Investigation of Different Coke Samples Adhering to Cyclone Walls of a Commercial RFCC Reactor
2016-03-22
(State Key Laboratory of Heavy Oil Processing, China University of Petroleum, Beijing 102249)
Investigation of Different Coke Samples Adhering to Cyclone Walls of a Commercial RFCC Reactor
Li Xiaoman; Song Jianfei; Sun Guogang; Yan Chaoyu; Wei Yaodong
(State Key Laboratory of Heavy Oil Processing, China University of Petroleum, Beijing 102249)
The microstructure and properties of the coke samples collected from 4 different wall regions of the cyclone in the reactor of a residue fl uid catalytic cracking unit (RFCCU) were analyzed by using the scanning-electron microscope (SEM), and the possible coke formation processes were investigated as well. The results showed that some of the heavy nonvolatile oil droplets entrained in the fl owing oil and gas mixture could possibly deposit or collide on the walls by gravity settling or turbulence diffusion, and then were gradually carbonized into solid coke by condensing and polymerization along with dehydrogenation. Meanwhile some of fi ne catalyst particles also built up and integrated into the solid coke. The coke can be classi fi ed into two types, namely, the hard coke and the soft coke, according to its property, composition and microstructure. The soft coke is formed in the oil and gas mixture’s stagnant region where the oil droplets and catalyst particles are freely settled on the wall. The soft coke appears to be loose and contains lots of large catalyst particles. However, the hard coke is formed in the oil and gas mixture’s fl owing region where the oil droplets and catalyst particles diffuse towards the wall. This kind of coke is nonporous and very hard, which contains a few fi ne catalyst particles. Therefore, it is clear that the oil and gas mixture not only carries the oil droplets and catalyst particles, but also has the effects on their deposition on the wall, which can in fl uence the composition and characteristics of deposited coke.
FCC reactor; cyclone; coke formation; microstructure; characteristics
1 Introduction
Over the past decades, the residue fl uid catalytic cracking (RFCC) process has rapidly evolved as a major process for upgrading heavier oil in China[1]. Because the quality of heavy fractions in the feedstock are becoming higher and higher, the amount of coke formed in the reactor also increases severely. However some coke species are deposited on the wall of the reactor instead of being conveyed to the regenerator for burning. This part of deposited coke has a potential harmful effect on the long cycle operation of RFCC unit. In some cases, the deposited coke could be detached from the wall and would plug up the catalyst particle fl ow area, such as the stripper or cyclone diplegs, which would result in a sudden and extreme loss of the catalyst inventory. As a result, the RFCC unit has to be unexpectedly shut down[2-4]. Minimizing the effect of deposited coke on the operation of RFCC unit has been a major challenge in achieving better economical benefits and a long cycle run.
The process of coke formation involves the complicated chemical reactions and physical variations. Many researches have been carried out to understand the coking formation process based on their sample analysis[4-9]and laboratoryscale studies[10-12]. Unconverted heavy oil droplets of feedstock and the cracked heavy products are formed in the reactor. This type of heavy oil, the boiling point of which is higher than the reactor temperature, covers the surface of catalysts or travels independently as droplets. When these oil droplets in combination with the catalyst particles are deposited on the wall, they are gradually carbonized into solid coke by condensing and polymerization coupled with dehydrogenation. Actually, in addition to chemical reactions, there are many factors in fl uencing the coke formation, such as the operating temperature, the residence time, the catalyst concentration, the oil and gas mixture fl ow pattern, etc[4,6,13]. Among these factors, the oil and gas mixture fl ow pattern plays an important role in the formation and build-up of the coke block[14-15].
The cyclone is an important device in the RFCC reactor for separating oil and gas mixture and catalyst particles. The coke block formed on the cyclone wall is one of the common causes of an unscheduled shutdown of RFCC unit. In this study, the coke samples collected from 4 different wall regions of the cyclone in a commercial RFCC reactor were analyzed. The characteristics of coke sample were investigated based on the macro- and micro-morphology. Furthermore, the effect of oil and gas mixture’s fl ow pattern on the coke property was discussed to provide an understanding of the mechanism of coke formation on the cyclone wall.
