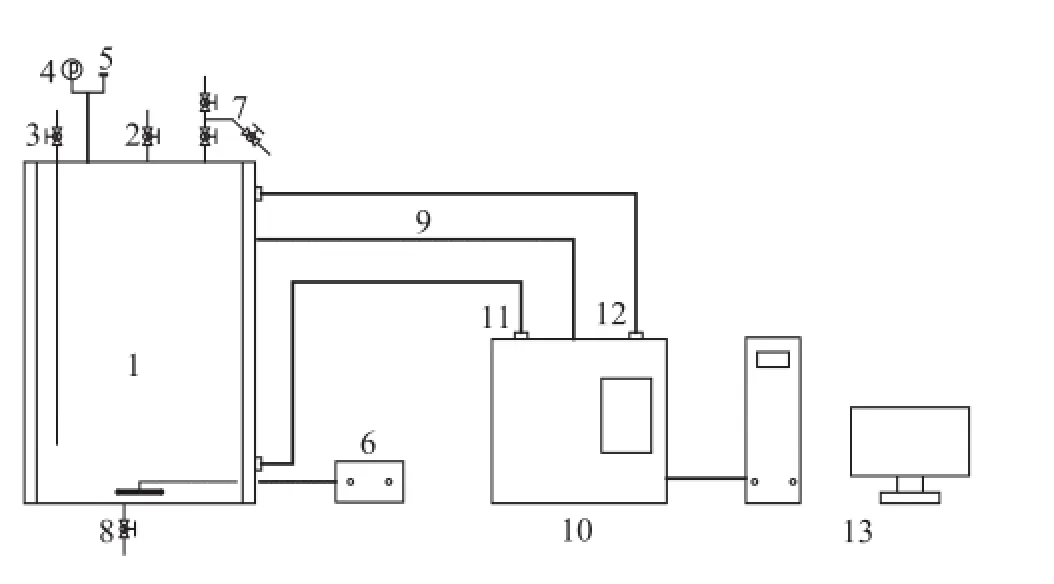
Figure 1 Schematic of the experimental apparatus
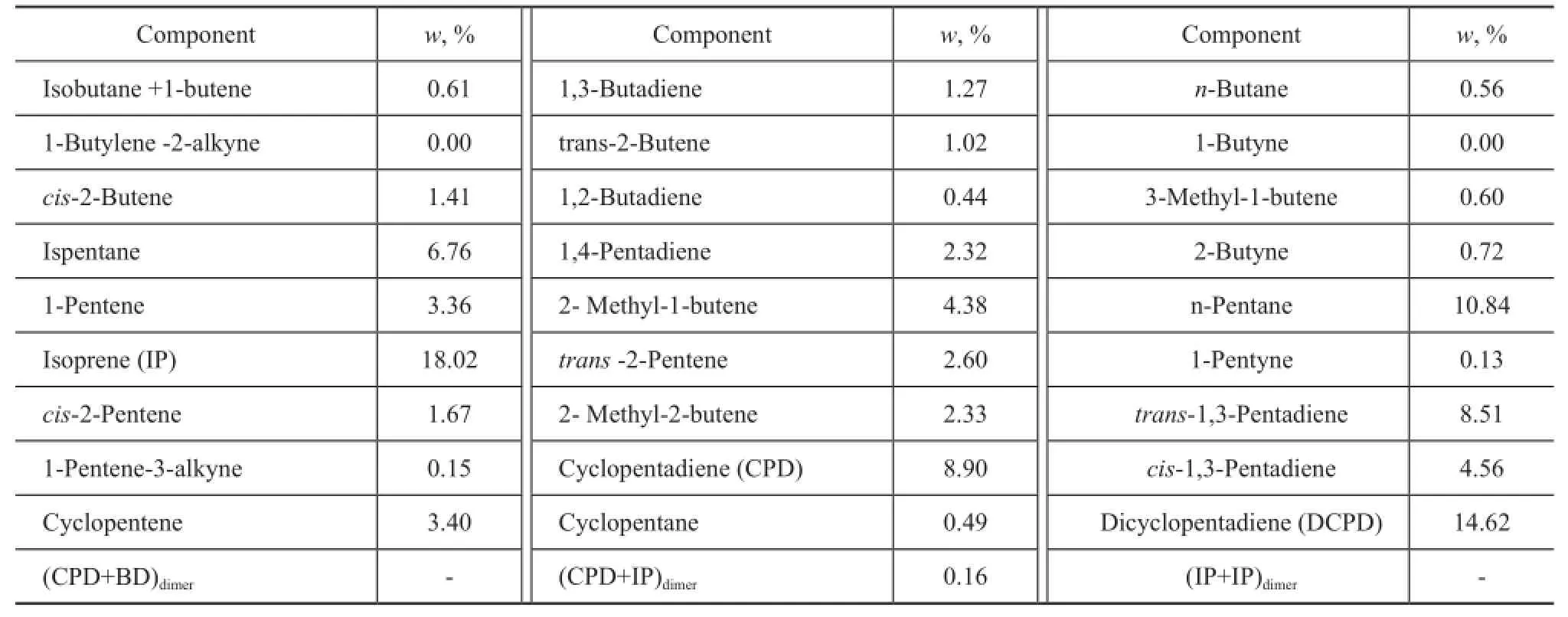
Table1 Analysis of C5fraction
The experiments are carried out in an 1000-mL stainless steel autoclave made by the Buchi Laboratory Equipment Trading (Shanghai) Ltd., as shown in Figure 1. The autoclave consists of an internal heating loop and an external oil-heating jacket and is equipped with an automatic temperature control system. The reactor has facilities for thesampling of the liquid phase. After charging the sealed reactor, C5fraction is pumped into the reactor from the valve #3 (C5fraction inlet). Then the reaction pressure is increased to 1.2 MPa by adding N2through the valve #3 (gas inlet), and then the reactor is heated to the required temperature. At the same time, the stirring speed is set at 100 rpm. It is found out that the diffusion effect has been eliminated when the temperature of the C5fraction increases to 50 ℃ and the stirring speed rises to 1 500 r/min. Finally, a liquid sample of 1 mL is taken through the valve #7 (sampling port) after a de fi nite period of reaction time.
