Preparation of Core-Shell Composite of Y@Mesoporous Alumina and Its Application in Heavy Oil Cracking
2016-03-22YuanChengyuanWangZhengwuZhangHaitaoTanZhengguoPanZhishuangGaoXionghou
Yuan Chengyuan; Wang Zhengwu; Zhang Haitao; Tan Zhengguo; Pan Zhishuang; Gao Xionghou
(1. Lanzhou Petrochemical Research Center, Petrochemical Research Institute, PetroChina, Lanzhou 730060; 2. PetroChina Dushanzi Petrochemical Company, Karamay 838600)
Preparation of Core-Shell Composite of Y@Mesoporous Alumina and Its Application in Heavy Oil Cracking
Yuan Chengyuan1; Wang Zhengwu2; Zhang Haitao1; Tan Zhengguo1; Pan Zhishuang1; Gao Xionghou1
(1. Lanzhou Petrochemical Research Center, Petrochemical Research Institute, PetroChina, Lanzhou 730060; 2. PetroChina Dushanzi Petrochemical Company, Karamay 838600)
A well core-shell composite of Y@meso-Al with a mesoporous alumina shell and a Y zeolite core was synthesized. The mesoporous alumina shell has a wormhole-like structure with large mesopores. The prepared catalytic cracking catalyst using this composite has exhibited excellent catalytic performance for heavy oil cracking thanks to its favorable physicochemical properties, such as high surface area, large pore volume and outstanding acid sites accessibility for large molecules provided by the composite. In comparison with the reference catalyst using pure Y zeolite, the oil conversion achieved by the above-mentioned catalyst increased by 2.73 percentage points, while the heavy oil yield and coke yield decreased by 2.23 percentage points and 1.28 percentage points, respectively, with the light oil yield increasing by 2.27 percentage points.
zeolite; mesoporous alumina; core-shell; accessibility; catalytic cracking
1 Introduction
As acid catalysts, Y zeolites have found wide applications in catalytic cracking area for their unique properties such as strong acidity, high hydrothermal stability and abundant micropores[1-3]. However, with the growing world oil shortage, the heavy components in petroleum feedstock have greatly increased. Due to the diffusion dif fi culty in small micropores of zeolites, the catalytic cracking of heavy oil has been restricted extremely[4-5]. Therefore, it is very urgent to create mesopores in catalytic cracking catalysts for the pre-cracking of heavy oil, which will greatly facilitate the further cracking of heavy oil to light products such as gasoline and diesel over Y zeolites[6].
To achieve this goal, various strategies such as dealumination and desilication for creating Y zeolites with hierarchical pore structures, in particular mesopores, which can facilitate the diffusion of heavy oil molecules, have been explored[7-13]. However, these methods inevitably suffer from several defects, such as limited pore volume, disordered pore channel, zeolite structure destruction and tremendous material loss, which greatly prevent their applications in practice[14]. Therefore, it is highly urgent to develop a more controllable methodology for preparing hierarchical Y zeolite materials.
Recently, the fabrication of core-shell structured composite materials has become a research hotspot for many potential uses of these materials in separation, catalysts, drug delivery, etc.[15-17]Among them, fabricating coreshell composites by coating mesoporous materials around zeolites is of particular importance, because of the combination of advantages of both mesoporous materials and microporous zeolites by these composites. For example, the core-shell composite of Y zeolite and silicabased MCM-41 mesoporous material was successfully prepared using cetyltrimethylammonium bromide as the template[18-21]. Composite with silicalite-1 or ZSM-5 as the core and SBA-15 silica-based sieves as the shell was also synthesized[22]. However, to the best of our knowledge, the preparation of core-shell composite of Y zeolite and mesoporous alumina, which should exhibit good performance in catalytic cracking of heavy petroleum feedstocks due to the excellent properties such as bigopened mesopores, large surface area, highly uniform channels, high thermal stability and abundant surface acid sites of the mesoporous alumina[23-24], has not been reported yet.
In this paper, a novel composite of Y@mesoporous alumina with a hierarchical pore system was successfully prepared. The obtained composite possessed a classic core-shell structure fabricated by mesoporous alumina shell and Y zeolite core. Furthermore, the composite was employed as the active component for the preparation of catalytic cracking catalyst, and the heavy oil cracking performance of the obtained catalyst was also evaluated.
2 Experimental
2.1 Preparation of Y@mesoporous alumina and catalytic cracking catalysts
2.1.1 Synthesis of Y@mesoporous alumina
As shown in Scheme 1, 4.0 g of triblock copolymer (P123, EO20PO70EO20), 15 g of Y zeolite (nSi/nAl=17) and 60 ml of anhydrous ethanol were fi rst mixed and stirred for 1 h at room temperature. Then 2.0 ml of nitric acid and 4.08 g of aluminum iso-propoxide (C9H21AlO3) were added to the above mixture under vigorous stirring. The obtained mixture was firstly stirred at room temperature for 30 min, and then kept static for another 3 h. After that, the obtained mixture was subject to ageing in a drying oven at 65 ℃ overnight to go through the solvent evaporation process. Finally, the obtained solid sample was calcined at 550 ℃ for 4 h in air. The fi nal product was labeled as Y@meso-Al.
