Linkage of Aromatic Ring Structures in Saturates, Aromatics, Resins and Asphaltenes Fractions of Vacuum Residues Determined by Collision-Induced Dissociation Technology
2016-03-22
(SINOPEC Research Institute of Petroleum Processing, Beijing 100083)
Linkage of Aromatic Ring Structures in Saturates, Aromatics, Resins and Asphaltenes Fractions of Vacuum Residues Determined by Collision-Induced Dissociation Technology
Wang Wei; Liu Yingrong; Liu Zelong; Hou Huandi; Tian Songbai
(SINOPEC Research Institute of Petroleum Processing, Beijing 100083)
The linkage of aromatic ring structures in vacuum residues was important for the re fi ning process. The Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR MS) combined with collision-induced dissociation (CID) is a powerful method to characterize the molecular structure of petroleum fractions. In this work, model compounds with different aromatic ring structures were measured by CID FT-ICR MS. The cracking of the parent ions and the generated fragment ions were able to distinguish different linkage of the model compounds. Then, vacuum residues were separated into saturates, aromatics, resins and asphaltenes fractions (SARA), and each fraction was characterized by CID technology. According to the experimental results, the aromatic rings in saturates and aromatics fractions were mainly of the island-type structures, while the aromatic rings in resins and asphaltenes fractions had a signi fi cant amount of archipelago-type structures.
FT-ICR MS; vacuum residues; SARA; collision-induced dissociation (CID)
1 Introduction
Heavy oils have been widely applied in the re fi ning process, and it is necessary to have a better comprehension of detailed chemical structure information on heavy oils. Vacuum residues with boiling points higher than 540 ℃ are considered as the heaviest fractions of crude oils. Typically, vacuum residues are separated into saturates, aromatics, resins and asphaltenes (SARA) fractions according to their solubility and polarity. The SARA fractions are measured by elemental analysis, IR, and NMR, resulting in the general information about their chemical compositions.
The Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR MS) has been used in the analysis of vacuum residues[1-14]. Thousands of peaks can be obtained in the mass spectra and most of the peaks can be assigned with a given chemical formula by the ultra-high mass power resolution and mass accuracy of FT-ICR MS[15-16]. The structural differences of SARA fractions in crude oils have also been studied by FT-ICR MS[17-19]. Typically, the double-bond equivalence (DBE) distribution and the carbon number distribution are used to describe the different composition of the fractions. However, the DBE distribution could not be used to distinguish the different types of aromatic structures, such as the island and archipelago structures.
Several analytical methods were developed to investigate the detailed structures of asphaltene fractions, including the laser-induced acoustic desorption/electron ionization (LIAD/EI)[20]and the thin fi lm pyrolysis[21]. The collisioninduced dissociation (CID) combined with mass spectrometry was a powerful tool for determining the structure of organic molecules[22-23]. The structures of several compounds obtained from the SARA fractions of bitumen were identified by the low-energy collision-induced dissociation tandem mass spectrometry (CID-MS/MS)[24]. The CID technique combined with FT-ICR MS was also applied in the measurement of vacuum gas oil[25](VGO) and vacuum residue[26](VR). Dealkylation of single-core structures[25]and cracking of the linkage of multi-core structures[26]were observed in the petroleum samples, revealing the structural information of petroleum molecules. The CID technique was further used to investigate the molecular structures of basic nitrogen compounds inasphaltene[27]. However, the differences of the aromatic structures in SARA fractions have not been discussed with respect to vacuum residues.
In this work, several model compounds and the SARA fractions of vacuum residues were characterized by CID FT-ICR MS. The collision energy was carefully tuned by the change of the collision voltage. The model compounds with the island- and archipelago-type structures behaved differently when the collision voltage increased. Then, the corresponding CID behavior of SARA fractions of vacuum residues can be used to distinguish the different aromatic structures.
2 Experimental
2.1 Model compounds
Several model compounds are listed in Table 1. The structures of compounds 1 and 2 were of the island-type, while the structures of compounds 3 and 4 were of the archipelago-type. Compound 5 was a polystyrene with an average molecular weight of 972 Da, which also belonged to an archipelago-type structure. Compounds 1 to 4 were synthesized and the model compound 5 was purchased from the Sigma-Aldrich Corporation.