2.3 Analysis
The liquid samples are analyzed with a gas chromatograph (Hewlett-Packard 6890) equipped with FID and a HP-PONA capillary column (50 m×0.2 mm×0.5 µm). The external standard normalization method is used for the quanti fi cation. The injector is operated in the split mode at 225 ℃ and 15.2 psi and the oven temperature program was set at 20 ℃(13 min)—20 ℃/min to 120 ℃(25 min)—10 ℃/min to 180 ℃(2min).
3 Results and Discussions
3.1 Establishment of the kinetic model
The main dimerization reactions of the C5fraction are as follows:
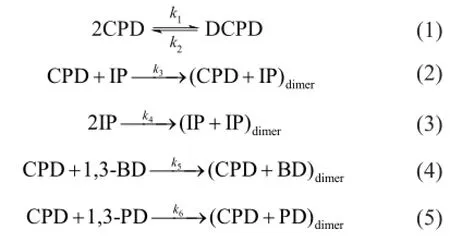
The reverse reactions of (2)—(5) are ignored because they are much slower than the corresponding positive reactions. As a result, the reaction rates are expressed as:
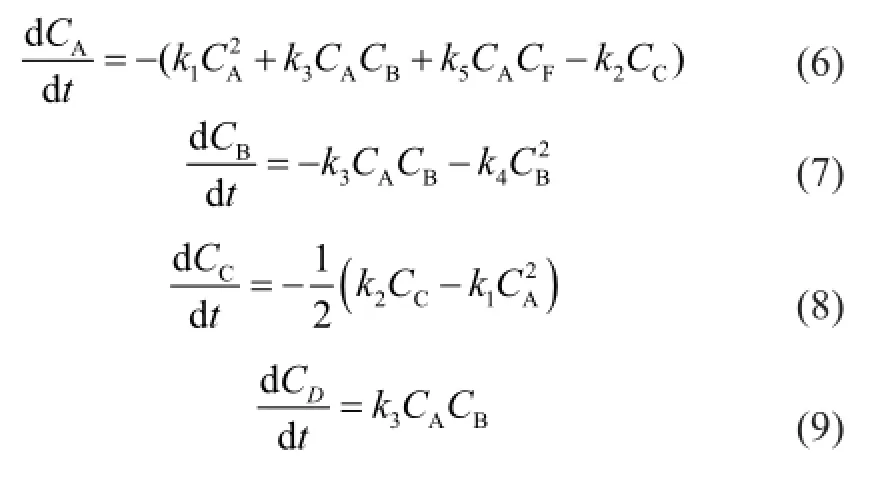
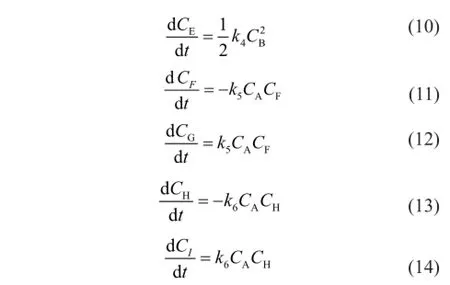
whereCis the component concentration (mol/L),tis the reaction time (s),kis the reaction rate constant (L/(mol·s)) and the subscripts A, B, C, D, E, F, G, H and I represent CPD, IP, DCPD, (CPD+IP)dimer, (IP+IP)dimer, 1,3-BD, (CPD+BD)dimer, 1,3-PD and (CPD+PD)dimer, respectively.
3.2 Parameters of reaction kinetics model

Figure 2 The concentration of 1,3-PD during the reaction at different temperatures
It can be seen from Figure 2 that the conversion of 1,3-PD is almost independent of the reaction temperature under the experimental conditions, covering a temperature rangeof from 50 ℃ to 100 ℃ and a reaction time of from 1 h to 8 h. Therefore, the reaction (5) and equations (13) and (14) were not taken into consideration in order to simplify the problem.