2.1.2 Preparation of catalytic cracking catalyst containing Y@meso-Al
30 g of kaolin clay and 15 g of Y@meso-Al were fi rstly mixed. Then the obtained solid mixture was added into 45.5 g of alumina sol solution under vigorous stirring. The obtained solution was stirred for 1 h at room temperature, and then was put in a drying oven to be subject to ageing overnight at 120 ℃. After that, the obtained solid sample was calcined at 450 ℃ for 3 h, and named as Cat-new.
2.1.3 Preparation of reference catalytic cracking catalyst
30 g of kaolin clay and 15 g of Y zeolite were fi rstly mixed. Then the obtained solid mixture was added to 45.5 g of alumina sol solution under vigorous stirring. The obtained mixture was stirred for 1 h at room temperature, and was then put into a drying oven to be subject to ageing overnight at 120 ℃. After that, the obtained solid sample was calcined at 450 ℃ for 3 h, and named as Cat-old.
2.2 Characterization
X-ray diffraction (XRD) was carried out on a PANalytical X’pert pro diffractometer. The BET surface area and the pore size distribution measurements were performed on a Micromeritics ASAP 2010 instrument operating at liquid nitrogen temperature. The transmission electron microscopy (TEM) micrographs were obtained with a JEM-2010 electron microscope. The Fourier transform infrared (FTIR) spectra of the samples after adsorption of pyridine (Py) and 2,6-di-tert-butylpyridine (DTBPy) were recorded on a Bruker Tensor 27 FT-IR spectrometer.
2.3 Cracking tests
The catalytic cracking of heavy oil was performed in a micro fixed fluid bed unit. Prior to testing, the catalysts were steam deactivated at 800 ℃ for 4 h. The reaction temperature was 500 ℃, with the catalyst/oil weight ratio equating to 4. The properties of the heavy oil feedstock are listed in Table 1.
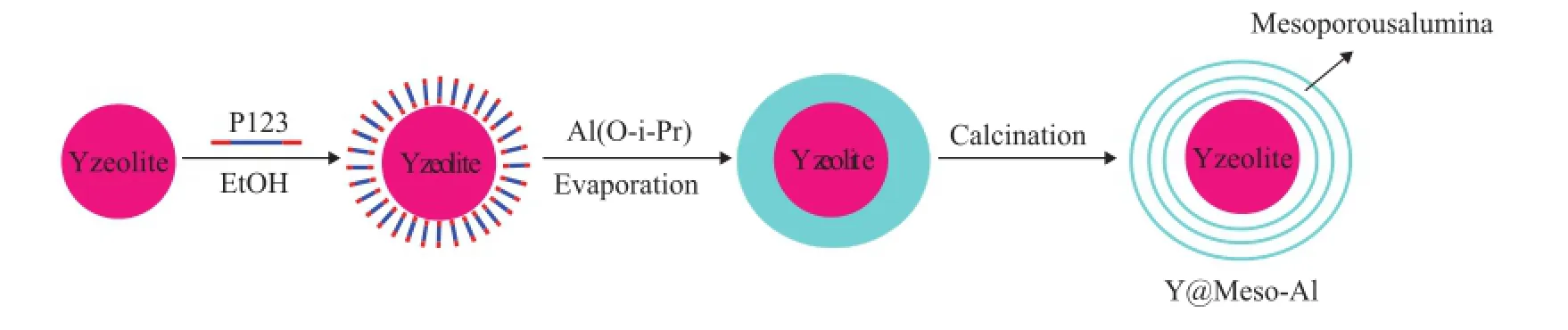
Scheme 1 Preparation of Y@meso-Al

Table 1 Properties of heavy oil feedstock
3 Results and Discussion
3.1 XRD analyses
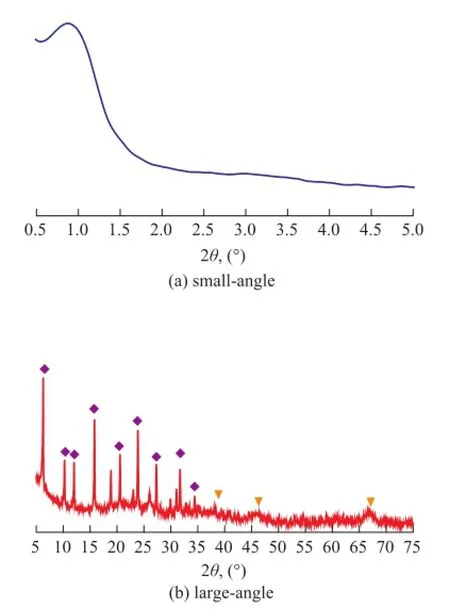
Figure 1 XRD patterns of the Y@meso-Al sample
Figure 1 shows the XRD patterns of the sample Y@meso-Al. It is shown that a resolved diffraction peak at a 2θ value of 0.8owas observed in the small-angle XRD pattern, corresponding to the (100) face diffraction peak of mesoporous materials with hexagonal pores[25]. The appearance of this diffraction peak con fi rmed that some mesoporous alumina shell had been formed in the sample of Y@meso-Al. In the large-angle XRD pattern, the sample of Y@ meso-Al exhibited the characteristic diffraction peaks of microporous Y zeolite and Al2O3, indicating to the coexistence of microporous Y zeolite and mesoporous alumina shell. These resolved diffraction peaks also illustrated that there was no remarkable degradation for Y zeolite during the covering process.