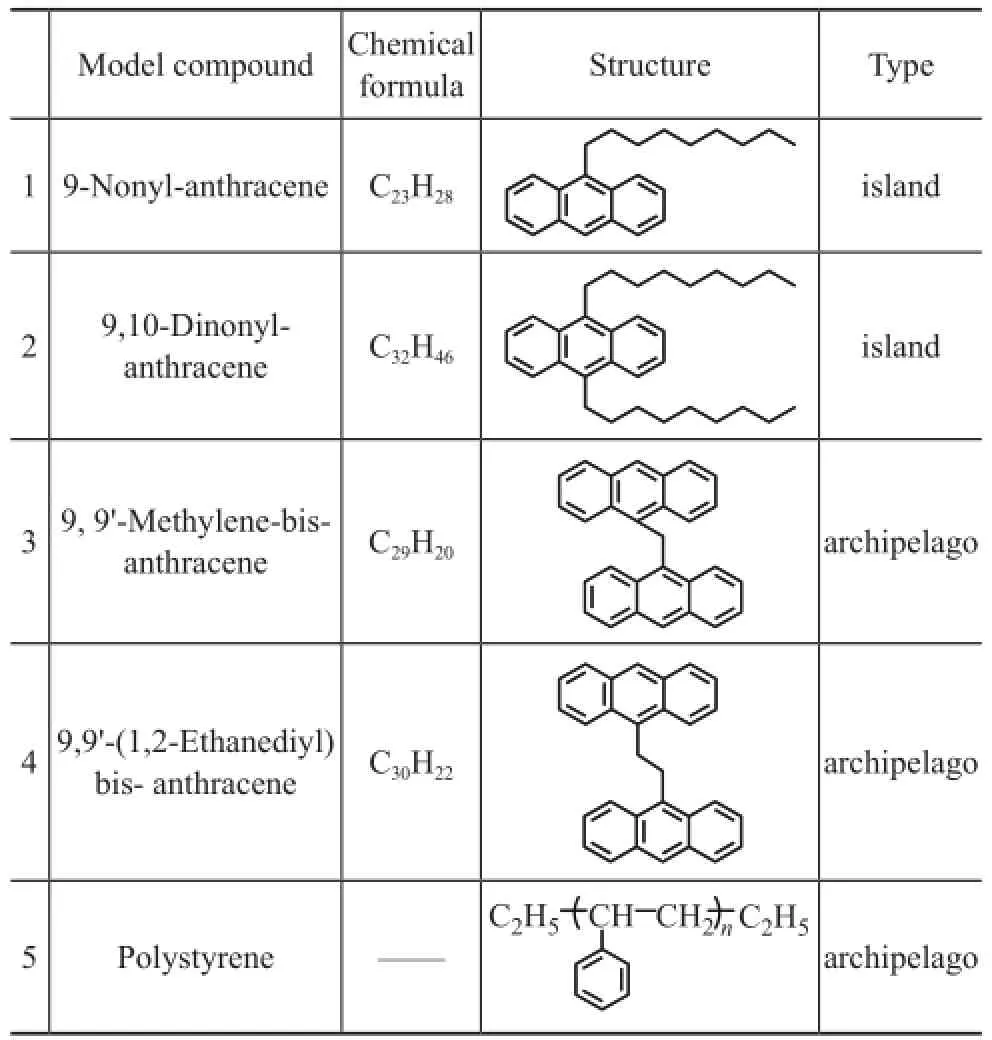
Table 1 Model compounds with island- and archipelagotype structures
2.2 Mass spectrometry analysis
Two vacuum residues (VR-1 and VR-2, as shown in Table 2) were separated into SARA fractions by liquid column chromatograph according to ASTM method D4124-97. The SARA fractions were dissolved in the HPLC-grade toluene (Dikma Corp.) at a concentration of 1.0 mg/mL. The prepared samples were injected into FT-ICR MS at a rate of 360 μL/h by a syringe pump (Hamilton Corp.). Analyses were conducted on a 9.4 T Bruker Apex FT-ICR MS. The positive mode atmospheric pressure photo ionization (APPI) was used as the ionization source. Nitrogen served as the drying gas and nebulizing gas. The drying gas fl ow rate was 4.0 L/min at a temperature of 200 ℃. The nebulizing gas fl ow rate was 1.0 L/min and the APPI temperature was 400 ℃. The collision gas was helium. Them/zranges of the SARA fractions were from 300 Da to 1200 Da. The CID experiments were operated by tuning the collision voltage from 0V to -42V for the model compounds and SARA fractions of vacuum residues.