Due to the strong coupling of the reactions, the changes ofCD,CFandCGbefore and after the reaction are much less than those ofCAandCB. Therefore, in the calculation process of the nonlinear regression, the values ofk2andk5are very small. In the regression ofk1—k5, the in fl uence ofk1on the residual value is much greater than that ofk5, which means that the regressed value ofk5has a low precision.
The available literature[18]shows thatk1is much larger thank2in the same temperature range used in this study. As a result, the impact of reverse reaction is relatively small for reaction (1), because it is easily concealed in the measurements and calculation errors of the positive reaction. In this sense, it is dif fi cult to regressk2in a required accuracy.
It can be seen from the above-mentioned case that it is necessary to decouple the whole reaction system in order to obtain accurate values ofk1—k5. As the values of Δ(CPD+IP)dimerand Δ(CPD+BD)dimerbefore and after the reaction are much smaller than that of ΔDCPD, so the contributions of reactions (2) and (4) to ΔCPD are negligible. As a result, the equation (6) is simpli fi ed as:
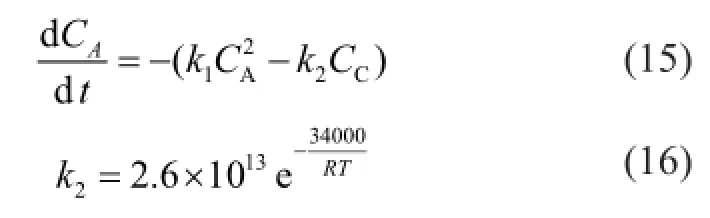
where the value ofk2is calculated by equation (16) as referred to in the literature[18].
The value ofk1is regressed by formulas (8) and (15) using the nonlinear regression method by minimizing the deviation between the calculated and the experimental values ofCAandCC. With the known values ofk1andk2,CA(t) can be calculated from equations (8) and (15). Subsequently, the values ofk3andk4can be determined by substitutingCA(t) into equations (7), (9) and (10) using the nonlinear regression method by minimizing the deviations between the calculated and experimental values ofCB,CD, andCE.
Finally, the value ofk5is obtained by substitutingCA(t) into equations (11) and (12) using the nonlinear regression method in order to minimize the deviations between the calculated and the experimental values ofCF, andCG.
3.3 In fl uence of reaction pressure
The experiments were carried out at 1.0, 1.1, 1.2, 1.3 and 1.4 MPa to study the effect of pressure on the thermal dimerization reactions. It can be seen from Figure 3 that the conversion rate of CPD and IP are both almost independent of the reaction pressure. This is actually expected because all the reactants are in liquid phase. In the following experiments, the reaction pressure was selected to be 1.2 MPa.
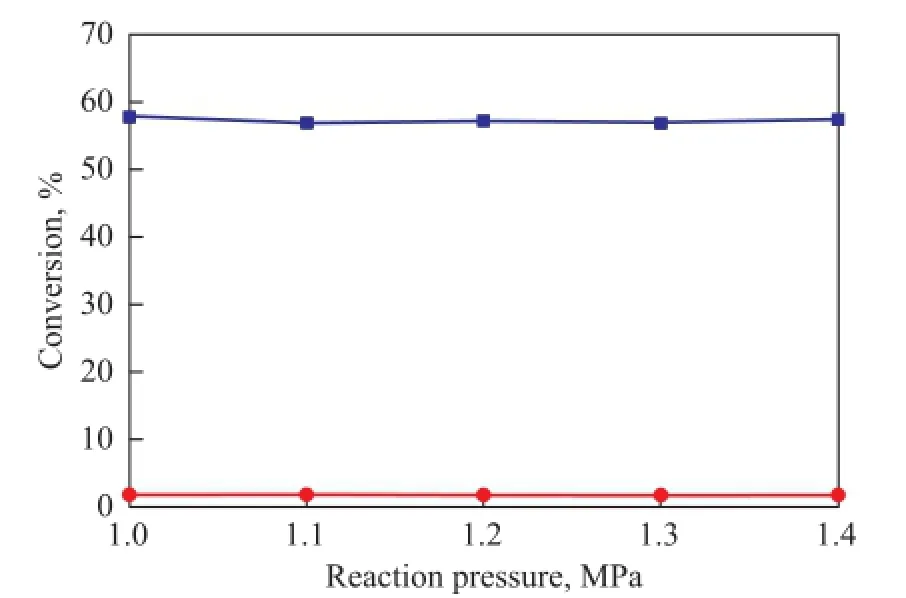
Figure 3 Effect of reaction pressure on the dimerization reaction at 90over a duration of 4 h
3.4 In fl uence of stirring speed
The variation in the CPD conversion rate with the stirring speed is given in Figure 4 for the dimerization of C5fraction proceeding at 90 ℃ and 1.2 MPa for 4h. It can be seen from Figure 4 that the conversion rate of CPD is high at low stirring speed. However, with the increase of the stirring speed, the conversion rate of CPD falls rapidly at first and approaches a flat value eventually. Just as shown in the literature[18], the influence of reaction temperature on the thermal dimerization reaction of CPD is signi fi cant. Both the mass and heat transfer in the autoclave is not uniform and the local overheating occurs at low stirring speed, so the dimerization reaction rate increases with an increasing stirring speed. When the stirring speed is large enough, however, the mass andheat transfer becomes basically uniform without diffusion effects, which can lead to a relatively stable reaction rate. In this work, we fi nd out that the conversion rate of CPD is already stable when the speed is greater than 1 500 r/min, so a stirring speed of 1 500 r/min is used in the experiments.