3.2 N2adsorption/desorption analyses
The N2adsorption/desorption isotherms and pore size distribution of different samples are shown in Figure 2. It can be seen that Y zeolite displayed a type I adsorption isotherm, with a fast adsorption volume increasing at low pressure followed by a platform appearing, which indicated the characteristics of micropores material[26]. The small hysteresis loop was caused by the secondary mesopores resulted from the desilication of Y zeolite in the alkaline environment[27]. Compared with the pure Y zeolite, the sample of Y@meso-Al showed a much bigger hysteresis loop ascribed to the synergistic function of microporous Y zeolite and mesoporous alumina in Y@meso-Al[28].
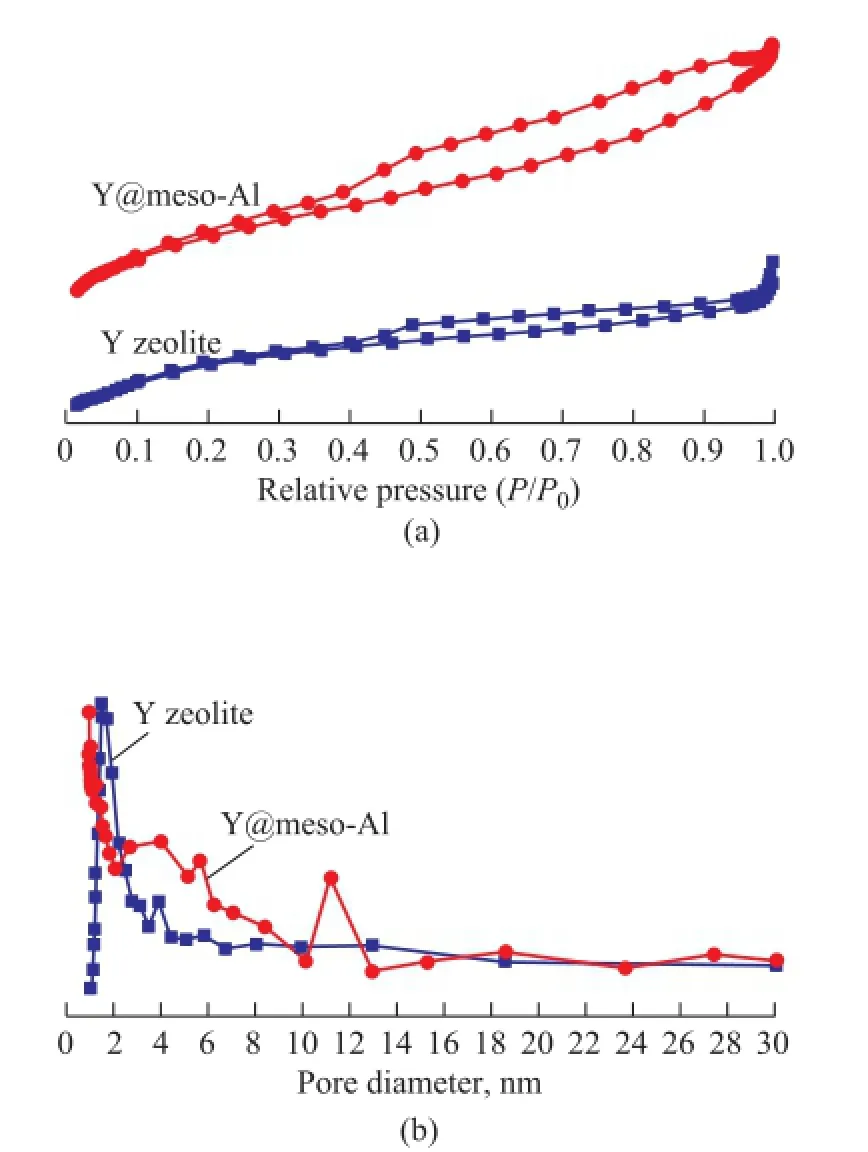
Figure 2 N2adsorption/desorption isotherms (a) and pore size distribution (b) of Y zeolite and Y@meso-Al
It can be seen from the pore size distribution that the main distribution peak for Y zeolite appeared in the microporous range. In contrast with the Y zeolite, besides a distribution peak in the microporous range, the sample Y@ meso-Al exhibited additionally two distribution peaks in the mesoporous range, indicating that the pores of Y zeolite were not blocked during the covering process along with the formation of mesoporous alumina shell.
