Methodology for Design of Reactive Distillation Column and Kinetics for Isoamylene Etheri fi cation Catalysed by Amberlyst 35
2016-03-22
(Key Laboratory of Coal Science and Technology (Taiyuan University of Technology), Ministry of Education and Shanxi Province, Taiyuan, 030024)
Methodology for Design of Reactive Distillation Column and Kinetics for Isoamylene Etheri fi cation Catalysed by Amberlyst 35
Wu Yanli; Li Wenying; Li Qiang; Feng Jie
(Key Laboratory of Coal Science and Technology (Taiyuan University of Technology), Ministry of Education and Shanxi Province, Taiyuan, 030024)
Isoamylene from the Fischer-Tropsch syncrude can be transformed to valuable fuel oxygenate additives through an equilibrium limited etherifcation reaction with methanol. A reactive distillation process is established to increase isoamylene conversion. Facing the challenge of improving product purity at the same time, an equilibrium stage model based design methodology is proposed and illustrated step-by-step for converting the Fischer-Tropsch C5olefns totert-amyl methyl ether (TAME) process by using Aspen Plus. Under the guide of the proposed methodology, the design leads to a TAME product purity of higher than 95% and an isoamylene conversion of higher than 90%. The etherifcation kinetics over Amberlyst 35 is also studied within a temperature range of 60 ℃ to 75 ℃to shed more light on the feasibility of process development. The methodology provides an effective reactive distillation column design to achieve the target reactant conversion and product purity simultaneously.
Amberlyst 35; design methodology; etherifcation; isoamylene; reactive distillation column
1 Introduction
The development and commercialization of Fischer-Tropsch (FT) fuel has rapidly expanded worldwide recently, as driven by concerns ranging from climate change to fuel security. Because of the limitation of olefn content in liquid fuel, C5olefns contained in FT syncrude is not allowed to be used as fuel additive, but they can be converted to high value-added chemicals. 1-Pentene can be extracted and further used as copolymers[1]. Isoamylene (IA) can be converted totert-amyl methyl ether (TAME) (Eq 1-2) by etherifcation reaction, which is a high-octane oxygenate additive to gasoline to improve the gasoline quality and reduce the carbon monoxide and particulates emissions during combustion[2]. The isoamylene etherifcation is also a crucial step for 1-pentene purifcation[3].
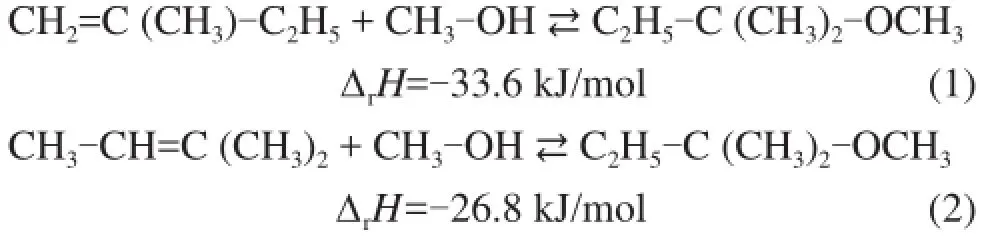
The etherifcation reaction is an equilibrium limited reaction. At 60 ℃ and 0.7 MPa, the equilibrium constant K is 39.6±2.5 when using 2-methyl-1-butene (2M1B) and is 4.1±0.3 when using 2-methyl-2-butene (2M2B) as the starting materials. The conversion of IA is limited to 56 % at 60 ℃ by chemical equilibrium when a stoichiometric ratio of IA to methanol is used. To increase the conversion, the dual-reactor in serial process[4], the side reactor process[5], and the reactive distillation (RD) process are developed. The limitation of the former two processes[6]suggests RD is preferred for achieving a high conversion rate. With the advantages of promoting equilibrium-limited chemical reactions and reduction of the reactor and recycle costs[7], RD process brings about signifcant benefts to TAME production system, and has been used in the newer commercial technologies (NExTAME, CDEtherol, etc.)[8]. The RD column combines the reaction with separation, so the product purity is the other important design issue, which is related to the product quality and the selling price. Ramkissoon, et al.[9]put emphasis on fnding optimal operating conditions of the RD column to obtain the maximum TAME purity, but the fnal optimum purity is only 0.76 after optimization. The diffculty to design RD column signifcantly increases especially with the increase of the number of componentsand chemical reactions.