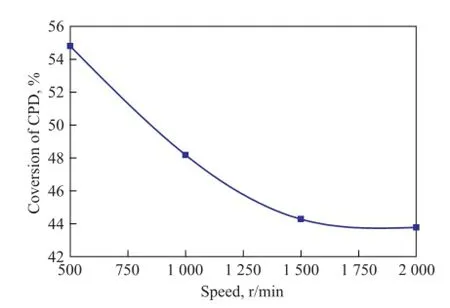
Figure 4 In fl uence of different speed on conversion rate of CPD at 90and 1.2 MPa in 4 h
3.5 In fl uence of reaction temperature and reaction time
As it has been mentioned above, C5fraction was a liquid at 1.2 MPa and the dimerization occurred without catalysts and solvents in this study, so the reaction was mainly in fl uenced by the reaction temperature and reaction time. In the process for separation of C5fraction, the selfdimerization of CPD is the main reaction while the copolymerization of IP with CPD and that of CPD with 1,3-BD are side reactions. As shown in Figures 5—7, the conversion rates of CPD, IP and 1,3-BD increase gradually with both an increasing reaction temperature and reaction time on one hand, while the conversion rate of CPD is higher than that of IP and 1,3-BD on the other hand. When the reaction temperature rises to 90 ℃, the reaction rate accelerates rapidly because the activity of CPD, IP and 1,3-BD is all improved greatly at such a high temperature. Figures 5—7 also reveal that the increasing trend in the reaction rate is gentle with an increasing reaction time since the concentrations of CPD, IP and 1,3-BD decrease with the increase in reaction time.
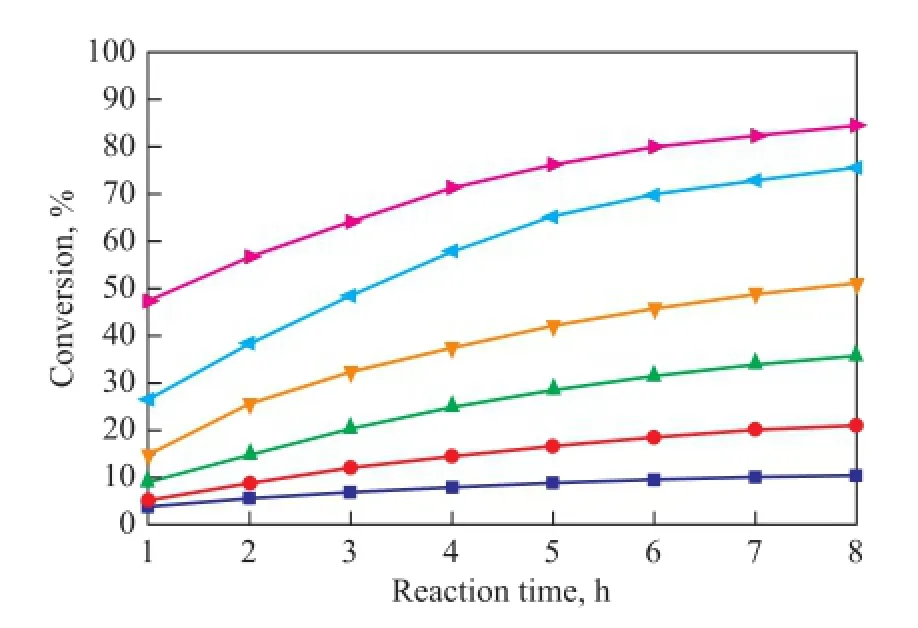
Figure 5 Variation in conversion of CPD during the reaction at different temperatures
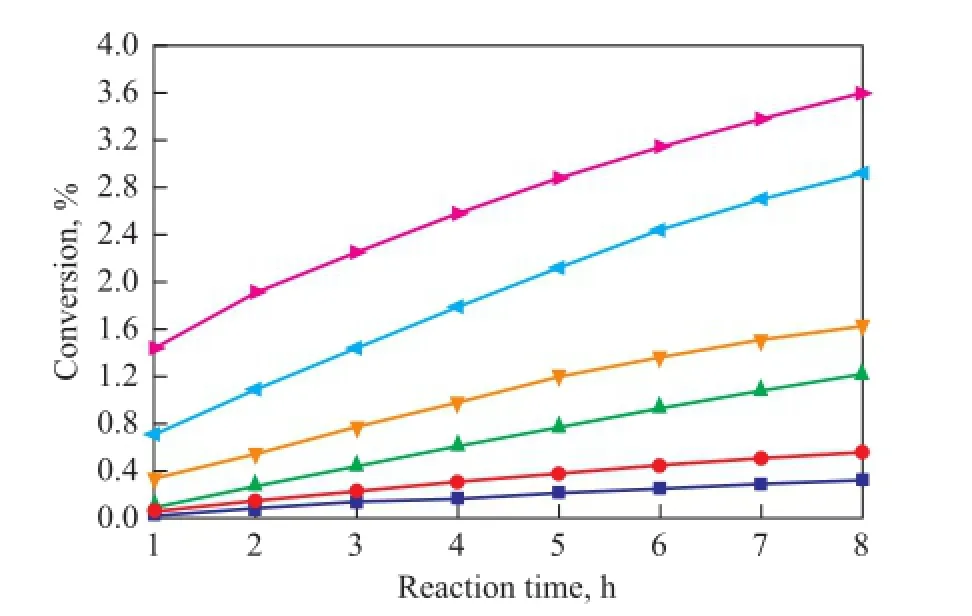
Figure 6 Variation in conversion of IP during the reaction at different temperatures
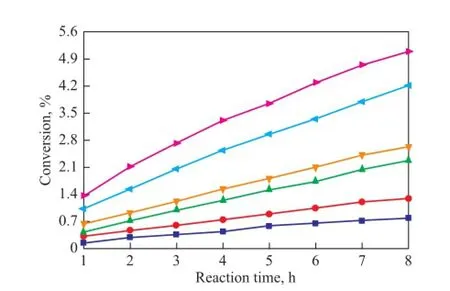
Figure 7 Variation in conversion of 1,3-BD during the reaction at different temperatures