As shown in Table 2, the total surface area (SBET) and pore volume (Vtotal) of the sample Y@meso-Al were smaller than that of Y zeolite. However, the sample Y@meso-Al possessed higher mesoporous surface area (Smeso) and higher mesoporous pore volume (Vmeso), indicating that there were more mesopores in Y@meso-Al. Compared to the sample Cat-old, the SBETandVtotalof the sample Catnew increased slightly owing to the existence of mesoporous alumina in Cat-new.
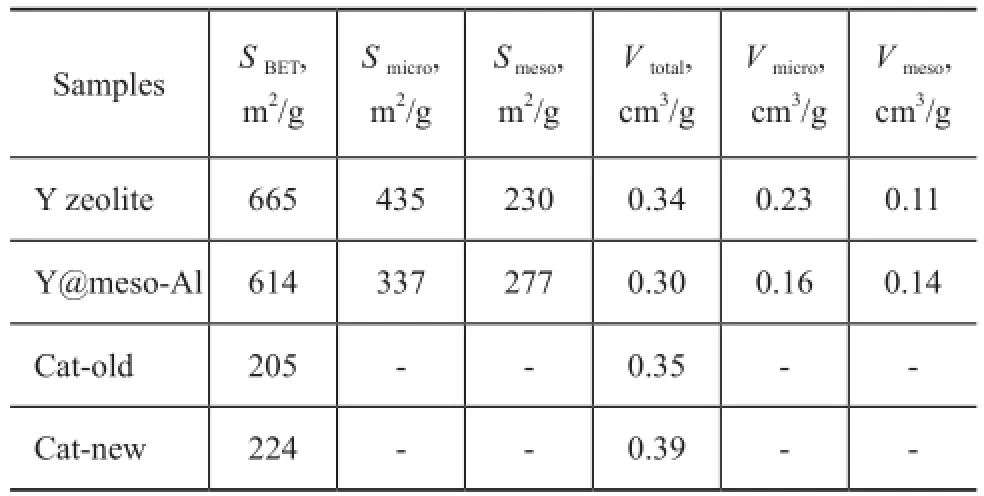
Table 2 Structural properties of different samples
3.3 TEM analyses
The TEM image of Figure 3(a) clearly shows that the sample Y@meso-Al had a classic core-shell structure, with Y zeolite being covered by a uniform mesoporous alumina shell. No separated pieces of Y zeolite and mesoporous alumina were observed. Judging from the image scale, the mesoporous alumina shell thickness was calculated to be about 30 nm. The structural detail of mesoporous alumina shell is shown in Figure 3(b). As it is shown in the TEM image, the mesoporous alumina shell of the composite Y@meso-Al possessed interconnected mesopores with a disordered arrangement.
3.4 Accessibility of active sites
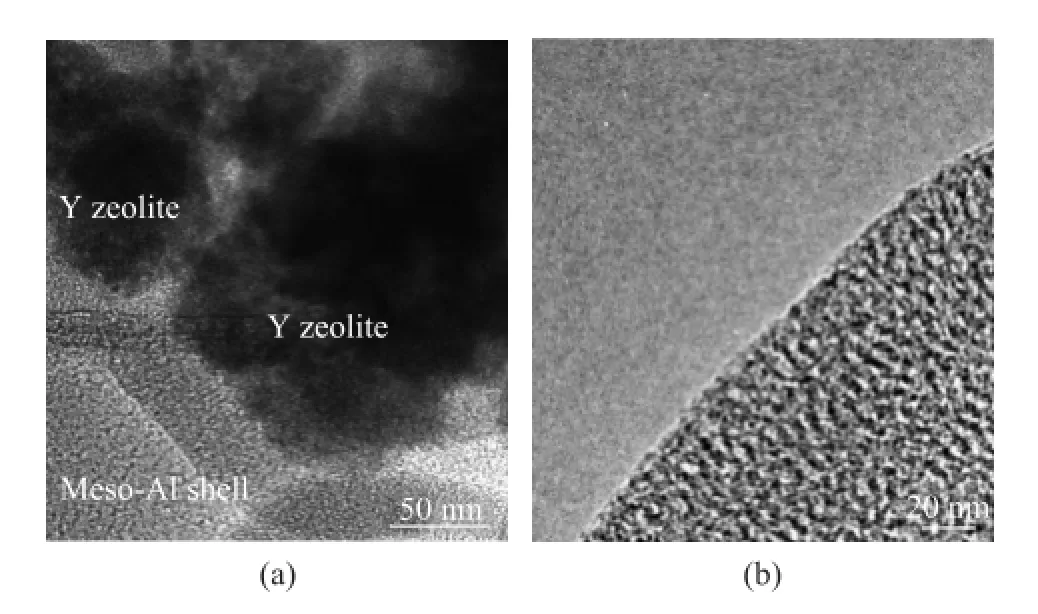
Figure 3 TEM images of Y@meso-Al
The accessibility of acid sites is a very important factor for the performance of catalytic cracking catalysts in heavy oil catalytic cracking[29]. Here, pyridine (Py) and 2,6-di-tert-butylpyridine (DTBPy) adsorption FT-IR spectra were used to estimate the accessibility of acid sites for the studied catalysts[30]. The area of corresponding adsorption peak (A) is linearly correlated to the quantity of the acid sites which probe molecules can reach. For the DTBPy probe molecule, because of its large diameters, it can only reach partially the acid sites, while pyridine molecule can reach all acid sites. Therefore, it is reasonable to take the value ofADTBPy/APyto estimate the acid sites accessibility of the catalyst for the large residual oil molecules. Higher value ofADTBPy/APymeans better accessibility of acid sites.