The RD column design methodology is expected to provide the estimation of column confguration variables and operating variables which can satisfy the design specifcations for given feed inputs. Graphical[10-11], optimizationbased[12-14], and heuristic-based method[15]are the most common methodologies reported for RD design as the three methods have their own merits and demerits[16]. A graphical design method to determine the reactive distillation design parameters has been developed for TAME synthesis[17]. But the graphical methods are limited by the graphical nature. The multicomponent system will increase the complexity for the optimization-based method application, because high computational effort should be paid. Heuristic equilibrium model based method is easy in principle and has less intensity in computation, which can be applied in multicomponent system, but fails to reach the optimum integration between the reaction and the separation process[18]. To the best of our knowledge, the effective method is not available to guide the design of a RD column that would simultaneously achieve the product purity and reactants conversion requirements. It is of great signifcance to develop such an effective method for the synthesis and design of reactive distillation column.
In this work, a design methodology based on equilibrium model is proposed for RD column design. To simulate the RD column completely, an IA etherifcation kinetic model is also presented using the Amberlyst 35 resin catalyst, which has exhibited excellent catalytic performance[19]. The RD process is developed in Aspen Plus process simulator for converting FT C5olefns to oxygenate additives. The conceptual design and operating parameters, as well as the mass and heat balance for the process are determined simultaneously.
2 Reactive Distillation Column Design Method
The RD column design aims at determining the column configuration and operating parameters which are able to achieve the targets previously specified. The column confguration involves the number of stages, the location and mumber of reactive stages, and the number of feed stages, while the operating parameters include the refux ratio, the heat duty, the fow rates, etc. The design method is based on the equilibrium model of RD column. The configuration and operating parameters are determined by successive iteration using an equilibrium model of RD column until the process specifcations are achieved. The RadFrac module in Aspen Plus is adopted herein, which is the most common equilibrium model for RD column design.
The number of theoretical stages (N) and the feed location (NF) are calculated with the Fenske-Underwood equation by the DSTWU module in Aspen Plus. Temperature and concentration profles are very helpful to monitor the reaction temperature and reactants concentration change in the reactive region. The catalyst loading can be estimated by the system in which the ideal reactors and fash separators are connected in series[15]. Pressure (P) is the key parameter that affects the temperature profle. Bottom mole fow (B) is the key to concentration profle, and an excess reactant can be added to adjust the concentration profle in RD column. The column design variables and operat-ing variables are searched in the three-iteration-loop involved in the methodology. The following steps (Figure 1) are presented to design a RD column that meets the specifed conversion and purity of product under constraints of reactive region temperature. The refux ratio (R) sent to the RadFrac module is 1.3 times as much as the minimum refux ratio obtained in the DSTWU module.
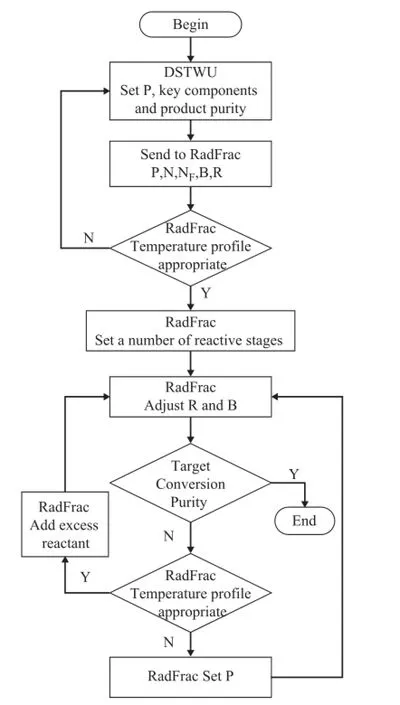
Figure 1 Structure of the design method in this work
3 Experimental
3.1 Materials and catalyst
The reactants include IA and methanol. The IA (≥99%, containing 10% of 2M1B and 89 wt.% of 2M2B) was purchased from the Zibo Liantan Chemical Company, Limited (Zibo, Shandong, China). Methanol (≥99%) was obtained from the Tianjin Fengchuan Chemical Reagent Company, Limited (Tianjin, China). TAME (99.7%) used for quantitative analysis, was supplied by the Aladdin Company (Shanghai, China). The Amberlyst 35 was supplied by the Rohm and Haas Company (USA). The water inside the catalyst was removed with methanol, and the catalyst was dried at 80 ℃ for 24 h prior to use.