As shown in Figures 8—10, the copolymerization rate of IP with CPD is much higher than the self-dimerization rate of IP. The yield of (CPD+IP)dimerat 80 ℃ and 100 ℃is 0.54% and 1.74%, respectively, after having been subject to reaction for 1 h, whereas the self-dimerization of IP is always very feeble when the reaction temperature is below 90 ℃. For example, the yield of (IP+IP)dimeris only 0.02% at 80 ℃ after having been subject to reaction for 8 hours. However, just as shown in Fig. 9, the selfdimerization rate increases rapidly when the temperatureexceeds 90 ℃. For example, the yield of (IP+IP)dimerrises up to 0.21% after having been subject to reaction for 4 hours at 90 ℃. The reason is that as the reaction temperature rises, the activity of the self-dimerization reaction of IP is also enhanced rapidly.
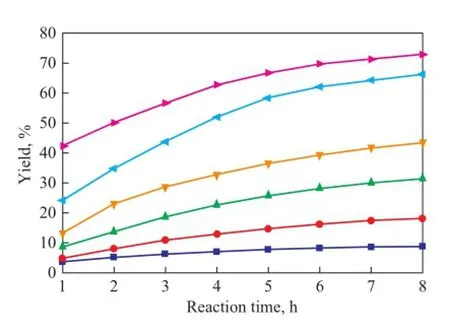
Figure 8 Variation in yield of DCPD during the reaction at different temperatures
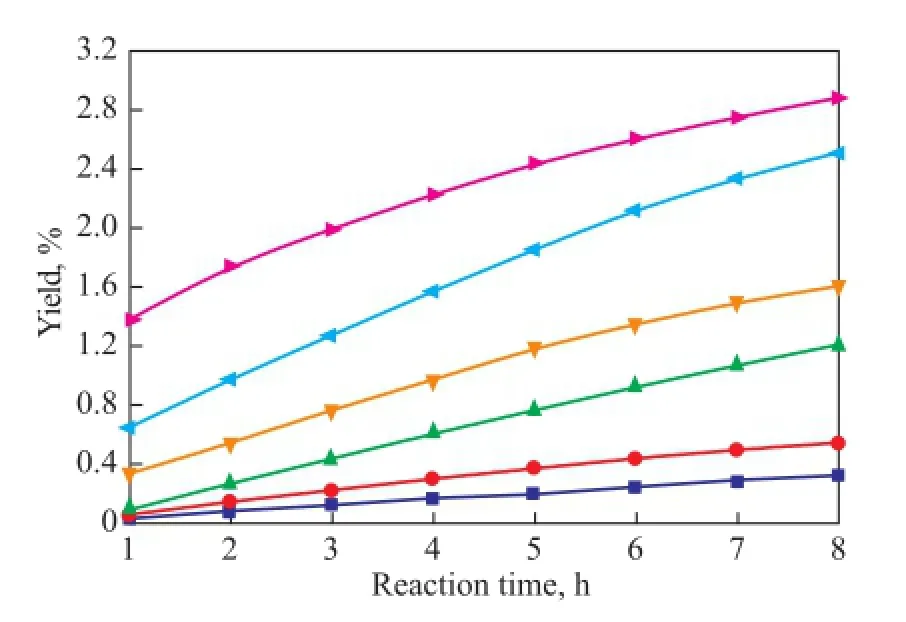
Figure 10 Variation in yield of (CPD+IP)dimerduring the reaction at different temperatures

Figure 9 Variation in yield of (IP+IP)dimerduring the reaction at different temperatures
The experimental results given above show that the reaction temperature below 90 ℃ is bene fi cial to increasing the conversion rate of CPD as well as reducing the loss of IP. Therefore, it is optimal to set the reaction temperature to be lower than 90 ℃. In this study, the operating temperature in the reactive distillation column was around 80 ℃ and hence the loss of IP caused by its self-polymerization decreased. Moreover, CPD and DCPD can be separated at the same time during the self-polymerization reaction of CPD by means of the separation effect of column plates. As a result, the equilibrium of reaction (1) is broken and the positive reaction is enhanced which leads to the increase of CPD conversion. As shown in the literature[5], the CPD conversion became already greater than 80% after having been subject to reaction for 0.65 h.