The FT-IR spectra of samples Cat-old and Cat-new after adsorption of Py and DTBPy are presented in Figure 4. It is shown that the absorption peaks at around 1 540 cm-1and 3 400 cm-1for all samples could be assigned to the Brønsted acid sites on which Py and DTBPy were adsorbed, respectively[31]. There was no distinct difference in Py adsorption peak area (APy) for the two samples. However, as to the big probe molecules of DTBPy, the corresponding adsorption peak area (ADTBPy) of the sample Cat-new was much higher than that of the sample Cat-old.
The values ofADTBPy/APyfor the two samples are shown in Figure 5. As it can be seen that the value ofADTBPy/APyfor the sample Cat-new was remarkably higher than that of the sample Cat-old, indicating to the better acid sites accessibility for the large molecules of Cat-new than Catold. The promotion of acid sites accessibility can be ascribed to the favorable mesopores in the sample Cat-new catalyst provided by Y@meso-Al, which could greatlyfacilitate the diffusion of large molecules in the Cat-new catalyst[32-34].
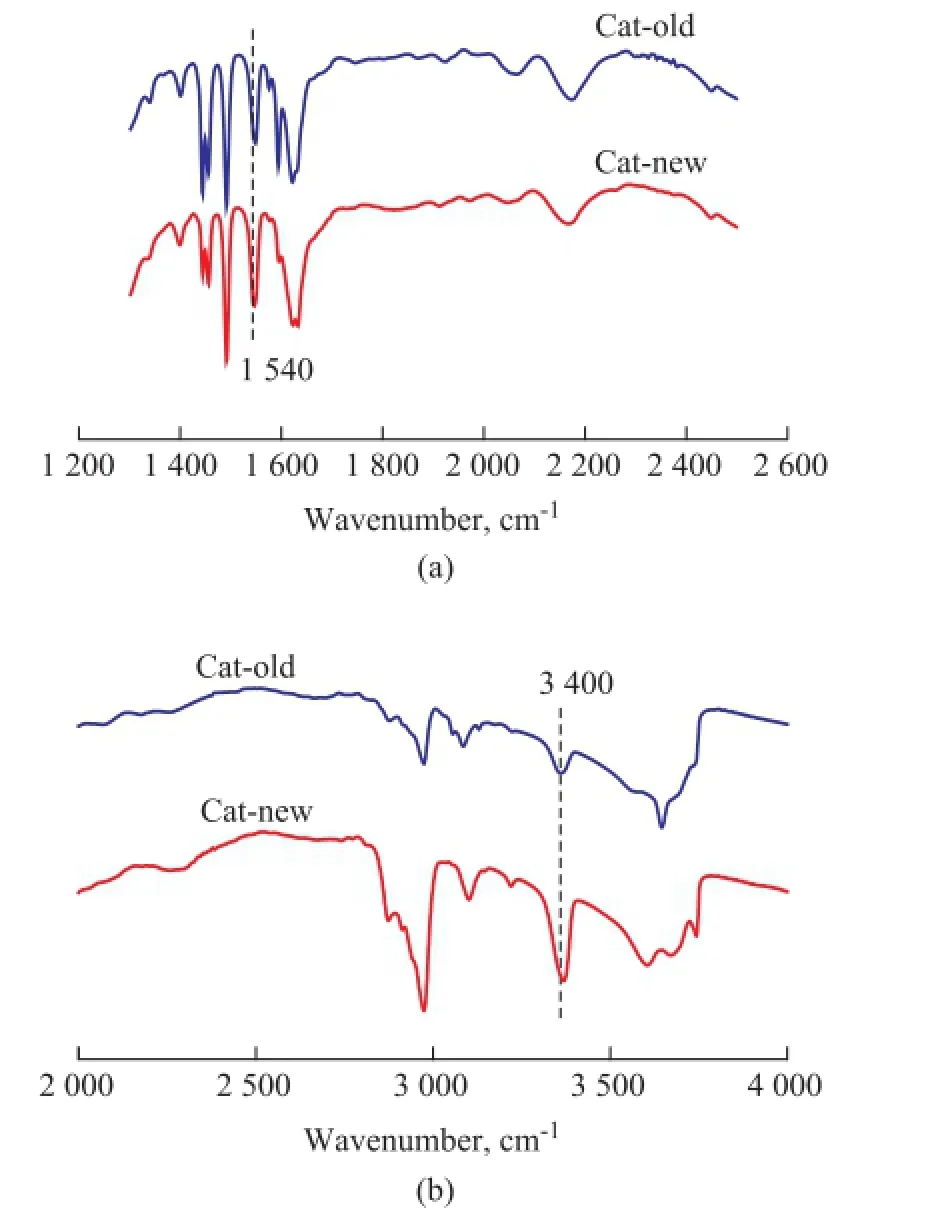
Figure 4 FT-IR spectra of samples Cat-old and Cat-new after adsorption of Py (a) and DTBPy (b)
