Highly Active and Stable Ni2P/SiO2Catalyst for Hydrogenation of C9Petroleum Resin
2016-03-22
(Industrial Catalysis Institute of Zhejiang University of Technology, State Key Laboratory Breeding Base of Green Chemistry Synthesis Technology, Zhejiang University of Technology, Hangzhou 310014)
Highly Active and Stable Ni2P/SiO2Catalyst for Hydrogenation of C9Petroleum Resin
Jiang Lin; Feng Feng; Jiang Dahao; Guan Zhengyu; Li Xiaonian
(Industrial Catalysis Institute of Zhejiang University of Technology, State Key Laboratory Breeding Base of Green Chemistry Synthesis Technology, Zhejiang University of Technology, Hangzhou 310014)
Catalytic hydrogenation is an appropriate method for the improvement of C9petroleum resin (C9PR) quality. In this study, the Ni2P/SiO2(containing 10% of Ni) catalyst prepared by the temperature-programmed reduction (TPR) method was used for hydrogenation of C9petroleum resins. The effect of reaction conditions on catalytic performance was studied, and the results showed that the optimum reaction temperature, pressure and liquid hourly space velocity (LHSV) was 250 ℃, 6.0 MPa, and 1.0 h-1, respectively. The bromine numbers of hydrogenated products were maintained at low values (~250 mgBr/100g) within 300 h, showing the high activity and stability of Ni2P/SiO2catalyst. The fresh and spent catalysts were characterized by X-ray diffraction (XRD), BET surface area (BET) analysis, scanning electron microscopy (SEM), transmission electron microscopy (TEM), Fourier transform infrared (FTIR) pyridine adsorption, and X-ray photoelectron spectroscopy (XPS). Compared with the traditional sulfurated-NiW catalysts, Ni2P possessed globe-like structure instead of layered structure like the active phase of NiWS, thereof exposing more active sites, which were responsible for the high activity of Ni2P/SiO2catalyst. The stability of Ni2P/SiO2catalyst was probably attributed to its high sulfur tolerance, antisintering, anti-coking and carbon-resistance ability. These properties might be further ascribed to the special Ni-P-S surface phase, high thermal stability of Ni2P nanoparticles and weak surface acidity for the Ni2P/SiO2catalyst.
Ni2P/SiO2, C9petroleum resin, catalytic hydrogenation, high activity, excellent stability
1 Introduction
C9petroleum resin (C9PR) was produced by polymerization of C9fraction, which is the by-product obtained from the steam cracking unit. C9PR is widely used in hot-melt adhesives, paints, inks, rubber and other fi elds[1]. However, the raw C9PR normally has yellow or amber color, poor thermal stability, poor oxidation resistance and other shortcomings, because of the high content of unsaturated bonds as well as sulfur and halogen residues[2-3]. Catalytic hydrogenation is certainly the most widely applicable method for the improvement of C9PR quality[4-5]. Traditionally, supported Pd or Pt catalysts exhibited excellent activity, but these catalysts are usually deactivated quickly due to the poisoning of noble metal by S, Cl and other impurities remaining in the raw materials[6-7]. Supported Ni-W-S and Ni-Mo-S catalysts have high ability of anti-poisoning, however, it can easily give rise to carbon deposition and obtain products with low softening point because of the strong acidity of these catalysts[3,8]. Therefore, it is still required to develop the catalysts with high sulfur tolerance and weak acidity for C9PR hydrogenation.
In recent decades, metal phosphides as a new type of hydroprocessing catalysts have been extensively studied. It has been reported that the activity of common phosphides decreases in the following order: Ni2P>WP>MoP>CoP>Fe2P[9]. It has been found that Ni2P catalyst shows excellent activity for many hydroprocessing reactions, such as hydrodesulfurization (HDS), hydrodenitrogenation (HDN), hydrodechlorination, and deoxygenation[10-13]. Furthermore, the application of Ni2P in some special selective hydrogenation of organic compounds such as cinnamaldehyde, phenylacetylene and naphthalene containing a few S, Cl and other impurities were also reported[14-18]. These results showed that Ni2P exhibited high activity and high resistance to those toxicants.
Considering the excellent catalytic performance of Ni2P, we explored the application of Ni2P for the hydrogenation of C9PR in this paper.
2 Experimental
2.1 Catalyst preparation
The Ni2P/SiO2(containing 10% of Ni) catalyst was prepared by the temperature-programmed reduction (TPR) method[19]. Typically, Ni(NO3)2·6H2O (2.48 g) and (NH4)2HPO4(1.12 g) were dissolved in 5 mL of distilled water, respectively, and mixed together, then a few drops of nitric acid were added to dissolve the precipitate. Subsequently, 5 g of SiO2was impregnated with the above solution. Excess water was removed on a rotary evaporator until dryness. The impregnate was dried at 110 ℃ for 4 h and was subsequently calcined at 500 ℃ for 6 h in a muf fl e furnace. The reduction of the sample was carried out in a fi xed-bed reactor (WFSM-3060). Firstly, the reactor was purged with H2(≥99.999%) at room temperature for 1 h, and then the temperature was raised to 300 ℃ at a rate of 10 ℃/min, and subsequently at a rate of 2 ℃/min to 650 ℃, which was maintained for 3 h in H2fl ow at a rate of 50 mL/min. Finally, the sample was cooled down to room temperature and passivated in a fl ow of 0.5 vol% O2/N2stream for 3 h.