3.2 Kinetic measurement
The kinetic experiments were conducted at 1 MPa in a vertical down-fow reactor with a length of 550 mm and an inner diameter of 8 mm, which was heated by an external electric oven. The oven was controlled by a proportional integral derivative (PID) temperature controller. The physical mixture composed of 0.2 to 1 g of catalyst and the special inert quartz particles formed a bed height of 5 cm. The temperature of the catalyst bed was measured by three thermocouples, and the temperature was controlled to within ±0.5 ℃. The temperature was varied from 60 ℃ to 75 ℃ with an interval of 5 ℃, and the catalyst mass was between 0.2 and 1 g with an interval of 0.2 g. The methanol and IA were pre-mixed in a raw material tank so that the reaction could be operated at different mole ratios ranging from 1:1 to 1.5:1 at a specifed total fow rate of 0.5 mL/min. The reactant mixture was introduced by a plunger pump and routed downward inside the reactor. Repeated measurements of each catalyst at each particular condition were made at least 3 times, and the average value was adopted. The standard deviation of each datum was less than 1.5%.
The composition of methanol, IA, and TAME at the outlet was determined by gas chromatography using an Agilent 6890N apparatus, equipped with a fame ionization detector and an RTX-1 capillary column (0.2 mm×30 m). The temperature of the detector and the injector was set at 250 ℃and 200 ℃, respectively. Helium was the carrier gas with a fow rate of 45 mL/min.
4 Results and Discussion
4.1 Preliminary study
Preliminary studies were conducted to determine the“reaction-limited” conditions for kinetics determination. The effects of external mass transfer were evaluated at the same residence time and at a liquid volumetric fow rate ranging from 0.2 to 0.6 mL/min, as given in Figure 2. It can be observed that there is no external mass transfer effect with a liquid superfcial velocity above 0.1 mm/s. A liquid volumetric fow rate of 0.5 mL/min (with a liquid velocity of 0.17 mm/s) is adopted. The Weisz–Prater criterion[20]is used to assess the intra-particle mass transfer limitation. The value in this study is 0.67, which is much less than 1. The result proves that internal mass transfer resistance does not exist with an Amberlyst 35 catalyst particle diameter less than 0.5 mm. This conclusion is confrmed by the etherification reaction using other catalysts[20-21]. The infuence of different particle size catalysts on methanol conversion is illustrated in Table 1.
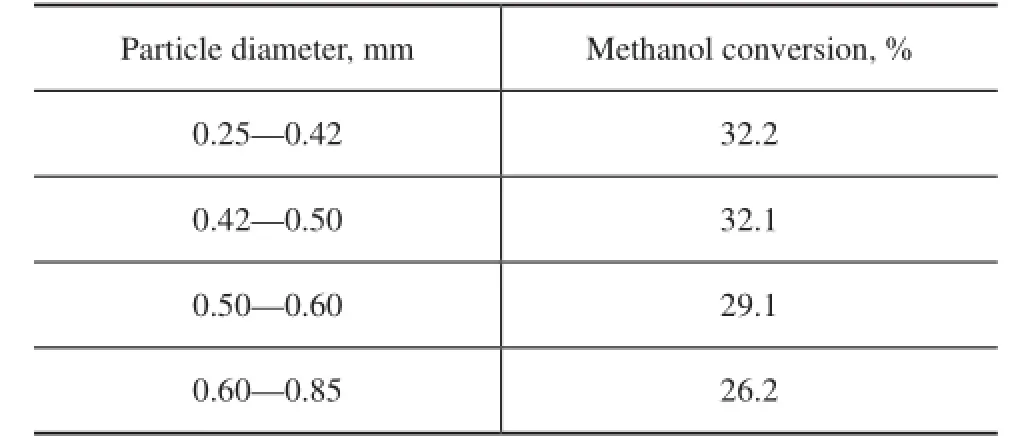
Table 1 Effect of different particle size of catalysts on conversion
The reactor used in this study was operated under isothermal conditions in a continuous plug-flow mode, which could meet the following conditions:1)db/dpwas greaterthan 10 to avoid channeling along its wall[22]. 2)Lb/dpwas greater than 100 to ignore the axial mixing effects[23]. Based on some justifed assumptions in the previous study[24], the material balance in a plug fow reactor can be described as follows.