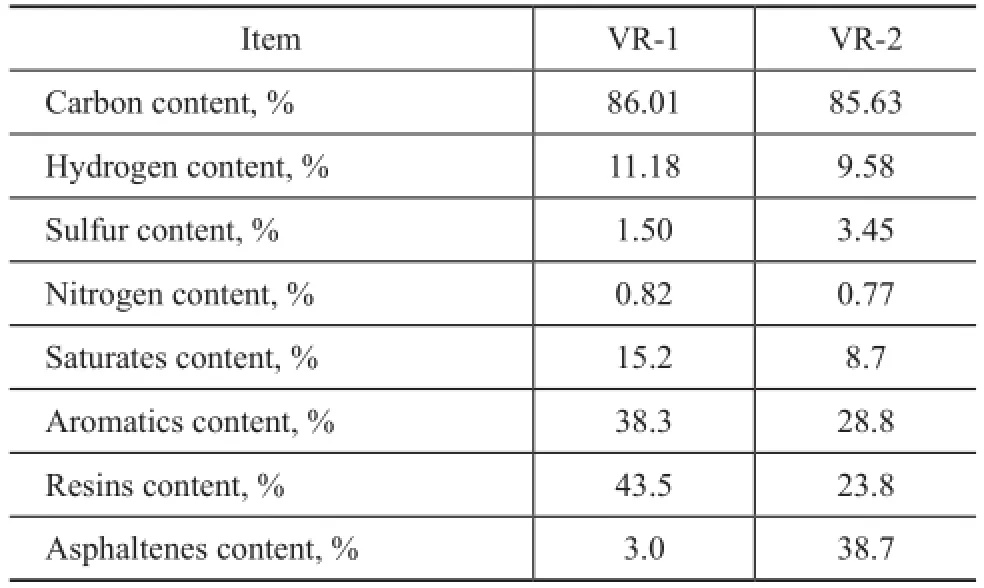
Table 2 Bulk properties of the vacuum residues
2.3 Mass calibration and data analysis
The spectra obtained by APPI FT-ICR MS were internally calibrated by Tuning Mix (Agilent Corp.). The peaks with a relative abundance greater than six standard deviations of baseline RMS noise (6σ) were selected for the data analysis. Chemical formulas (CcHhNnOoSs) were calculated according to them/zvalues within ±1 ×10-6.
3 Results and Discussion
3.1 CID behavior of model compounds
The CID mass spectra of model compounds 1 to 5 at different collision voltages are illustrated in Figures 1—5. As regards compounds 1 and 2 with the island-type structures, only the cracking of side-chains was observedwhen the collision voltage was higher than -18 V. As a result, the DBE of the fragment ions were the same as the parent ions. However, for the archipelago-type compound 3, both the rearrangement of the aromatic rings and the cracking of the bridged linkage took place when the collision voltages were above -18 V. The breaking of the bridged linkage in compound 3 resulted in a significant reduction of DBE, from 20 for the parent ion to 10 for the fragment ion. Compound 4 behaved similarly with compound 3 under CID conditions, but breaking of the linkage became more dominant in compound 4 as it had a C2-linkage which was longer and softer than the C1-linkage in compound 3.