The procedures for preparation of NiWS/SiO2(containing 1.5% of NiO and 20% of WO3) catalysts were as follows[3]: At fi rst, NiW/SiO2catalysts were prepared by coimpregnation of SiO2with an aqueous solution of mixed Ni(NO3)2·6H2O and (NH4)6W12O39·H2O. After impregnation, the catalyst samples were dried at 110 ℃ for 5 h and then were calcined at 450 ℃ for 4 h in a muf fl e furnace. Subsequently, the NiW/SiO2catalyst was sul fi ded in situ with a 10% (v/v) H2S/H2mixture for 4 h at 400 ℃ to obtain NiWS/SiO2. Finally, the sample was cooled down to room temperature and passivated in a flow of 0.5 vol% O2/N2stream for 3 h.
2.2 Catalyst characterization
X-ray diffraction (XRD) patterns of both the fresh and spent samples were determined with an X′Pert diffractometer (PNAlytical Co.) equipped with a Cu Kα radiation source that was operated at 45 kV and 40 mA. The particle sizes of Ni2P were determined by a Tecnai G2 F30 S-Twin TEM at an operating voltage of 300 kV. The BET surface area and pore structure of catalysts were obtained on a Quantachrome NOVA 2100e instrument. Scanning electron microscopy (SEM) was obtained on a Hitachi S-4700 instrument at an operating voltage of 15 kV. Fourier transform infrared (FTIR) pyridine adsorption was determined with an FTS3000 infrared spectroscopy to detect the kind and number of the acid sites on the surface of catalysts. X-ray photoelectron spectroscopy (XPS) was performed with a Kratos AXIS Ultra DLD instrument. Al Kα radiation at an energy scale calibrated versus adventitious silicon (Si 2p peak at 103.4 eV) was used.
2.3 Activity test
The hydrogenation of C9PR solution (containing 15% of C9PR in cyclohexane) was carried out in a fi xed-bed reactor (WFSM-3060, with an inner diameter of 8 mm and a length of 260 mm). The raw C9PR was purchased from the Taiwan Beifa Xingye Co., Ltd., and the bromine number of the C9PR solution was about 3.67 gBr/100g. A total of 4 mL of passivated catalyst (20—40 mesh) was loaded in the reactor. The passivated Ni2P/SiO2and NiWS/SiO2catalysts were activated at 300 ℃ for 1.5 h in a H2fl ow (at a fl ow rate of 9.5 mL/min), respectively. After the activation, the temperature and H2pressure were adjusted to the desired values and C9PR solution was then fed into the reactor by a liquid pump. The ef fl uent from the reactor was separated in the gas-liquid separator, and then the liquid product was collected to determine the bromine number by a BR-1 bromine/bromine index detector (made in China Guorui). Bromine index electrolyte (1—1 000 mgBr/100 g) was used when the bromine number of product was lower than 1 000 mgBr/100 g for accuracy purpose, otherwise the bromine valence electrolyte (0—300 gBr/100 g) was adopted. All the bromine number in this paper was tested in solutions (15% of stocks or products in cyclohexane). Softening point of the hydrogenated products was determined by an automatic asphalt softening point tester (model SYD-2806F, made in China Changji).