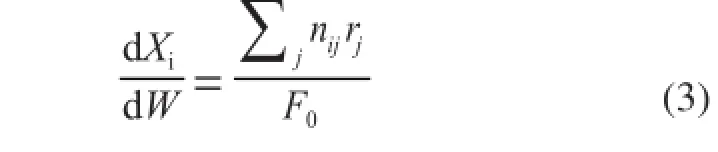

Figure 2 Effect of liquid super fi cial velocity on methanol conversion (70 ℃, methanol/IA=1.5)
4.2 Parameter estimation
For the etherifcation reaction over Amberlyst 35 catalyst, TAME can be formed by the two reactions given in Eq 1-2. The Eley-Rideal general rate expressions[25]for the above two reactions are presented in Eq 4-5.
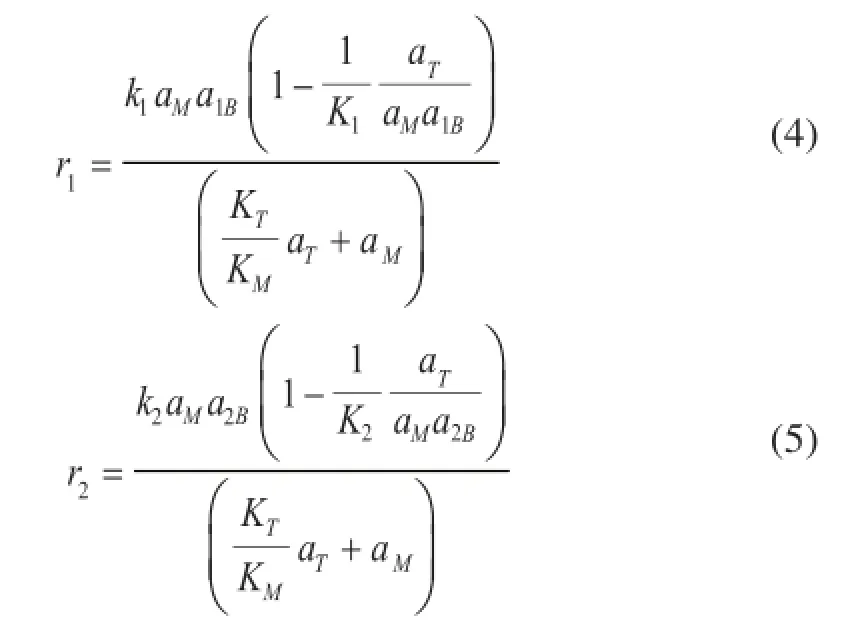
The Wilson model was chosen to calculate activity coefficients[26], while the details about Wilson parameters can be found elsewhere[27]. The equilibrium constantsK1andK2were temperature-independent in the range of experimental conditions, which were adequately investigated in the previous study[28]. And the adsorption equilibrium constants can be given by the Van’t Ho ff equation[29]. The rate constantsk1andk2can be further expressed in the Arrhenius form[30]as shown in Eq 6.