Unlike compounds 1 to 4, the compound 5 was a polymer which gave a series of parent ions in the mass spectra. The distribution of the parent ions shifted to the low molecular weight direction when the collision voltage increased from 0 V to -18 V. The average DBE of compound 5 also reduced a lot when the collision voltage increased, because the central peaks changed fromn=10 ton=8. The much lower collision voltage required for breaking the structure of compound 5 may be ascribed to the larger molecular weight and longer linkage in compound 5, which was more sensitive to the collision voltage[28]. This explanation could also be supported by the dramatic reduction of the ion peaks which contained more repeating units, such asn=10 to 14. Moreover, some of the fragment ions of compound 5 were not detected.
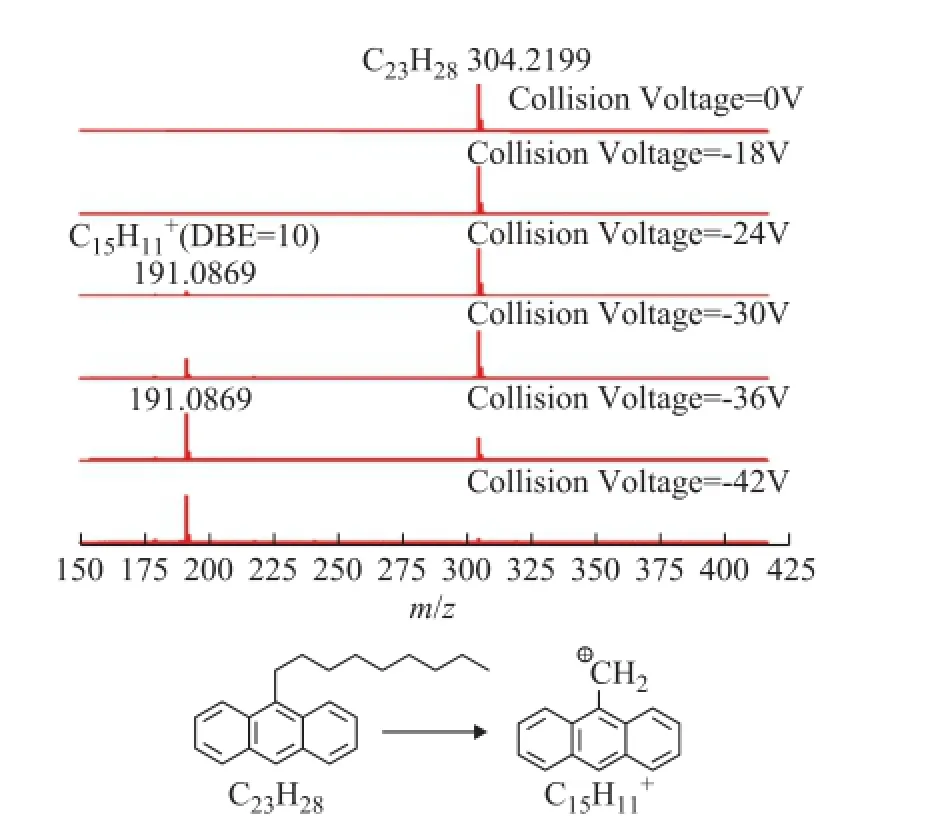
Figure 1 APPI FT-ICR MS spectra of model compound 1 at different collision voltages and the suggested mechanism for the generation of fragment ions
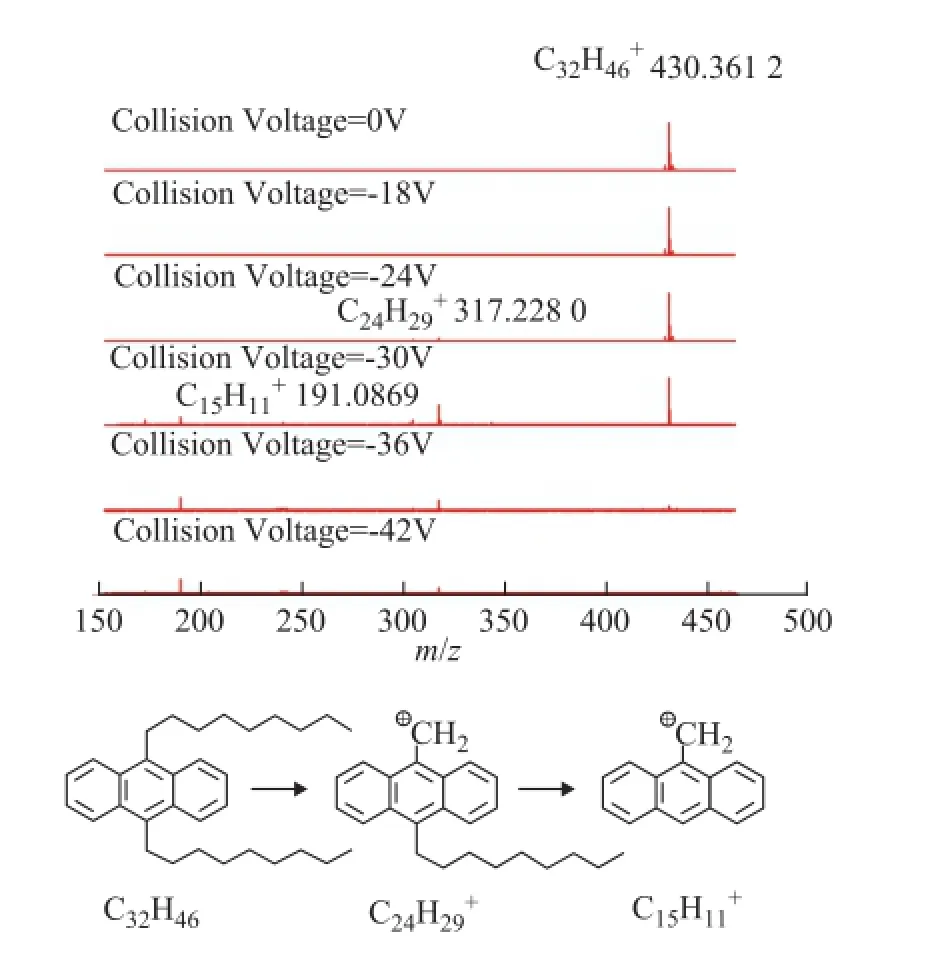
Figure 2 APPI FT-ICR MS spectra of model compound 2 at different collision voltages and the suggested mechanism for the generation of fragment ions
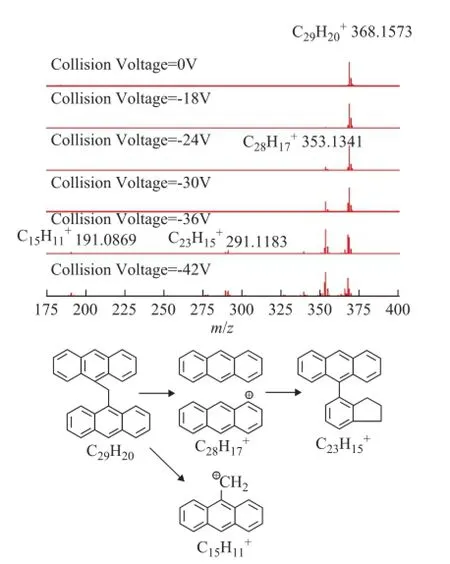
Figure 3 APPI FT-ICR MS spectra of model compound 3 at different collision voltages and the suggested mechanism for the generation of fragment ions
One of the possible reasons was that these fragment ions of polystyrene would be much smaller than the parent ions[29-30]and they could hardly transmit the ion tunnels in FT-ICR MS.3.2 CID behavior of SARA fractions of vacuum residues