3 Results and Discussion
3.1 Activity and stability of Ni2P/SiO2
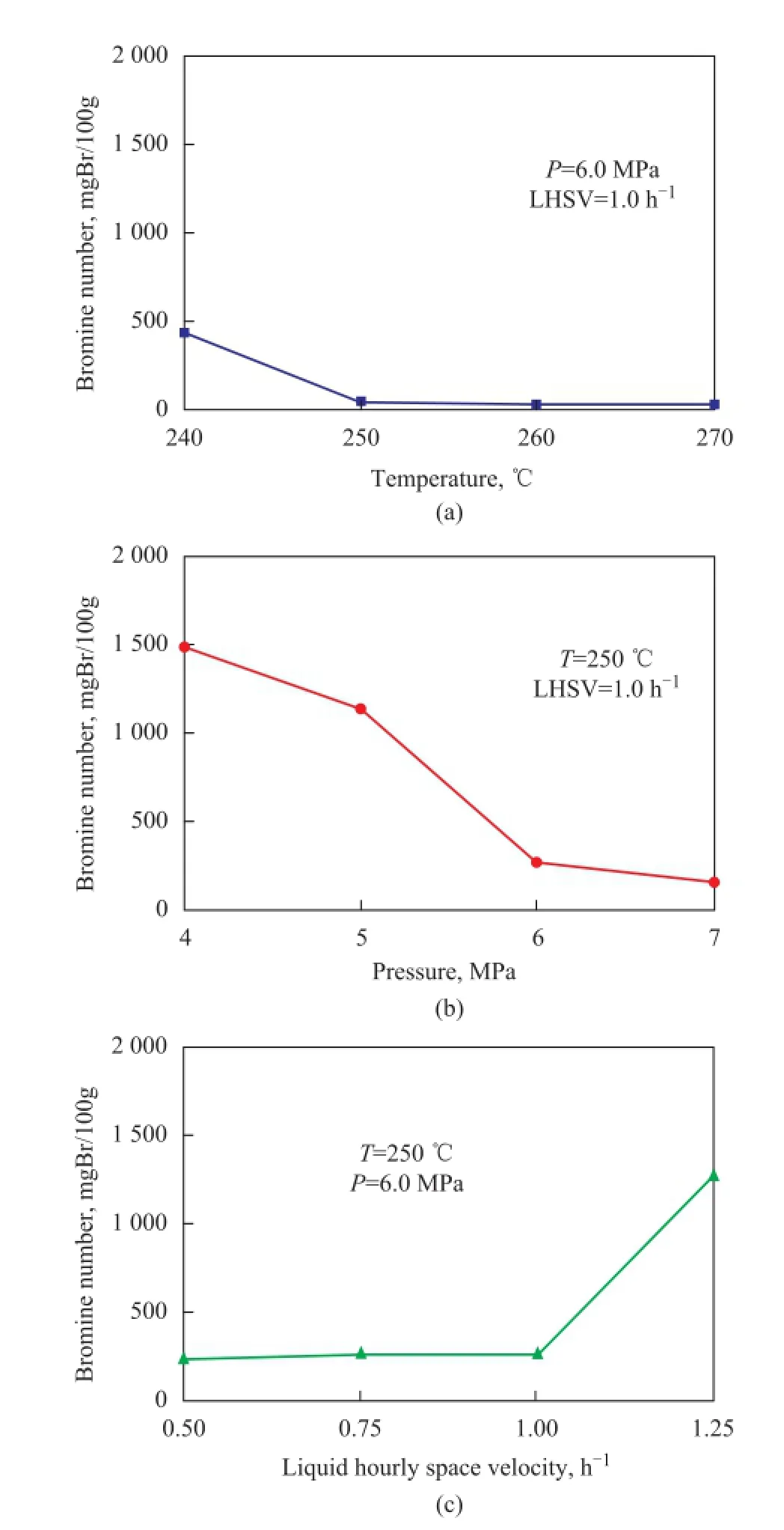
Figure 1 Effect of temperature (a), pressure (b), and liquid hourly space velocity (c) on the activity of Ni2P/SiO2for C9PR hydrogenation
Figure 1 presents the effect of reaction conditions on the performance of the Ni2P/SiO2catalyst for C9PR hydrogenation. As shown in Figure 1(a), the bromine number of product decreased as the reaction temperature increased. When the reaction temperature was higher than 250 ℃, the bromine number of products was all lower than 100 mgBr/100g. However, the petroleum resin will be degraded at higher temperature, which would lower the softening point of product. As shown in Figure 1(b), the bromine number of the product decreased dramatically as the hydrogen pressure increased from 4.0 MPa to 6.0 MPa. When the pressure was higher than 6.0 MPa, the decrease of bromine number was not obvious. The in fl uence of liquid hourly space velocity (LHSV) was also prominent (Figure 1(c)). When the LHSV was lower than 1.0 h-1, the bromine number of hydrogenated products could be maintained at low values (about 250 mgBr/100g), but the bromine number increased sharply when the LHSV was further increased. Therefore, the optimum conditions for C9PR hydrogenation over the Ni2P/SiO2catalyst covered a temperature of 250 ℃, a hydrogen pressure of 6.0 MPa and a LHSV of 1.0 h-1.
The results of stability test of Ni2P/SiO2are shown in Figure 2. It can be seen that the bromine number was maintained at around 250 mgBr/100g within 300 h. This result indicated that the Ni2P/SiO2catalyst not only exhibited high activity, but also exhibited excellent stability. Thus, the Ni2P/SiO2catalyst might have good prospects for the commercialization of C9PR hydrogenation process.
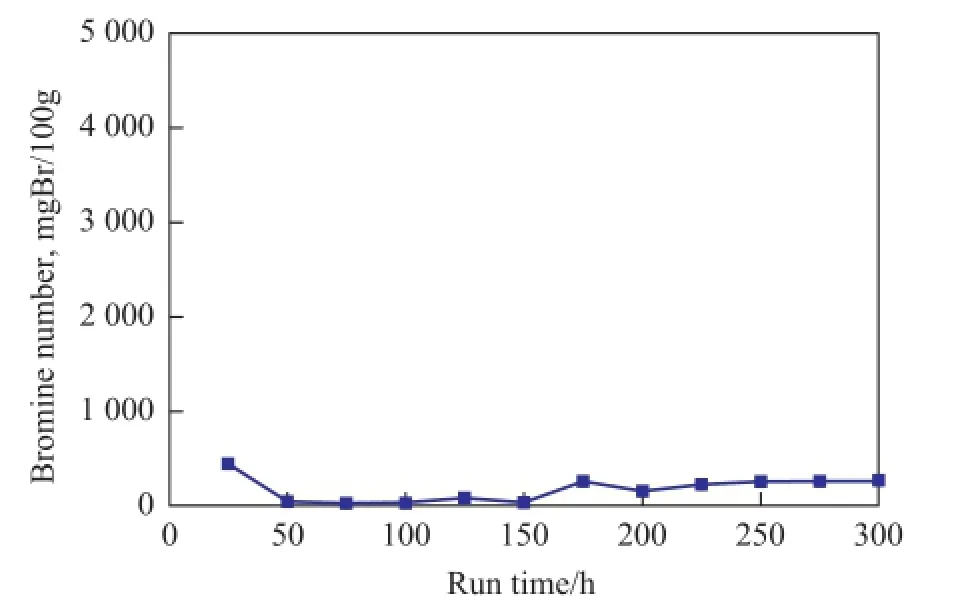
Figure 2 Stability of the Ni2P/SiO2catalyst for C9PR hydrogenation
3.2 Catalyst characterizations
In order to fi nd out the reasons for the excellent performance of the Ni2P/SiO2catalyst, the catalysts before and after reaction (300 h) were characterized by various tech-nologies. Figure 3 shows the wide-angle XRD patterns. The broad peak at around 22.5° is ascribed to amorphous silica. The diffraction peaks at 40.9°, 44.7°, 47.4°, and 54.6° are attributed to the (111), (201), (210) and (300) re fl ections of Ni2P crystalline phase[12]. It is notable that there is no other nickel phosphide phase such as Ni12P5in the prepared catalysts. The mean particle size of Ni2P in the fresh Ni2P/SiO2catalyst was 13.7 nm according to the Scherrer equation, while that on the spent Ni2P/SiO2catalyst was only 15.1 nm, which indicated that the Ni2P nanoparticles were rather stable during the reaction.