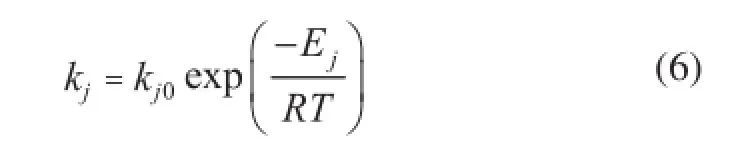
Consequently, there are six kinetic parameters in total that need to be estimated, in whichkj0andEjare associated with eachkj. The estimation of the pre-exponential constantsk10andk20, and their activation energy was refned in a common two-step procedure. Firstly, estimates ofk1andk2were obtained for each temperature level, and then estimates ofE1andE2were derived from these rate constant estimates as a function of temperature.
It can be seen from Eq 3 that the slope of the tangent of the curveXiversusW/F0is the reaction rate ofireactant at the conversionXi. The reaction rate is calculated by the differential method of kinetic analysis. The curveXiversusW/F0is given in Figure 3. The reaction rate of methanol at the conversionXifor the parameter estimation study is then obtained.
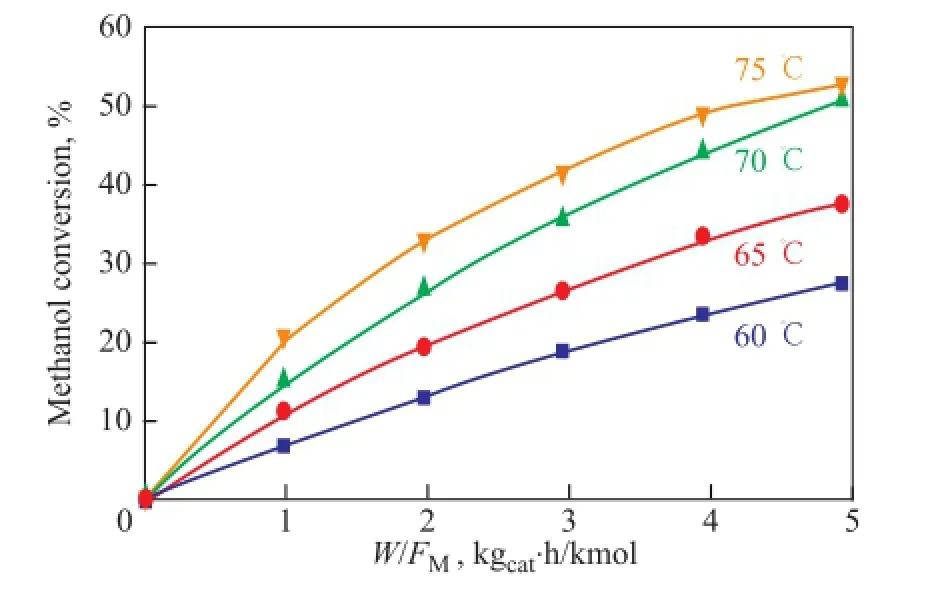
Figure 3 The curveXiversusW/F0(Methanol: IA=1:1)
The estimation of kinetic constantsk1andk2requires the solution of multi-response nonlinear systems. Several nonlinear kinetic parameters,θj,and independent variables,Xi,are contained in the reaction rates. The reaction rates at each reaction condition are assumed to be well represented by a nonlinear model given by Eq 7

For a nonlinear multi-response system, the nonlinear least-square analysis was used as the criterion to estimate the kinetic parameters and to compare the predicted reaction rates with the actual reaction rates.
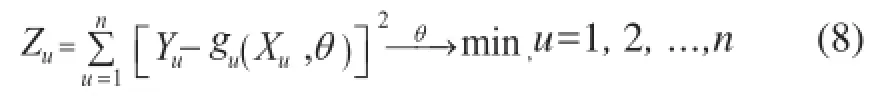
The objective function is given in Eq 8. Simultaneously, the kinetic constantsk1andk2were determined by applying a Levenberg-Marquardt method, which was an effec-tive search technique for optimization. Then the estimation of the pre-exponential constantsk10andk20, and their activation energy have been refned by statistical ftting of linear relationship between ln (k) vs 1/T, as shown in Figure 4. The values of the kinetic parameters and standard error along with their 90% likelihood intervals for nonlinear systems are presented in Table 2.