Figure 4 APPI FT-ICR MS spectra of model compound 4 at different collision voltages and the suggested mechanism for the generation of fragment ions
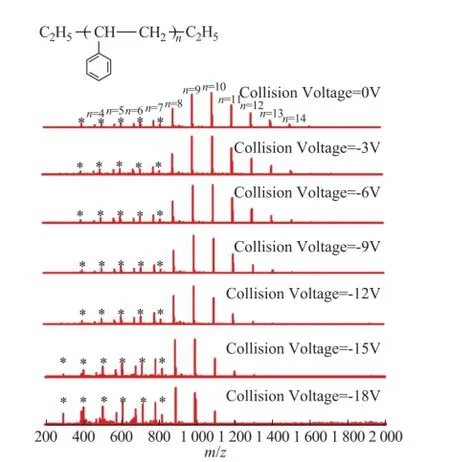
Figure 5 APPI FT-ICR MS spectra of model compound 5 at different collision voltages. The peaks marked with stars were considered as impurities
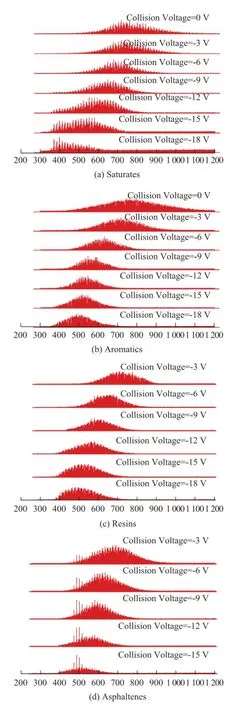
Figure 6 APPI FT-ICR MS spectra of SARA fractions of VR-2: (a) saturates, (b) aromatics, (c) resins, and (d) asphaltenes at different collision voltages
The SARA fractions of VR-1 and VR-2 were characterized by CID APPI FT-ICR MS. The CID mass spectra of SARA fractions of VR-2 are illustrated in Figure 6. Theoretically, the saturated hydrocarbons could not besufficiently ionized in APPI source[31]. However, strong signals were still detected in the saturates fractions. Detailed analysis according to the exact molecular weight of saturates fractions indicated the existence of aromatic hydrocarbons with low DBE and long side chains, such as C56H106(DBE=4) and C60H108(DBE=7). These kinds of aromatic hydrocarbons had similar polarity with the saturated hydrocarbons, which could not be fully removed from the saturated fractions in the course of separation.
It was also noted that the CID behavior of the SARA fractions was close to that of the model compound 5. Signi ficant reduction of the molecular weight distribution was observed for each fraction when the collision voltage was below -18V. The reason was attributed to the large molecular size of the petroleum molecules.