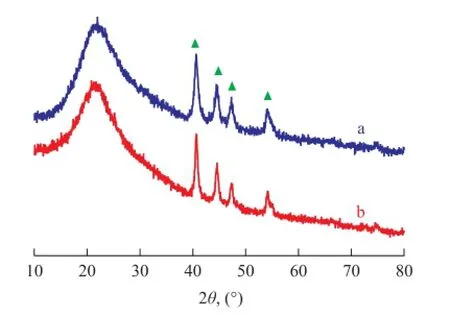
Figure 3 XRD patterns of fresh (a) and spent (b) Ni2P/SiO2catalyst
The textural properties of the SiO2and Ni2P/SiO2catalysts before and after reaction are listed in Table 1. The fresh Ni2P/SiO2catalyst underwent a slight loss in the BET surface area and pore volume due to the Ni2P loadings. In comparison, the loss of surface area and pore volume for spent Ni2P/SiO2was much larger. In view of the excellent stability and activity of Ni2P/SiO2catalyst during the reaction, the loss of surface area and pore volume could be attributed to the accumulation of the raw materials and products remaining in the pores of Ni2P/SiO2catalyst,which should not affect the performance of Ni2P/SiO2catalyst signi fi cantly.

Table 1 Textural properties of SiO2support and Ni2P/SiO2catalysts
Figure 4 shows the SEM images of fresh and spent Ni2P/SiO2catalysts. It can be found out that the particles of the fresh catalyst were spherical, which had a relatively uniform particle size and a clearly observed porosity. Compared with the fresh catalyst, no obvious changes in morphology and porosity were observed for the spent catalyst, indicating to a good mechanical property of the Ni2P/SiO2catalyst.
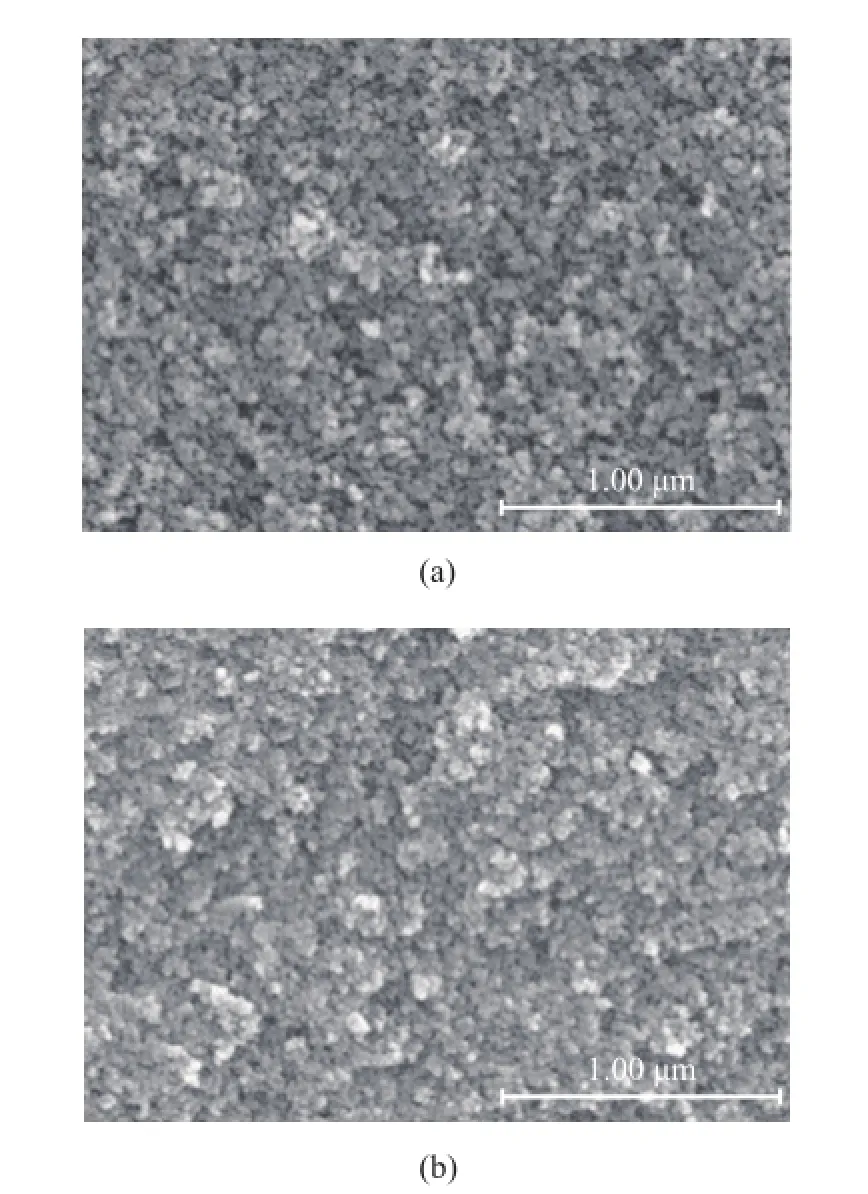
Figure 4 SEM images of (a) fresh and (b) spent Ni2P/SiO2catalysts
Figure 5 shows the TEM images and N2P particle size distribution of fresh (a, c) and spent (b, d) Ni2P/SiO2catalysts. It can be seen that the Ni2P nanoparticles adopted a globular morphology on the silica support. As for the fresh catalyst, the Ni2P nanoparticles were uniform although some aggregation were observed (Figure 5 (a) and (c)), which could be attributed to the high temperature adopted for the preparation of catalysts. In comparison, the mean particle size of Ni2P nanoparticles in spent catalyst (Figure 5 (b) and (d)) increased by only 2 nm, indicating that the Ni2P particles were fairly stable under our reaction conditions. In general, it can be concludedfrom TEM results that there was neither obvious sintering taking place nor change of crystalline phase for Ni2P nanoparticles. This result agreed well with the result of XRD analysis.