3.6 The kinetic model parameters
The rate constants kiat different temperatures were calculated according to the above steps. The calculation results are list in Table 2.
It is well known that the Arrhenius dependence has thefollowing form:
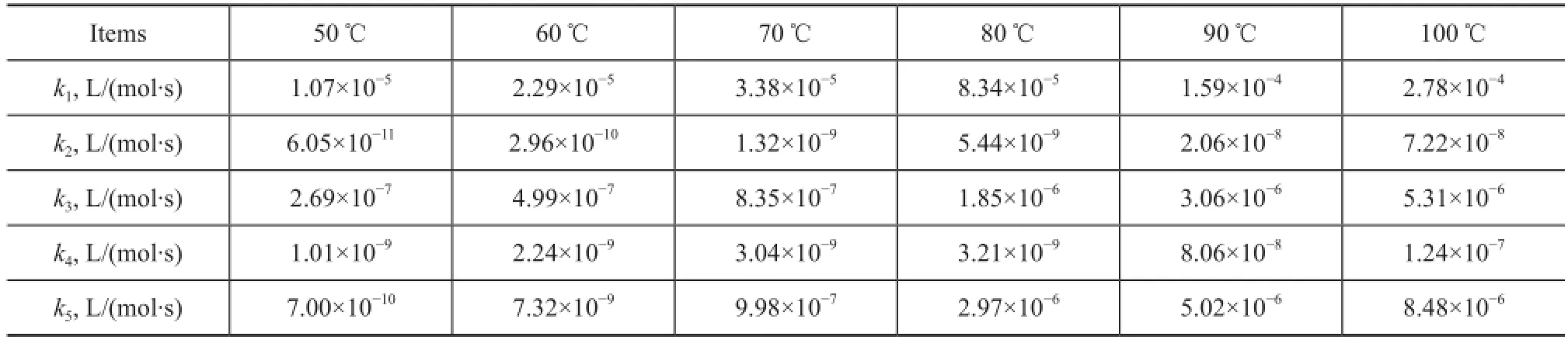
Table 2 Values ofkifor thermal dimerization of C5fraction solved by the model
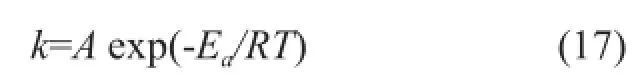
The pre-exponential factor and the activation energy of the reactions were calculated by the linear fi tting method. The results are shown in Table 3.
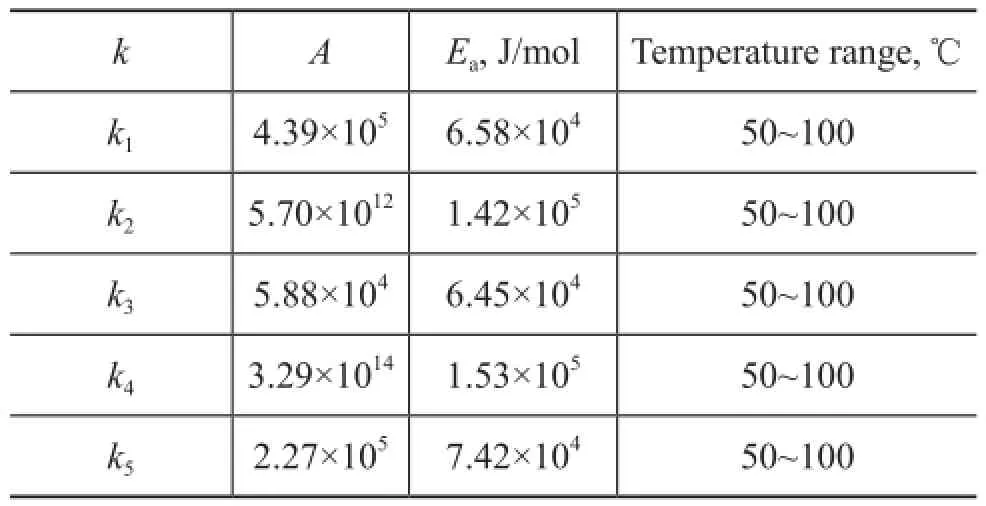
Table 3 Parameter values of kinetic model
3.7 Validation of kinetic model
To verify the prediction ability of the kinetic model, the concentrations of all compounds were calculated and compared with the experimental data. The parity plot of the experimental and the calculated concentrations of CPD, IP, 1,3-BD, DCPD, (IP+IP)dimer, (CPD+BD)dimerand (CPD+IP)dimerat reaction temperatures of 50 ℃, 70 ℃and 90 ℃ over different reaction times are shown in Figures 11—13, indicating to a good agreement between the experimental and the calculated concentrations. These results con fi rm that the kinetic model proposed in the present work is highly acceptable and reliable.