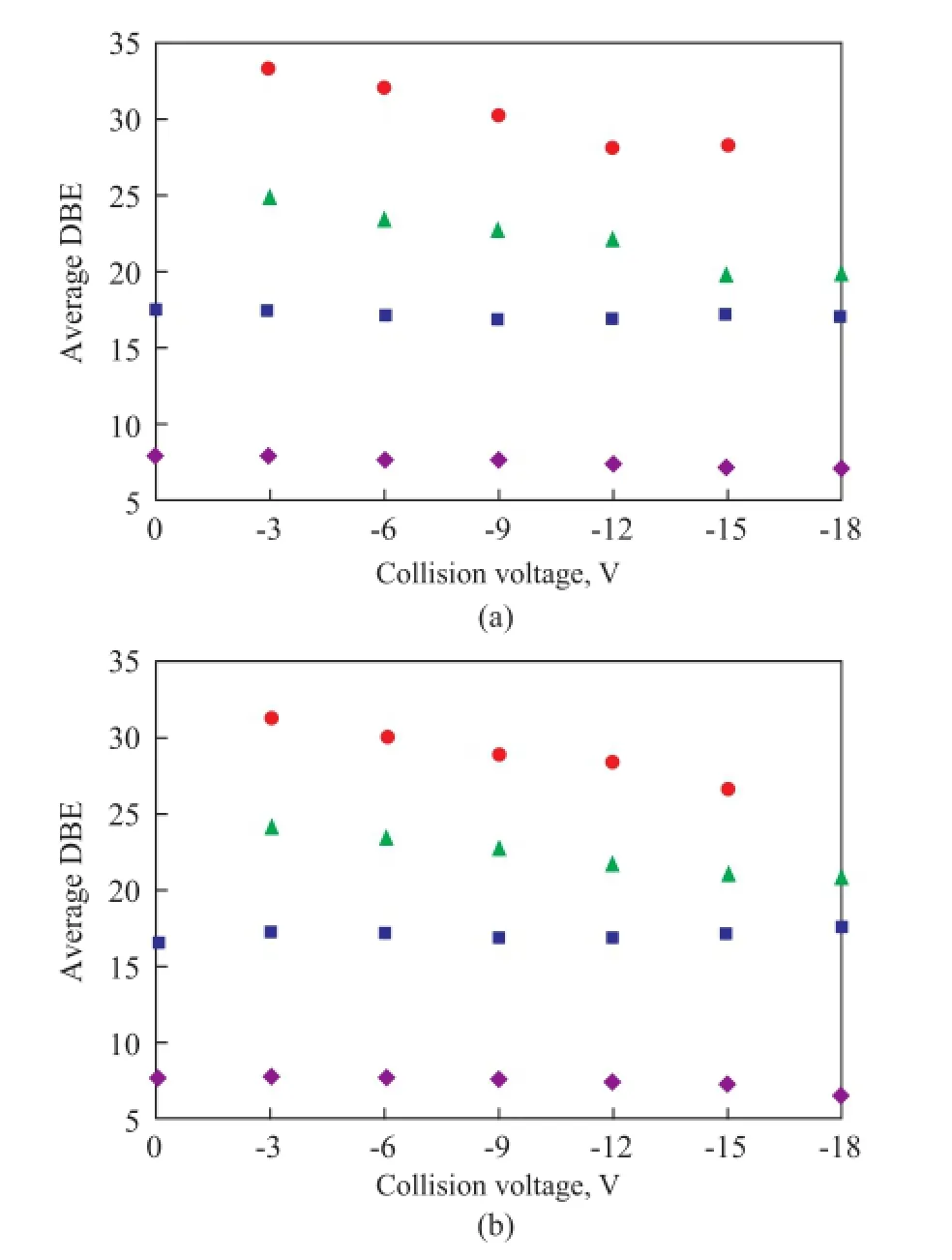
Figure 7 The average DBE of SARA fractions of VR-1 (a) and VR-2 (b) versus collision voltage
The average DBE of the aromatic hydrocarbons in SARA fractions of VR-1 and VR-2 versus collision voltage are shown in Figure 7. It can be seen clearly that the average DBE increased from saturates fractions to asphaltenes fractions, which was consistent with the polarity of the SARA fractions. Moreover, the average DBE of saturates and aromatics fractions remained unchanged while that of resins and asphaltenes fractions reduced with the increase of collision voltage. The CID behavior of saturates/ aromatics fractions fi tted well with that of compounds 1 and 2 having the island-type structures. Meanwhile, the similar results of resins/asphaltenes fractions compared with model compounds 3—5 strongly indicated the existence of archipelago-type structures. As a result, the average molecular structures of the SARA fractions in VR-1 and VR-2 are suggested in Figure 8. The differences between the saturates/aromatics fractions and the resins/ asphaltenes fractions were not only in the DBE value but also in the linkage of the aromatic rings.
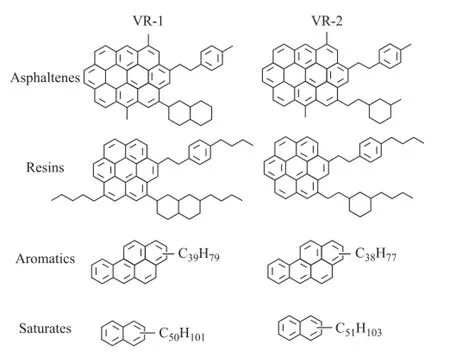
Figure 8 The suggested average structures of SARA fractions according to CID results
4 Conclusions
This study applied the CID technology coupled with the FT-ICR MS to determine the aromatic ring structure of vacuum residues. For model compounds with the islandtype structures, only the cracking of side chains was observed, with the aromatic ring structures unchanged. However, for the archipelago-type model compounds, the breaking down of the linkage chains generated different aromatic ring structures. As a result, the CID mass spectra could be used to distinguish the diversity of aromatic compounds in vacuum residues. The average DBE of saturates and aromatics fractions remained almost unchanged, while that of resins and asphaltenes fractionsreduced with an increasing collision voltage. These results proved that most aromatic compounds in saturates/aromatics fractions were of the island-type structures and some aromatic compounds in resins/asphaltenes should be of the archipelago-type structures. This work reveals that the CID technique combined with FT-ICR MS is a hopeful way to obtain more compositional information about the petroleum samples, which is valuable for the re fi ning process of heavy oils.