2 Experimental
2.1 Preparation of coke samples
The RFCC unit, from which the deposited coke samples in this study have been acquired, boasts an annual processing capacity of 1.4 Mt/a. The RFCC unit mainly consists of a riser, a reactor, and a regenerator. The schematic diagram of the reactor is shown in Figure 1. The riser utilizes a close-coupled cyclone system to separate catalyst particles from cracked products, which has 3 sets of parallel two-stage cyclones directly connected to the riser. The oil and gas mixture’s outlet of the primary cyclone is closely coupled to the inlet of the secondary cyclone. There is a gap between the primary cyclone outlet and the secondary inlet to allow stripper vapors to enter the secondary cyclones. The reactor operates at a pressure of 0.25 MPa (a) and a temperature of 492 ℃ at the outlet of the riser. The diameter of the reactor is 6.4 m. The barrel of the primary cyclone has a diameter of 1.392 m, while the barrel of the secondary cyclone has a diameter of 1.344 m. The outer diameter of the exit tube in the secondary cyclone is 0.47 m, with its penetrated length equating to 0.75 m.
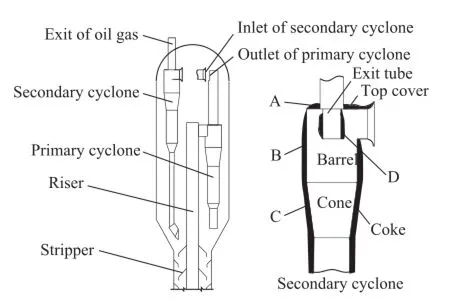
Figure 1 Coke sampling locations on the cyclone in RFCC reactor
The deposited coke was found not only on the outer walls of the primary cyclone and secondary cyclone, but also on the outer wall of the exit tube of the secondary cyclone when the cyclones were subject to inspection after the scheduled shutdown of the RFCC unit, as shown in Figure 1 and Figure 2. The coke samples used in this work were carefully collected from the wall of the secondary cyclone, with 4 different locations labeled in Figure 1 and Figure 2. In these pictures A is the top cover, B is the wallof the barrel, C is the wall of the cone, and D is the wall of the exit tube.

Figure 2 Photos of the coke deposited on the cyclone wall
2.2 Analyses of coke samples
Scanning electron microscope (SEM) and elemental analysis were used to characterize the morphology and composition of the coke samples, respectively. The SEM (model S-360, Cambridge Instrument Company) was equipped with an energy dispersive spectrometer (EDS) to obtain elemental composition of the surface. To examine the residual catalyst presented in the coke, coke samples were placed in a muf fl e oven at a temperature of 650 ℃ to burn off the carbonaceous materials. Then a Multi-sizer Coulter Counter instrument was used to analyze the catalyst particle size distribution.
3 Results and Discussion
3.1 Macro- and micro-morphology of the deposited coke
According to the macrograph and property of the coke samples on the wall of the cyclone, the deposited coke can be classi fi ed into two typical kinds: the soft coke and the hard coke. The soft coke appears mostly to be light gray, and its surface is not smooth. The soft coke block is loose and can be easily crushed. These soft coke samples were taken from the stagnant regions, where the oil and gas mixture could be retained, such as the outer walls of the cyclone, the top cover of the cyclone and the walls of the reactor, where the fl ow velocity of oil and gas mixture is very low. However, the hard coke has a dark–black color, and its surface is smooth and has striations caused by the movement of oil and gas mixture. The hard coke block has a compact structure, and gives a metal sound upon being percussed. The hard coke samples were taken from the region where the oil and gas mixture fl owed at a high velocity, such as the outer wall of the exit tube of the cyclone, the inner wall of the oil and gas mixture transfer pipelines, etc. However, a larger quantity of coke from the reactor was detected in zones where an intermediate velocity between 0.1~1.5 m/s dominated, and this kind of coke had properties similar to those between the soft coke and hard coke, since it was neither loose nor dense and hard in its structure.