Figure 5 TEM images and Ni2P particle size distribution of fresh (a, c) and spent (b, d) Ni2P/SiO2catalysts
The XPS spectra in the Ni (2p) and P (2p) regions for fresh and spent Ni2P/SiO2catalysts are shown in Figure 6. For the fresh catalyst, the peaks at 856.4 eV and 133.9 eV are assigned to Ni2+and P5+species, respectively, in the passivation layer formed on the Ni2P particles during synthesis. The peaks at 853.0 eV and 129.1 eV are assigned to the reduced Ni and P species in Ni2P. These binding energies of Ni and P indicate that the Ni species in Ni2P bear a partially positive charge (Niδ+, 0<δ<2), while the P species in Ni2P bear a partially negative charge (Pδ−, 0<δ<1). Therefore, the XPS analysis indicates a slight transfer of electron density from Ni to P[20]. The above resultsare consistent with those reported in the references[20-22]. For the spent catalyst, the peaks at 853.0 eV and 856.4 eV for Niδ+and Ni2+, respectively, are detected, too. But the peaks of Niδ+(853.0 eV) and Pδ−(129.1 eV) become weaker, while the peaks of Ni2+(856.4 eV) and P5+(134.2 eV) tend to be stronger. These results come from the fact that the spent catalyst without passivation treatment was oxidized on the surface during the long time contact with air. By combining both the XPS and XRD results, it could be concluded that the active component Ni2P was stable under reaction conditions.
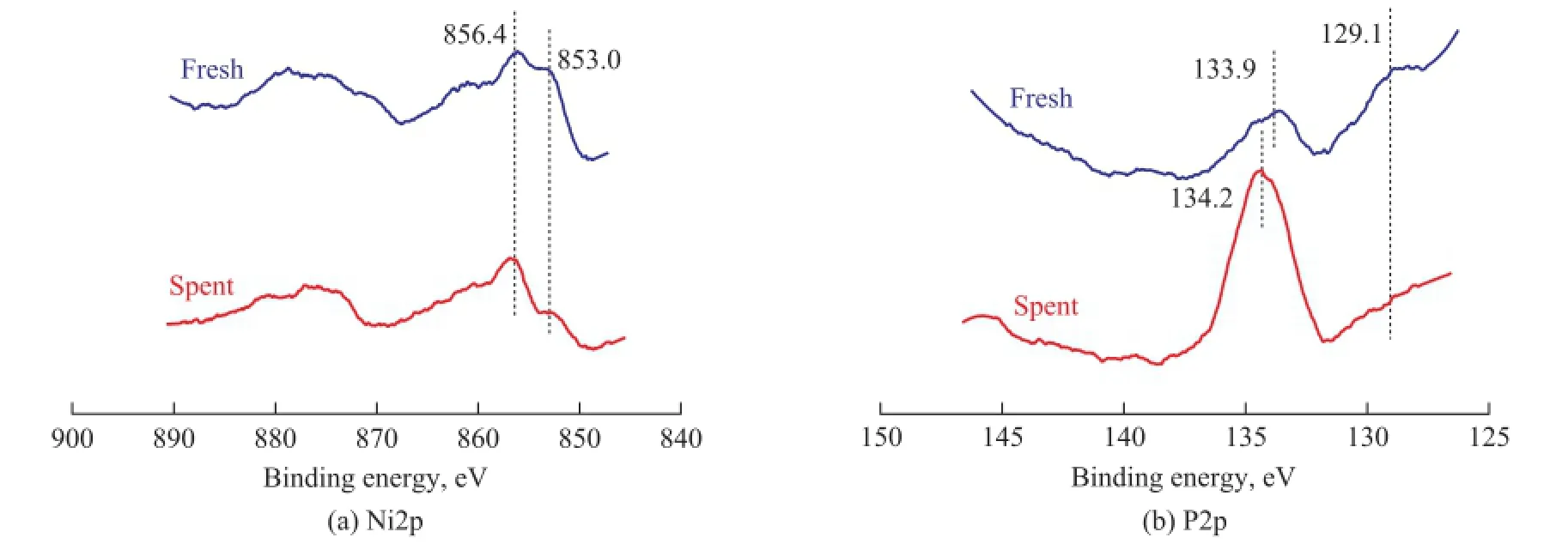
Figure 6 XPS spectra in the Ni2p and P2p regions for fresh and spent Ni2P/SiO2catalysts
3.3 Discussions
The purpose of catalytic hydrogenation of C9PR is to remove the C=C bonds, in order to improve the color, oxidation resistance and heat stability of the resin. The key to realize this purpose is to develop a catalyst with high activity and stability. Our experimental results indicated that Ni2P was a suitable catalyst to satisfy the above demand.