Acknowledgements: This work was supported by the Major State Basic Research Development Program of China (973 Program, No. 2012CB224801).
[1] Comisarow M B, Marshall A G. Frequency-sweep Fourier transform ion cyclotron resonance spectroscopy[J]. Chem Phys Lett, 1974, 26(4): 489-490
[2] Wang Wei, Liu Yingrong, Liu Zelong, et al. Quantitative analysis using Fourier transform ion cyclotron resonance mass spectrometry and correlation between mass spectrometry data and sulfur content of crude oils[J]. China Petroleum Processing and Petrochemical Technology, 2015, 17(4): 71-80
[3] Qian K, Rodgers R P, Hendrickson C L, et al. Reading chemical fine print: Resolution and identification of 3000 nitrogen-containing aromatic compounds from a single electrospray ionization Fourier transform ion cyclotron resonance mass spectrum of heavy petroleum crude oil[J]. Energy Fuels, 2001, 15(2): 492-498
[4] Qian K, Robbins W K, Hughey C A, et al. Resolution and identification of elemental compositions for more than 3000 crude acids in heavy petroleum by negative-ion microelectrospray high-field Fourier transform ion cyclotron resonance mass spectrometry[J]. Energy Fuels, 2001, 15(6): 1505-1511
[5] Chen Xiaobo, Li Teng, Liu Yibin, et al. Characterization of nitrogen compounds in vacuum residue and their structure comparison with coker gas oil[J]. China Petroleum Processing and Petrochemical Technology, 2014, 16(3): 33-41
[6] Schaub T M, Rodgers R P, Marshall A G. Speciation of aromatic compounds in petroleum refinery streams by continuous flow field desorption ionization FT-ICR mass spectrometry[J]. Energy Fuels, 2005, 19(4): 1566-1573
[7] Liu Yingrong; Zhang Qundan; Wang Wei; et al. Changes of petroleum acid distribution characterized by FT-ICR MS in heavy acidic crude oil after true boiling point distillation[J]. China Petroleum Processing and Petrochemical Technology, 2014, 16(1): 8-12
[8] Wang Xiaowei, Long Jun, Tian Songbai, et al. Study on side chains of aromatics in Tahe vacuum gas oil[J]. Petroleum Processing and Petrochemicals, 2015, 46(5): 1-6 (in Chinese)
[9] Wang Naixin, Liu Zelong, Zhu Xinyi, et al. Boiling range distributions of hydrocarbon molecular compositions in diesel by gas chromatography-time of fl ight mass spectrometry[J]. Petroleum Processing and Petrochemicals, 2015, 46(1): 89-95 (in Chinese)
[10] Panda S K, Schrader W, al-Hajjj A, et al. Distribution of polycyclic aromatic sulfur heterocycles in three Saudi Arabian crude oils as determined by Fourier transform ion cyclotron resonance mass spectrometry[J]. Energy Fuels, 2007, 21(2): 1071-1077
[11] Purcell J M, Juyal P, Kim D, et al, Hendrickson C L, Marshall A G. Sulfur speciation in petroleum: Atmospheric pressure photoionization or chemical derivatization and electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry[J]. Energy Fuels, 2007, 21(5): 2869-2874
[12] Shi Q, Zhao S, Xu Z, et al. Distribution of acids and neutral nitrogen compounds in a Chinese crude oil and its fractions: Characterized by negative-ion electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry[J]. Energy Fuels, 2010, 24(7): 4005-4011
[13] Zhang L, Xu Z, Shi Q, et al. Molecular characterization of polar heteroatom species in Venezuela Orinoco petroleum vacuum residue and its supercritical fl uid extraction subfractions[J]. Energy Fuels, 2012, 26(9): 5795-5803
[14] Gaspar A, Zellermann E, Lababidi S, et al. Impact of different ionization methods on the molecular assignments of asphaltenes by FT-ICR mass spectrometry[J]. Anal Chem, 2012, 84(12): 5257-5267
[15] Marshall A G, Hendrickson C L, Jackson G S. Fourier transform ion cyclotron resonance mass spectrometry: A primer[J]. Mass Spectrom Rev, 1998, 17(1): 1-35
[16] Marshall A G, Hendrickson C L. Fourier transform ion cyclotron resonance detection: Principles and experimental configurations[J]. Int J Mass Spectrom, 2002, 215(1-3): 59-75