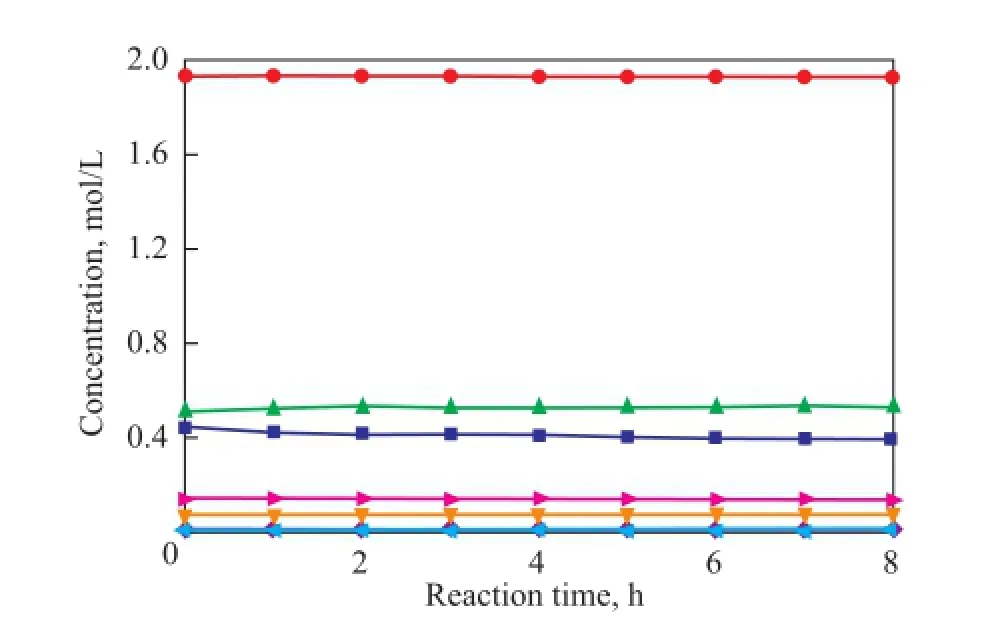
Figure 11 Concentration curve of CPD, IP, 1,3-BD, DCPD,(IP+IP)dimer, (CPD+BD)dimerand (CPD+IP)dimerat 50over different reaction time
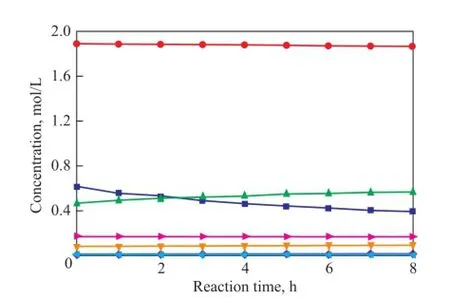
Figure 12 Concentration curve of CPD, IP, 1,3-BD, DCPD,(IP+IP)dimer, (CPD+BD)dimerand (CPD+IP)dimerat 70℃over different reaction time
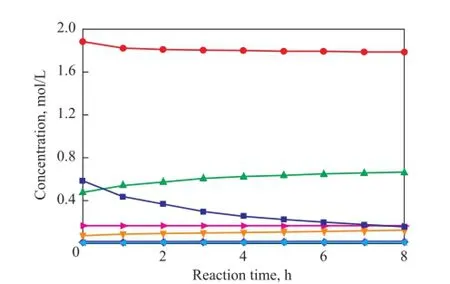
Figure 13 Concentration curve of CPD, IP, 1,3-BD, DCPD,(IP+IP)dimer, (CPD+BD)dimerand (CPD+IP)dimerat 90over different reaction time
4 Conclusions
The intrinsic kinetics of thermal dimerization of C5fraction in the reactive distillation process was investigated in an 1000-mL stainless steel autoclave under reaction conditions covering a range of temperatures (50, 60, 70, 80, 90, and 100 ℃), a scope of reaction times (1, 2, 3, 4, 5, 6, 7, and 8 h), a range of reaction pressures (1.0, 1.1, 1.2, 1.3, and 1.4 MPa), and a string of stirring speeds (500, 1 000, 1 500, and 2 000 r/min). The experimental results andtheoretical analysis can lead to the following conclusions:
1. The pre-exponential factors and the activation energies for self-dimerization of CPD, IP and 1,3-BD , the depolymerization of DCPD and the copolymerization of IP with CPD and those of 1,3-BD with CPD were obtained. The intrinsic kinetic model derived thereby was con fi rmed to be highly acceptable and reliable in comparison with the experimental results.
2. The experimental results showed that the reaction temperature below 90 ℃ was bene fi cial to increasing the conversion rate of CPD as well as reducing the loss of IP. When the operating temperature in the reactive distillation column is around 80 ℃, CPD and DCPD can be separated while the self-polymerization reaction of CPD is proceeding due to the separation effect of column plates. As a result, the equilibrium of reaction (1) is broken and the positive reaction is enhanced which can lead to the increase of CPD conversion, so the intrinsic kinetic model can be used in the design of reactive distillation column for treating C5fraction.