The XRD results showed that Ni2P was the only crystalline phase and the Ni2P nanoparticles were quite stable under reaction conditions. As we know, both phosphides and sul fi des have hydrogenation activity, and NiWS has been used as the traditional catalyst in the hydrogenation of petroleum resin. Therefore, the bromine number of hydrogenated products obtained over the Ni2P/SiO2and NiWS/SiO2catalysts was compared, as shown in Table 2. It can be found out that the average bromine number of the products over Ni2P/SiO2(244 mgBr/100g) was much lower than that of NiWS/SiO2(1193 mgBr/100g), showing the higher activity of Ni2P/SiO2catalysts.
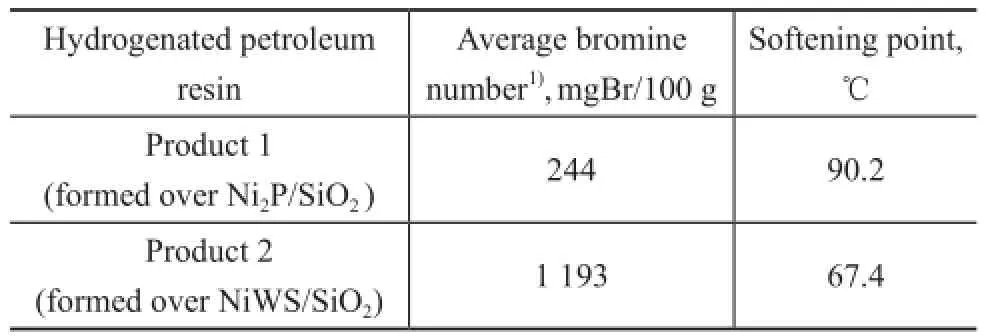
Table 2 Catalytic performance for hydrogenation of C9PR over Ni2P/SiO2and NiWS/SiO2catalysts
It is well known that for the sulfides a common trend is the adoption of layered structures, whereas the phosphides generally tend to form a globular shape. The consequence for catalytic activity can be substantial, because the phosphides potentially can expose a greater number of coordinatively unsaturated surface atoms, and hence can give rise to higher site density[23]. The coordinatively unsaturated surface atoms and high site density make the properties of Ni2P similar to those of noble metals. Thus, as it has been discussed above, the high activity of Ni2P/SiO2catalysts for the hydrogenation of C9PR in this study should be mostly attributed to the special crystal structure of Ni2P. High stability of the N2P/SiO2catalyst could be ascribed to its high sulfur tolerance, anti-sintering, anti-coking and carbon-resistance abilities. Actually, the high sulfur resistance of Ni2P is still not very clear. Most of the research believe that the catalyst underwent a surface phase change, which resulted in the formation of a phosphosulfi de layer on the top of the Ni2P phase, and Ni-P-S might be the real activity sites[24-25]. Meanwhile, it was suggested that the amount of S species incorporation into Ni2P phase was much fewer than the case with other NixPyphases, such as Ni12P5[26]. And others suggested that the incorporation of S into Ni2P was labile and could be easily removed as H2S through hydrogenation, regenerating Ni sites[10]. Therefore, Ni2P catalyst showed superior tolerance towards potential catalyst poisons, such as sulfur[17,25-26]. Judging from the results of XRD and TEM analyses, it was noted that Ni2P nanoparticles were quite stable under reaction conditions, showing the excellent anti-sintering ability.
In addition, the surface acidity of the Ni2P/SiO2and NiWS/SiO2catalysts was compared by FTIR pyridine adsorption. As shown in Figure 7, pyridine molecules bonded to the Lewis acid sites were absorbed at 1 604 cm−1and 1 444 cm−1, whereas those responsible for the Brönsted acid sites (pyridinium ion) showed absorbance at 1 534 cm−1and 1 636 cm−1. The band at 1 485 cm−1is a combined band originating from pyridine bonded to both the Brönsted and the Lewis acid sites[27-29]. The spectra of NiWS/SiO2showed the sharp pyridine absorption bands at 1 444 cm−1, 1 485 cm−1, and 1 604 cm−1, and the weak pyridine absorption bands at 1 534 cm−1, and 1 636 cm−1, showing that the NiWS/SiO2catalyst owned a number of Lewis acid sites and a few Brön-sted acid sites. The strong acidity of NiWS was ascribed to the unsaturated coordination of W atoms. However, the pyridine absorption bands at 1 444 cm−1, 1 485 cm−1, and 1 604 cm−1, and those at 1 534 cm−1and 1 636 cm−1for Ni2P/SiO2catalyst were both weak, showing that the number of the Lewis acid sites and the Brönsted acid sites on the Ni2P/SiO2catalyst was both few. Thus, it could be concluded that the acidity of Ni2P/SiO2was much weaker than that of NiWS/SiO2. The weak surface acidity can effectively prevent the occurrence of coking and carbon deposition over the Ni2P/SiO2catalyst to further improve its stability[30-31].