[17] Cho Y, Kim Y H, Kim S. Planar limit-assisted structural interpretation of saturates/aromatics/resins/asphaltenesfractionated crude oil compounds observed by Fourier transform ion cyclotron resonance mass spectrometry[J]. Anal Chem, 2011, 83(15): 6068-6073
[18] Cho Y, Na J G, Nho N S, et al. Application of saturates, aromatics, resins, and asphaltenes crude oil fractionation for detailed chemical characterization of heavy crude oils by Fourier transform ion cyclotron resonance mass spectrometry equipped with atmospheric pressure photoionization[J]. Energy Fuels, 2012, 26(5): 2558-2565
[19] Gaspar A, Zellermann E, Lababidi S, et al. Characterization of saturates, aromatics, resins, and asphaltenes heavy crude oil fractions by atmospheric pressure laser ionization Fourier transform ion cyclotron resonance mass spectrometry[J]. Energy Fuels, 2012, 26(6): 3481-3487
[20] Borton II D, Pinkston D S, Hurt M R, et al. Molecular structures of asphaltenes based on the dissociation reactions of their ions in mass spectrometry[J]. Energy Fuels, 2010, 24(10): 5548-5559
[21] Karimi A, Qian K, Olmstead W N, et al. Quantitative evidence for bridged structures in asphaltenes by thin film pyrolysis[J]. Energy Fuels, 2011, 25(8): 3581-3589
[22] Chawla R, Shukla A, Futrell J. Collision-induced dissociation of nitrobenzene molecular cations at low energies by crossed-beam tandem mass spectrometry[J]. J Phys Chem A, 2001, 105(2): 349-353
[23] Muntean F, Armentrout P B. Modeling kinetic shifts and competition in threshold collision-induced dissociation. Case study: n-Butylbenzene cation dissociation[J]. J Phys Chem A, 2003, 107(38): 7413-7422
[24] Tachon N, Jahouh F, Delmas M, et al. Structural determination by atmospheric pressure photoionization tandem mass spectrometry of some compounds isolated from the SARA fractions obtained from bitumen[J]. Rapid Commun Mass Spectrom, 2011, 25(18): 2657-2671
[25] Zhang L, Hou Z, Horton S R, et al. Molecular representation of petroleum vacuum resid[J]. Energy Fuels, 2014, 28(3): 1736-1749
[26] Qian K, Edwards K E, Mennito A S, et al. Determination of structural building blocks in heavy petroleum systems by collision-induced dissociation Fourier transform ion cyclotron resonance mass spectrometry[J]. Anal Chem, 2012, 84(10): 4544-4551
[27] Mullins O C. The modified Yen model[J]. Energy Fuels, 2010, 24(4): 2179-2207
[28] Penn S G, Cancilla M T, Lebrilla C B. Collision-induced dissociation of branched oligosaccharide ions with analysis and calculation of relative dissociation thresholds[J]. Anal Chem, 1996, 68(14): 2331-2339
[29] Zhang Z, Hirose T, Nishio S, et al. Chemical recycling of waste polystyrene into styrene over solid acids and bases[J]. Ind Eng Chem Res, 1995, 34(12): 4514-4519
[30] Kruse T M, Wong H, Broadbelt L J. Modeling the evolution of the full polystyrene molecular weight distribution during polystyrene pyrolysis[J]. Ind Eng Chem Res, 2003, 42(12): 2722-2735
[31] McKenna A M, Purcell J M, Rodgers R P, et al. Heavy petroleum composition. 1. Exhaustive compositional analysis of Athabasca bitumen HVGO distillates by Fourier transform ion cyclotron resonance mass spectrometry: A de fi nitive test of the Boduszynski model[J]. Energy Fuels, 2010, 24(5): 2929-2938
Received date: 2015-11-25; Accepted date: 2015-12-29.
Dr. Wang Wei, Telephone: +86-10-82368443 ; E-mail: wangwei3.ripp@sinopec.com.
杂志排行
中国炼油与石油化工的其它文章
- Experimental Study of UDS Solvents for Purifying Highly Sour Natural Gas at Industrial Side-stream Plant
- Highly Active and Stable Ni2P/SiO2Catalyst for Hydrogenation of C9Petroleum Resin
- Enhanced Performance of Denitrifying Sul fi de Removal Process by 1,2-Naphthoquinone-4-Sulphonate
- Investigation of Different Coke Samples Adhering to Cyclone Walls of a Commercial RFCC Reactor
- Numerical Study of Air Nozzles on Mild Combustion for Application to Forward Flow Furnace
- Preparation of Core-Shell Composite of Y@Mesoporous Alumina and Its Application in Heavy Oil Cracking