[1] Guo L, Li D F, Wang J F. Progresses in the separation of steam cracking C5fraction at home[J]. Petrochemical Technology, 2015, 44(2): 252–260 (in Chinese)
[2] Thomas R. C5extraction process uses acetonitrile[J]. Chemical Processing Engineer, 1972, 53(1): 34–36.
[3] Li D F, Ma L G. Progress in research for separation of steam cracking C5fraction[J]. Petrochemical Technology, 2007, 36(8): 755–762 (in Chinese)
[4] Wen J H, Bao Z H. Progress in C5fraction separation from steam cracking[J]. Chemical Industry and Engineering Progress, 2010, 29(2): 205–210 (in Chinese)
[5] Guo L, Li D F, Wang J F. Simulation of reactive distillation process for separation of steam cracking C5[J]. Petrochemical Technology, 2014, 43(12):1394–1400 (in Chinese)
[6] Goodrich Company. Extractive Distillation of C5Hydrocarbons Using Acetonitrile and Additives: US. 4081332[P], 1978
[7] Sha Y, Ge C F. Research & development of advanced C5separation process[J], Petroleum Refinery Engineering, 2010, 40(7): 5–8 (in Chinese)
[8] Japan Synthetic Rubber Company. Process for production of 1,3-butadiene or 2-methyl-1,3-butadiene having high purity: US, 3401515 [P], 1983
[9] Cui X M. Separation technology and utilization prospect of isoprene[J], Chemical Industry, 2011, 29(12): 38–45 (in Chinese)
[10] The Japanese Geon Company. Isoprene Puri fi cation Process: US, 3510405[P], 1970
[11] The Goodyear Tire & Rubber Company. Recovery of Isoprene: US, 3947506[P], 1976
[12] Hu T Y, Wei H L, Analysis of the composition of C5-dimer extracted by DMF from distillation residual of isoprene[J], Petrochemical Technology, 1987, 16(11):792–796 (in Chinese)
[13] Li S Q, Guo S P, Liu Y, et al. Separation of cyclopentadiene from C5cut fraction by thermal dimerization[J], Petrochemical Technology & Application, 2009, 27(5): 433–435 (in Chinese)
[14] Sun S Q, Dong L Y, Hao X G, et al. Studies on cyclopentadiene removal from pyrolysis C5distillates with thermal polymerization[J]. Qilu Petrochemical Technology, 2007, 35(1): 6–9 (in Chinese)
[15] Cheng J, Han B Y, Yang H, et al. The kinetic study on the anionic polymerization of isoprene[J]. Chinese Journal of Chemical Engineer, 1999, 7(2):154–160 (in Chinese)
[16] Wang X M, Bao Z H. Study on thermal dimerization of dienes in C5fraction[J]. Journal of Nanjing University of Technology, 2005,27(4): 53–57 (in Chinese)
[17] Chen X W, Bao Z H. Polymerization of dienes in C5fraction by ionic liquid catalyst[J]. Petrochemical Technology, 2007, 36(3): 232–236 (in Chinese)
[18] Hu J M, Li X, Xu H F, et al. Study of co-dimerization in C5reactive-distillation[J]. Petrochemical Technology, 2001, 30(6): 441–443 (in Chinese)
[19] Tian B L, Tang G Q, Zhang Q, et al. Simulations of thermal dimerization and reactive distillation processes in separation of cyclopentadiene from steam cracking C5[J]. Petrochemical Technology, 2008, 37(12): 1276–1281 (in Chinese)
[20] Bai G X. Kinetic study of dimerization of CPD[J], Petrochemical Technology, 1981, 10(2): 84–94 (in Chinese)
[21] Wang F S, Li L, Li R, et al. Thermopolymerization in C5fraction of ethylene pyrolysis[J]. Journal of Lanzhou University (Natural Sciences), 2007, 43(5): 74–77 (in Chinese)
[22] Li W H, Ren H L, Chen J, et al. Kinetics on cyclopentadiene thermal dimerization of cracking C5fraction[J], Journal of Jilin Institute of Chemical Technology, 2010, 27(3): 20–23 (in Chinese)
Received date: 2016-01-11; Accepted date: 2016-01-23.
Wang Tiefeng E-mail: wangtf@tsinghua.edu.cn.
杂志排行
中国炼油与石油化工的其它文章
- Experimental Study of UDS Solvents for Purifying Highly Sour Natural Gas at Industrial Side-stream Plant
- Highly Active and Stable Ni2P/SiO2Catalyst for Hydrogenation of C9Petroleum Resin
- Enhanced Performance of Denitrifying Sul fi de Removal Process by 1,2-Naphthoquinone-4-Sulphonate
- Investigation of Different Coke Samples Adhering to Cyclone Walls of a Commercial RFCC Reactor
- Numerical Study of Air Nozzles on Mild Combustion for Application to Forward Flow Furnace
- Preparation of Core-Shell Composite of Y@Mesoporous Alumina and Its Application in Heavy Oil Cracking