The SEM micrographs of the surface of the coke samples are shown in Figure 3. Figure 3(a) shows the micrographs of the coke sample collected at the location A. The coke consisted of spherical particles about 20—40 μm in diameter. The EDS spectral analysis shows that the spherical particles mainly consisted of aluminum and silicon, suggesting that the spherical particles were catalyst, whereas the main composition of the cover layer was carbon. So the spherical particles comprising catalyst particles were surrounded by the carbonaceous matter. These particles were randomly piled up to form a massive bulk matter. The coke layer seemed to have a role of bridging between the wall and catalyst particles or between the catalyst particles together, making the particles stuck together. As a result, there were a lot of random holes and voids. Figure 3(b) gives a micrograph of the coke sample collected at the location B. The coke sample B contained less catalyst particles and more coke in comparison with the coke sample A. The particle diameter in coke sample B was also smaller than that in coke sample A. The particles aggregated together in the form of clusters. The micrographs of the coke sample collected at the location C, as shown in Figure 3(c), are similar to those in Figure 3(a). Figure 3(d) provides micrographs of the coke sample collected at the location D. The coke sample contained mainly coke and a few small catalyst particles. In some area, the fi ne catalyst particles and coke merged together to form a massive bulk matter without porosity. In other area, the fi ne catalyst particles and coke spherules adhered together with a few voids. The coke spherules with smooth surface originated from the solidi fi ed oil droplets and adhered to each other in a grape-like form.
3.2 Elemental analysis of deposited coke
The elemental composition and H/C atomic ratio of coke samples from positions A, B and D are given in Table 1, which demonstrates that carbon and hydrogen are the major organic components along with small amounts of sulfur and nitrogen. On an ash-free basis, the carbon content reached more than 75 %. The H/C atomic ratio ranged from 0.23 to 0.33. These results indicated that the coke fraction was carbonized severely and its degree of chemical condensation was very high. On the other hand, the ash was the remainder of the deposited coke after the carbonaceous material was burned off in a muf fl e oven,which comprised primarily the catalyst powder, with its mass fraction ranging from 34.4% to 72.5%. The result suggests that the ratio of catalyst to coke could affect the coke hardness. As the content of the catalyst decreased, the coke hardness did increase. In fact, judging from the different contents of catalyst, a considerable part of coke was considered to be an intermediate between the soft coke and hard coke, or a mixture of soft coke and hard coke.
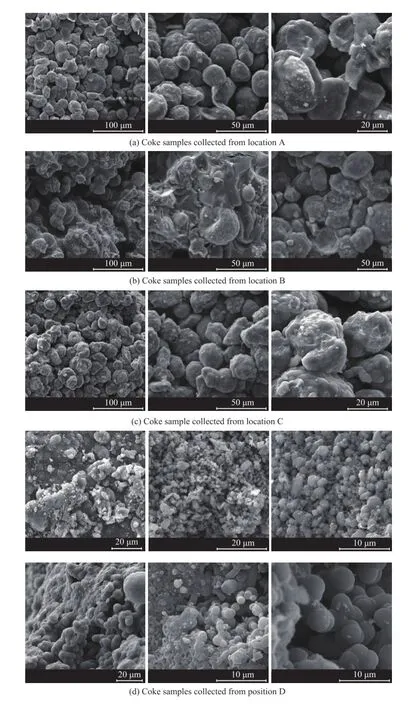
Figure 3 SEM micrographs of coke samples

Table 1 Elemental composition and H/C ratio of coke
3.3 Size analysis of catalyst particle in the coke sample
The particle size distribution of catalyst trapped in the deposited coke varied with its locations. In the oil and gas mixture’s stagnant region, more large particles were identi fi ed in the coke samples, as shown in Figure 4. The average particle diameter of coke samples A and B was about 20 μm and 32 μm, respectively. This size distribution could well match with that of catalyst entering the secondary cyclone from the primary cyclone featuring a more than 99% efficiency for separation of the catalyst with particle size in the range of between 10 μm and 120 μm (with an average size of 65 μm). Hence in the oil and gas mixture’s high velocity region, the particles were very tiny and the dominating particles were less than 10 µm in diameter. Figure 5 shows the micrographs of catalyst particles in the coke samples collected at the locations A and D after the carbonaceous matter was burned off. A lot of these samples were the fine fragments of the broken catalyst particles.

Figure 4 Particle size distribution of catalyst in the coke sample

Figure 5 SEM micrographs of particles after carbonaceous matter was burned off
3.4 Effect of fl ow pattern on the coke formation
Figure 6 indicates the basic process of the coking formation. In the reactor, if the boiling point of heavy oil is higher than the reactor temperature, the heavy hydrocarbon oil could be converted into oil mists and further be aggregated to droplets by coalescence. These heavy nonvolatile oil droplets are entrained by the flowing oil and gas mixture and catalyst particles. However, some of them possibly may deposit or collide on the wall surface via gravity settlement or turbulence diffusion. Meanwhile some of fi ne catalyst particles carried by the fl owing oil and gas mixture may deposit on the wall and can integrateinto a liquid fi lm formed by oil droplets. These deposited droplets are gradually carbonized to form the solid coke through condensing and polymerization coupled with dehydrogenation.