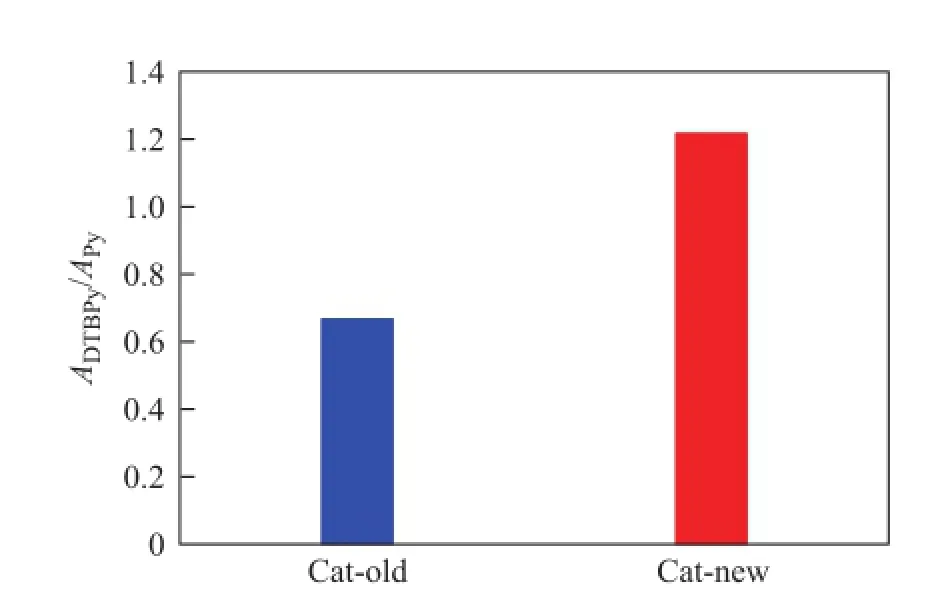
Figure 5 Acid sites accessibility analysis of samples Catold and Cat-new
3.5 Cracking tests
The activity and product distribution of residue cracking using different catalysts are shown in Table 3. The data showed that compared with the catalyst Cat-old, the feed oil conversion of the catalyst Cat-new increased by 2.73 percentage points, while the heavy oil yield and coke yield decreased by 2.23 percentage points and 1.28 percentage points, respectively, with light oil yield increasing by 2.27 percentage points. Therefore, the overall reaction performance of the Cat-new catalyst was obviously better than that of the Cat-old catalyst. Moreover, despite the decreased Y zeolite content in contrast with the Catold catalyst, the activity of the Cat-new catalyst was improved, which fully demonstrated the advantages of the Y@meso-Al sample. The excellent catalytic performance of the Cat-new catalyst can be attributed to its excellent structural properties, such as high surface area, big pore volume and outstanding acid sites accessibility provided by the composite of Y@meso-Al.