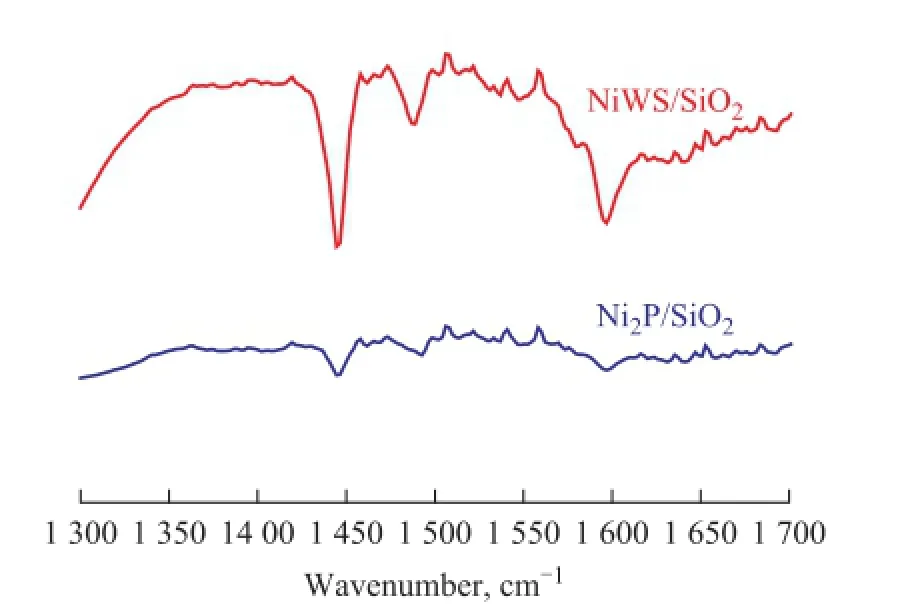
Figure 7 FTIR pyridine adsorption spectra of Ni2P/SiO2and NiWS/SiO2catalyst
At the same time, the softening point of the hydrogenated products formed over the Ni2P/SiO2and NiWS/SiO2catalysts are compared in Table 2. It can be seen that the softening point of hydrogenated products over the NiWS/SiO2catalyst was much lower than that obtained over the Ni2P/SiO2catalyst. The hydrogenated petroleum resin with low softening point could be obtained over the NiWS catalyst because of its strong cracking activity resulted from its strong acidity[3,8], while the lower acidity of Ni2P species could avoid the production of cracking products as far as possible. Therefore, the high activity was mainly resulted from the great number of coordinatively unsaturated surface atoms of Ni2P. And the high stability was mainly ascribed to its superior sulfur tolerance, high anti-sintering, anti-coking and carbon-resistance abilities of Ni2P. And the high quality of product (in terms of less cracking products) on Ni2P/SiO2should be attributed to the less acidity of Ni2P catalysts.
4 Conclusions
In conclusion, the Ni2P/SiO2catalyst was quite active and stable for the hydrogenation of C9petroleum resin. The special globe-like structure, high sulfur resistance, anti-sintering, anti-coking and carbon-resistance of Ni2P were accountable for its high activity and stability. The Ni2P/SiO2catalyst might have a potential application in commercial process of C9PR hydrogenation.
Acknowledgements: This work was financially supported by the Scienti fi c Research Fund of Zhejiang Provincial Education Department (Y201225114) and the Natural Science Foundation of Zhejiang Province (LY13B030006).
[1] Chai Zhongyi. Progress in hydrogenation technology for C9petroleum resin[J]. China Synthetic Resin and Plastics, 2009, 26(6): 71-74 (in Chinese)
[2] Yu Lujun, Jiang Dahao, Xu Jiao, et al. Color cause and hydrogenation modification of C9petroleum resin[J]. Petrochemical Technology, 2012, 41(10): 1181-1185 (in Chinese)
[3] Yu Lujun, Jiang Dahao, Xu Jiao, et al. Two-stage hydrogenation modification of C9petroleum resin over NiWS/ γ-Al2O3and PdRu/γ-Al2O3catalysts in series[J]. China Petroleum Processing & Petrochemical Technology, 2012, 14(3): 83-89
[4] Li Jianzhou, Cao Yaoqiang, Tan Wenxing. Research and development of hydrogenation catalysts for C5petroleum resins[J]. Petrochemical Industry Application, 2008, 27(3): 25-37 (in Chinese)
[5] Zhang Qiang, Li Changbo, Zhang Honglin. Investigation of piperylene C5petroleum resin hydrogenation upgrading process[J]. Applied Chemical Industry, 2009, 38(12): 1724-1726 (in Chinese)
[6] Yamakawa F, Kitamura T, Chinda T. Catalyst for petroleum resin hydrogenation and process for producing hydrogenated petroleum resin: The United States, US 0228143A1[P]. 2005
[7] Guan Zhengyu, Feng Feng, Xu Jiao, et al. Pd/Al2O3catalyst for hydrogenation modification of C5petroleum resin and its deactivation reason[J]. China Adhesives, 2014, 23(10): 13-17 (in Chinese)
[8] Xu Jiao, Feng Feng, Jiang Dahao, et al. Research progressof hydrogenation process and catalyst for C5petroleum resin[J]. China Adhesives, 2012, 21(6): 53-58 (in Chinese)
[9] Zhao H, Oyama S T, Freund H J, et al. Nature of active sites in Ni2P hydrotreating catalysts as probed by iron substitution[J]. Appl Catal B: Environ, 2015, 164: 204-216
[10] Oyama S T, Wang X, Lee Y K, et al. Active phase of Ni2P/SiO2in hydroprocessing reactions[J]. J Catal, 2004, 221(2): 263-273
[11] Chen J, Sun L, Wang R, et al. Hydrodechlorination of chlorobenzene over Ni2P/SiO2catalysts: in fl uence of Ni2P loading[J]. Catal Lett, 2009, 133(3): 346-353
[12] Yang Y, Chen J, Shi H. Deoxygenation of methyl laurate as a model compound to hydrocarbons on Ni2P/SiO2, Ni2P/MCM-41, and Ni2P/SBA-15 catalysts with different dispersions[J]. Energy Fuels, 2013, 27(6): 3400-3409