Figure 6 Diagram showing the formation of deposited coke
Many factors in fl uence the process of the coke formation, such as the operating temperature, the residence time, the feedstock properties, the catalyst performance, etc. However, the gas flow state is closely related to the process of the coke formation. The gas fl ow not only carries the oil droplets and catalyst particles, but also determines the form of the oil droplets depositing on the wall, which can affect the characterization and structure of the coke.
The reason why the catalyst particles and oil droplets are deposited on the wall surface during the movement of oil and gas fl ow may be ascribed to the effect of the gravity, the diffusion and the fl uid drag force. So, different fl ow patterns can result in different deposition types. Figure 7 describes the forms of the oil droplets and catalyst particles deposited on the cyclone wall. In the reactor, there are many regions where the gas stream is in a static state or moves at a relatively low speed, as it can be found at the top cover of the cyclone, at the inner wall of the reactor and at the outer wall of the cyclone. A lot of large catalyst particles and a few oil droplets may possibly accumulate on the wall resulted from free settlement and collision, which can form the accumulated coke after a period of aging. The accumulated coke is the soft coke, since the catalyst particle diameter is larger with more internal gaps and holes, as shown in Figure 7 (a, b). The size of accumulated coke can increase continuously if there is enough space. As a result, the accumulated coke can form a large size of coke block. In the internal space of cyclone, the oil and gas mixture is fl owing in a state of high-velocity swirling. But in the boundary layer on the outer wall of exit tube of cyclone, the concentration of catalyst particles and oil droplets is very low due to the centrifugal separation action. There are some fi ne catalyst particles and oil droplets that could possibly collide on the wall under the action of strong turbulent diffusion. When the droplets are deposited on the wall, they would merge together and form a liquid film to adhere to and encapsulate the catalyst particles. During the coking reaction, a coke layer is formed and fi xed on the wall surface to form a deposited coke. Then the catalyst particles and oil droplets are subsequently deposited on the surface of the coke layer, as shown in Figure 7 (c), so that the thickness of coke layer increases. When the coke layer reaches a certain thickness, the fl ow channel area outside of the exit tube reduces so that the flow velocity of oil and gas mixture increases. Therefore, the catalyst particles and droplets cannot be easily deposited on the surface of coke layer, which further restrains the growth of the coke layer. The deposited coke belongs to the hard coke as the catalyst particles are very tiny with less internal gaps and holes.
In areas with a relatively high catalyst concentration, such as the inner wall of the cyclone, the fl ow of catalyst particles scours the wall surface severely. No any deposition of oil droplets and catalyst particles would take place. Under this condition, the erosion problem may occur andmust be considered carefully.

Figure 7 Depositing forms of the oil droplets and catalyst particles on the cyclone wall
4 Conclusions
The characteristics of the coke are analyzed based on the microstructure and the properties of coke samples taken from 4 different wall regions of a cyclone in a commercial residue fluid catalytic cracking (RFCC) reactor. The coke consisted mainly of carbonaceous matter and catalyst particles. There are two kinds of coke, the accumulated coke (soft coke) and the deposited coke (hard coke), the formation of which depends substantially on the fl ow patterns of oil and gas mixture. The accumulated coke is formed on the outer wall of the cyclone, where oil and gas mixture is in a stagnant state. The oil droplets and catalyst particles are accumulated together on the surface of the equipment by gravity settlement, and then are converted to the coke with lots of catalyst particles. The deposited coke is formed on the outer wall of exit tube of the cyclone, where oil and gas mixture is fl owing at a high velocity. The oil droplets and catalyst particles are deposited on the wall surface via turbulent diffusion, and are converted into coke with a few fi ne catalyst particles. It is clear that the oil and gas mixture’s fl ow patterns have a signi fi cant effect on the forms relating to the deposition of oil droplets and catalyst particles, which determines the incorporation of oil droplets with catalyst particles and their proportion, as well as the composition and characteristics of the coke.