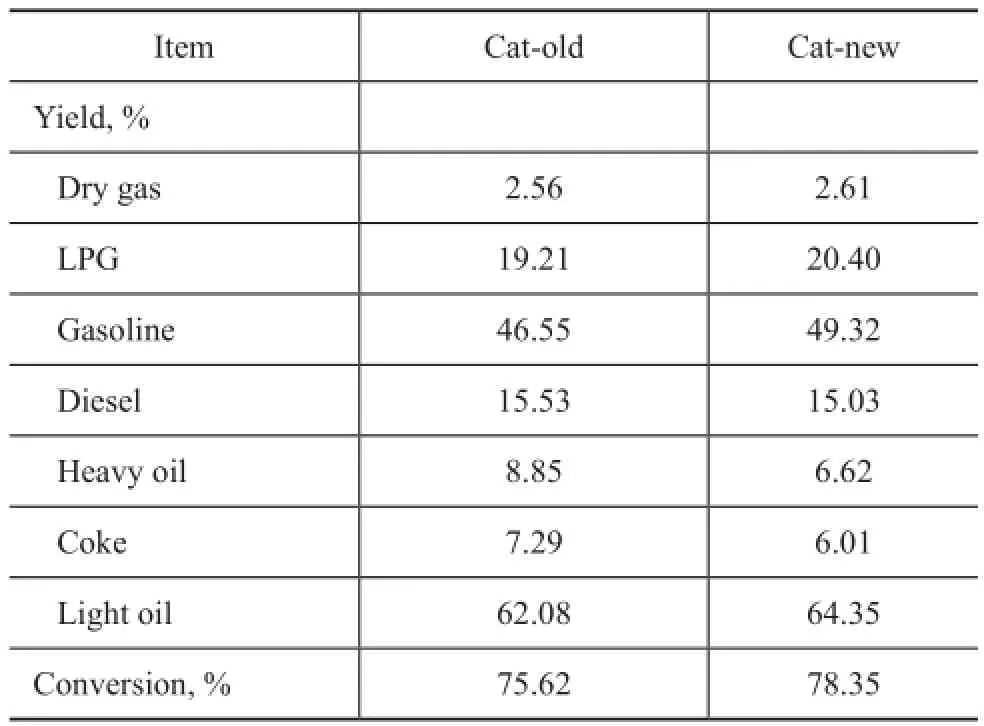
Table 3 Residue cracking data for different catalysts
4 Conclusions
The uniform core-shell composite consisting of the Y zeolite core and mesoporous alumina shell was prepared, and employed as the active component for preparing catalytic cracking catalyst. The physicochemical properties of the newly prepared catalyst were greatly improved by the obtained composite characteristic of high surface area, large pore volume and outstanding acid sites accessibility. As a result, the corresponding catalyst showed excellent performance for heavy oil cracking. In comparison with the reference catalyst, the heavy oil conversion of the newly prepared catalyst increased by 2.73 percentage points, while the heavy oil yield and coke yield decreased by 2.23 percentage points and 1.28 percentage points, respectively, with the light oil yield increasing by 2.27 percentage points.
Acknowledgements: This work was financially supported by the Department of Science and Technology Management of PetroChina (No. 2011B-2404-0102).
[1] Corma A, Martinez A, Martinez-Soria V, et al. Hydrocracking of vacuum gasoil on the novel mesoporous MCM-41 aluminosilicate catalyst [J]. J Catal, 1995, 153(1): 25-31
[2] Yilmaz B, Muller U. Catalytic applications of zeolites in chemical industry [J]. Top Catal, 2009, 52(11): 888-895
[3] Hosseinpour N, Mortazavi A, Bazyari A, et al. Synergetic effects of Y-zeolite and amorphous silica-alumina as main FCC catalyst components on triisopropylbenzene cracking and coke formation [J]. Fuel Process Technol, 2009, 90(2): 171-179
[4] Qi Y P, Chen S L, Dong P, et al. Novel macroporous residua FCC catalysts [J]. J Fuel Chem Technol, 2006, 34(6): 685-689
[5] Li H, Yu J, Xu J, et al. Identification of key oil refining technologies for China National Petroleum Co. (CNPC) [J]. Energy Policy, 2007, 35(4): 2635-2647
[6] Maselli J, Peters A W. Preparation and properties of fluid cracking catalysts for residual oil conversion [J]. Catal Rev, 1984, 26(1): 525-554
[7] Qumi Y, Takahashi J, Takesshima K, et al. Realumination of zeolite Y under acidic conditions [J]. J Porous Mater, 2007, 14(4): 19-26
[8] Yu Z, Zheng A, Wang Q, et al. Insights into the dealumination of zeolite HY revealed by sensitivity-enhanced27Al DQ-MAS NMR spectroscopy at high field [J]. Angew Chem Int Ed, 2010, 49(46): 8657-8661
[9] Tomiyama T, Okumura K, Niwa M. Enhancement in cracking activity of USY zeolites treated with ammonium nitrate solution [J]. Chem Lett, 2011, 40(1): 49-51
[10] Qin Z, Shen B, Gao X, et al. Mesoporous Y zeolite with homogeneous aluminum distribution obtained by sequential desilication-dealumination and its performance in the catalytic cracking of cumene and 1,3,5-triisopropylbenzene [J]. J Catal, 2011, 278(2): 266-275
[11] Chandra Shekara BM, Jai Prakash B S, Bhat Y S. Dealumination of zeolite BEA under microwave irradiation [J]. ACS Catal, 2011, 1(3): 193-199
[12] de Jong K P, Zecevic J, Friedrich H, et al. Zeolite Y crystals with trimodal porosity as ideal hydrocracking catalysts [J]. Angew Chem Int Ed, 2010, 49(52): 10074-10078.