[13] Feng L, Vrubel H, Bensimon M, et al. Easily-prepared dinickel phosphide (Ni2P) nanoparticles as an efficient and robust electrocatalyst for hydrogen evolution[J]. Phys Chem Chem Phys, 2014,16: 5917-5921
[14] Li X, Zhang Y, Wang A, et al. In fl uence of TiO2and CeO2on the hydrogenation activity of bulk Ni2P[J]. Catal Commun, 2010, 11(14): 1129-1132
[15] Zhang X, Zhang Q, Guan J, et al. Hydrogenation of naphthalene on nickel phosphide supported on silica[J]. Asia-Pac J Chem Eng, 2009, 4(5): 574-580
[16] Wang H, Shu Y, Zheng M, et al. Selective hydrogenation of cinnamaldehyde to hydrocinnamaldehyde over SiO2supported nickel phosphide catalysts[J]. Catal Lett, 2008, 124(3): 219-225
[17] Ruan M, Guan J, He D, et al. The hydrogenation of aromatic-naphthalene with Ni2P/CNTs[J]. RSC Adv, 2015, 5: 57700-57703
[18] Carenco S, Leyva-Pérez A, Concepción P, et al. Nickel phosphide nanocatalysts for the chemoselective hydrogenation of alkynes[J]. Nano Today, 2012, 7(1): 21-28
[19] Moon J S, Kim E G, Lee Y K. Active sites of Ni2P/SiO2catalyst for hydrodeoxygenation of guaiacol: A joint XAFS and DFT study[J]. J Catal, 2014, 311: 144-152
[20] Sawhill S J, Phillips D C, Bussell M E. Thiophene hydrodesulfurization over supported nickel phosphide catalysts[J]. J Catal, 2003, 215(2): 208-219
[21] Song L, Zhang S, Wu X, et al. A novel synthesis of Ni2P catalysts by reducing nickel sul fi de at low temperature[J]. Vacuum, 2015, 111: 68-72
[22] Sawhill S J, Layman K A, Van Wyk D R, et al. Thiophene hydrodesulfurization over nickel phosphide catalysts: effect of the precursor composition and support[J]. J Catal, 2005, 231(2): 300-313
[23] Oyama S T. Novel catalysts for advanced hydroprocessing: transition metal phosphides[J]. J Catal, 2003, 216(1/2): 343-352
[24] Layman K A, Bussell M E. Infrared spectroscopic investigation of CO adsorption on silica-supported nickel phosphide catalysts[J]. J Phys Chem B, 2004, 108(30): 10930-10941
[25] Oyama S T, Wang X, Lee Y K, et al. Effect of phosphorus content in nickel phosphide catalysts studied by XAFS and other techniques[J]. J Catal, 2002, 210(1): 207-217
[26] Sawhill S J, Layman K A, Van Wyk D R, et al. Thiophene hydrodesulfurization over nickel phosphide catalysts: effect of the precursor composition and support[J]. J Catal, 2005, 231(2): 300-313
[27] Busca G. Spectroscopic characterization of the acid properties of metal oxide catalysts[J]. Catal Today, 1998, 41: 191-206
[28] Liang Liping, Zhu Qing, Zhao Yongxiang, et al. Effects of heat treatment on the structure and properties of the tungstophosphoric acid-modified zirconia aerogel solid acids[J]. Acta Phys Chim Sin, 2011, 27(8): 1933-1940 (in Chinese)
[29] Devassy B M, Lefebvre F, Halligudi S B. Zirconia-supported 12-tungstophosphoric acid as a solid catalyst for the synthesis of linear alkyl benzenes[J]. J Catal, 2005, 231(1): 1-10
[30] Shen Zhihong, Fu Yumei, Jiang Ming, et al. Effect of chemical modification on hydrogen transfer activity of cracking catalyst[J]. Chinese Journal of Catalysis, 2004, 25(3): 227-230
[31] Liu Lihua, Liu Shuqun. Ni2P-MoS2/γ-Al2O3catalyst for deep hydrodesulfurization via the hydrogenation reaction pathway[J]. China Petroleum Processing and Petrochemical Technology, 2014, 16(4): 12-18
Received date: 2015-09-28; Accepted date: 2015-12-12.
Dr. Feng Feng, Telephone: +86-13958141001; E-mail: ffeng@zjut.edu.cn.
杂志排行
中国炼油与石油化工的其它文章
- Experimental Study of UDS Solvents for Purifying Highly Sour Natural Gas at Industrial Side-stream Plant
- Effects of Silylation on Zn-IM5 and Its Catalytic Activity for Butane Aromatization
- Enhanced Performance of Denitrifying Sul fi de Removal Process by 1,2-Naphthoquinone-4-Sulphonate
- Investigation of Different Coke Samples Adhering to Cyclone Walls of a Commercial RFCC Reactor
- Numerical Study of Air Nozzles on Mild Combustion for Application to Forward Flow Furnace
- Preparation of Core-Shell Composite of Y@Mesoporous Alumina and Its Application in Heavy Oil Cracking