Acknowledgement: The authors thank the financial support from the National Natural Science Foundation of China (No. 21176250, No. 21566038)
[1] Chen Junwu, Xu Youhao. Catalytic Cracking Processes and Engineering [M]. the 3rdEdition. Beijing: China Petrochemical Press, 2015: 81-96 (in Chinese)
[2] Investigation Group of Long Cycle Running of FCCU, SINOPEC. Investigation on long period running of FCCU [J]. Petroleum Re fi nery Engineering, 1998, 28(2): 6-13( in Chinese)
[3] McPherson L J. Cause of FCC reactor coke deposits identi fi ed[J]. Oil & Gas J, 1984(9): 139-143
[4] Kim S W, Lee J W, Koh J S, et al. Formation and characterization of deposits in cyclone dipleg of a commercial residue fl uid catalytic reactor[J]. Ind Eng Chem Res, 2012, 51(43): 14279-14288
[5] Kim S W, Lee J W, Kim C J, et al. Characteristics of deposits formed in cyclones in commercial RFCC reactor[J]. Ind Eng Chem Res, 2012, 51(30): 10238-10246
[6] Xing Yingchun, Song Jianfei, Wei Yaodong, et al. Investigation of the factors in fl uencing coke formation process in FCC disengager [J].Acta Petrolei Sinica (Petroleum Processing Section), 2008, 24 (6): 702-708 ( in Chinese)
[7] Song Jianfei, Wei Yaodong, Gao Jinsen, et al. Characteristics of coke in FCC disengager and effect of oil gas fl ow on coke formation process[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2008, 24 (1): 9-14 ( in Chinese)
[8] Wei Yaodong, Song Jianfei, Zhang Kai, et al. Analysis of microstructure of coke and coking mechanism in the reactors of fluid catalytic cracking units[J]. Journal of Fuel Chemistry and Technology, 2005, 33(4): 445-449 ( in Chinese)
[9] Babita Behera, Piyush Gupta, Siddharth S. Ray. Structure and composition of hard coke deposited on industrial fl uid catalytic cracking catalysts by solid state13C nuclear magnetic resonance[J]. Applied Catalysis A: General. 2013, 466: 123-130
[10] Albrght L F, Marek J C. Coke formation during pyrolysis: role of residence time, reactor geometry, and time of operation[J]. Ind Eng Chem Res, 1988, 27(5):743-751
[11] Albrght L F, Marek J C. Mechanistic for formation of coke in pyrolysis units producing ethylene [J]. Ind Eng Chem Res, 1988, 27(5):755-759
[12] Valerio Cozzani. Characterization of coke formed in the pyrolysis of polyethylene[J]. Ind Eng Chem Res, 1997, 36(12): 5090-5095
[13] Gao Jinsen, Xu Chunming, Gao Daiwei, et al. Coking mechanisms within RFCC disengagers[J]. Pet Sci Technol, 2004, 22(5): 601-615
[14] Song J F, Sun G G, Chao Z X, et al. Gas flow behavior and residence time distribution in a FCC disengager vessel with different coupling con fi gurations between two-stage separators[J]. Powder Technology, 2010, 201(3): 258-265
[15] Song J F, Wei Y D, Shi M X. Characteristics and formation of carbonaceous deposits within a commercial RFCC disengager[C]. 8th World Congress of Chemical Engineering, Montreal, Canada, August 23-27, 2009, No. 54
Received date: 2015-10-28; Accepted date: 2015-11-21.
Dr. Song Jianfei, Tel:+86-10-89739018; E-mail: Songjf@cup.edu.cn
杂志排行
中国炼油与石油化工的其它文章
- Enhanced Performance of Denitrifying Sul fi de Removal Process by 1,2-Naphthoquinone-4-Sulphonate
- Effects of Silylation on Zn-IM5 and Its Catalytic Activity for Butane Aromatization
- Preparation of Core-Shell Composite of Y@Mesoporous Alumina and Its Application in Heavy Oil Cracking
- Experimental Study of UDS Solvents for Purifying Highly Sour Natural Gas at Industrial Side-stream Plant
- Numerical Study of Air Nozzles on Mild Combustion for Application to Forward Flow Furnace
- Highly Active and Stable Ni2P/SiO2Catalyst for Hydrogenation of C9Petroleum Resin