[13] Verboekend D, Vile G, Perez-Ramiez J. Hierarchical Y and USY zeolites designed by post-synthetic strategies [J]. Adv Funct Mater, 2012, 22(5): 916-928
[14] Li K, Valla J, Garcia-Martinez J. Realizing the commercial potential of hierarchical zeolites: New opportunities in catalytic cracking [J]. Chem Cat Chem, 2014, 6(1): 46-66
[15] Lu Y, Yin Y, Li Z, et al. Synthesis and self-assembly of Au@SiO2core-shell colloids [J]. Nano Lett, 2002, 2(7): 785-788
[16] Bouizi Y, Rouleau L, Valtchev V P. Factors controlling the formation of core-shell zeolite-zeolite composites [J]. Chem Mater, 2006, 18(20): 4959-4966
[17] Deng Y, Qi D, Deng C, et al. Superparamagnetic highmagnetization microspheres with an Fe3O4@SiO2core and perpendicularly aligned mesoporous SiO2shell for removal of microcystins [J]. J Am Chem Soc, 2008, 130(1): 28-29
[18] Xu L, Le Y J, Wu H H, et al. Core/shell-structured TS-1@ mesoporous silica-supported Au nanoparticles for selective epoxidation of propylene with H2and O2[J]. J Mater Chem, 2011, 21: 10852-10858
[19] Han Y, Pitukmanorom P, Zhao L, et al. Generalized synthesis of mesoporous shells on zeolite crystals [J]. Small, 2011, 7(3): 326-332
[20] Zhang Y H, Liu Y C, Li Y X. Synthesis and characteristics of Y-zeolite/MCM-48 biporous molecular sieve [J]. Appl Catal A, 2008, 345(1): 73-79
[21] Waller P, Shan Z, Marchese L, et al. Zeolite nanocrystals inside mesoporous TUD-1: A high-performance catalytic composite [J]. Chem Eur J, 2004, 10(20): 4970-4976
[22] Qian X F, Du J M, Li B, et al. Controllable fabrication of uniform core-shell structured zeolite@SBA-15 composites [J]. Chem Sci, 2011, 2: 2006-2016
[23] Yuan Q, Yin A X, Luo C, et al. Facile synthesis for ordered mesoporous γ-aluminas with high thermal stability [J]. J Am Chem Soc, 2008, 130(11): 3465-3472
[24] Yuan Q, Duan H H, Li L L, et al. Homogeneously dispersed ceria nanocatalyst stabilized with ordered mesoporous alumina [J]. Adv Mater, 2010, 22(13): 1475-1478
[25] Zhao D, Feng J, Huo Q, et al. Triblock copolymer synthesis of mesoporous silica with periodic 50 to 300 angstrom pores [J]. Science, 1998, 279(23): 548-552
[26] Sing K S W, Everett D H, Haul R A W, et al. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity [J].Pure Appl Chem, 1985, 57(4): 603-619
[27] Qin Z, Shen B, Yu Z, et al. A defect-based strategy for the preparation of mesoporous zeolite Y for high-performance catalytic cracking [J]. J Catal, 2013, 298: 102-111
[28] Jia L, Sun X, Ye X, et al. Core-shell composites of USY@ mesosilica: Synthesis and application in cracking heavy molecules with high liquid yield [J]. Micropor Mesopor Mater, 2013, 176: 16-24
[29] Thibault-Starzyk F, Stan I, Abello S, et al. Quanti fi cation of enhanced acid site accessibility in hierarchical zeolites -The accessibility index [J]. J Catal, 2009, 264(1): 11-14
[30] Thomas O, Guillaume C, Houalla M. Quantitative IR characterization of the acidity of various oxide catalysts [J]. Micropor Mesopor Mater, 2005, 82(1/2): 99-104
[31] Corma A, Fornes V, Forni L, et al. 2,6-Di-tert-butylpyridine as a probe molecule to measure external acidity of zeolites [J]. J Catal, 1998, 179(2): 451-458
[32] Xue Z, Zhang T, Ma J, et al. Accessibility and catalysis of acidic sites in hierarchical ZSM-5 prepared by silanization [J]. Micropor Mesopor Mater, 2012, 151: 271-276
[33] Zhang Baozhong, Liu Xiaopeng. Catalytic performance of MFI/MFI core-shell zeolites in benzene methylation[J]. China Petroleum Processing and Petrochemical Technology, 2014, 16(4): 94-99
[34] Liu Dongmei, Kong Feifei, Zhai Yuchun, et al. Secondary crystallization of Na2CO3-modified HZSM-5 zeolites with tetrapropylammonium hydroxide and their catalytic performance in thiophene alkylation reaction[J]. China Petroleum Processing and Petrochemical Technology, 2015, 17(3): 53-60
Received date: 2015-10-29; Accepted date: 2016-01-07.
Yuan Chengyuan, Telephone: +86-931-7981621; E-mail: yuanchengyuan@petrochina.com.cn.
杂志排行
中国炼油与石油化工的其它文章
- Experimental Study of UDS Solvents for Purifying Highly Sour Natural Gas at Industrial Side-stream Plant
- Effects of Silylation on Zn-IM5 and Its Catalytic Activity for Butane Aromatization
- Enhanced Performance of Denitrifying Sul fi de Removal Process by 1,2-Naphthoquinone-4-Sulphonate
- Investigation of Different Coke Samples Adhering to Cyclone Walls of a Commercial RFCC Reactor
- Numerical Study of Air Nozzles on Mild Combustion for Application to Forward Flow Furnace
- Highly Active and Stable Ni2P/SiO2Catalyst for Hydrogenation of C9Petroleum Resin