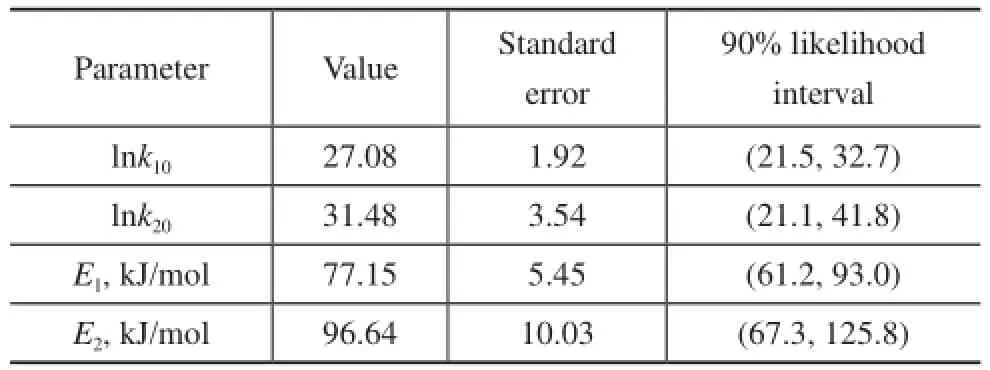
Table 2 Kinetic parameters, standard errors, and 90% likelihood interval for the kinetic
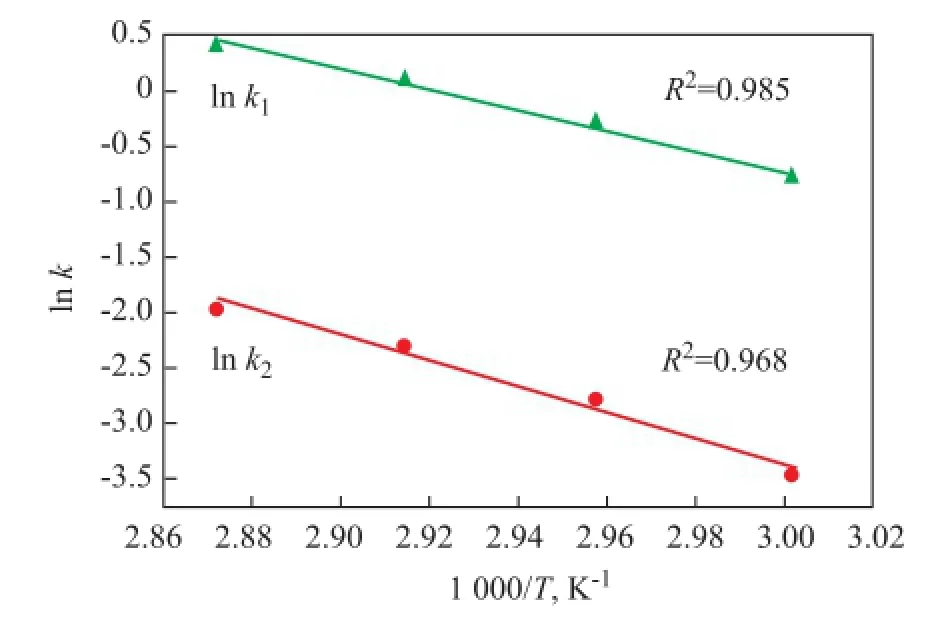
Figure 4 Arrhenius plot of rate coef fi cient for the kinetics study
The standard error and likelihood intervals suggest that the estimates of the kinetic parameters are reasonable. It can be observed that the pre-exponential factor for the 2M1B etherification reaction is smaller than that for the 2M2B etherification reaction. The activation energy is 77.15 kJ/mol (E1) for the etherifcation of 2M1B to TAME, which is less than that of 96.64 kJ/mol (E2) for the etherifcation of 2M2B. The activation energy for 2M2B etherifcation is greater than that for 2M1B etherifcation. Similar trends can be found in the previous studies listed in Table 3. And the activation energy for 2M1B etherifcation is also in the range from 72.6 kJ/mol to 101 kJ/mol and that for 2M2B etherifcation is in the range from 85.7 kJ/mol to 102.4 kJ/mol.
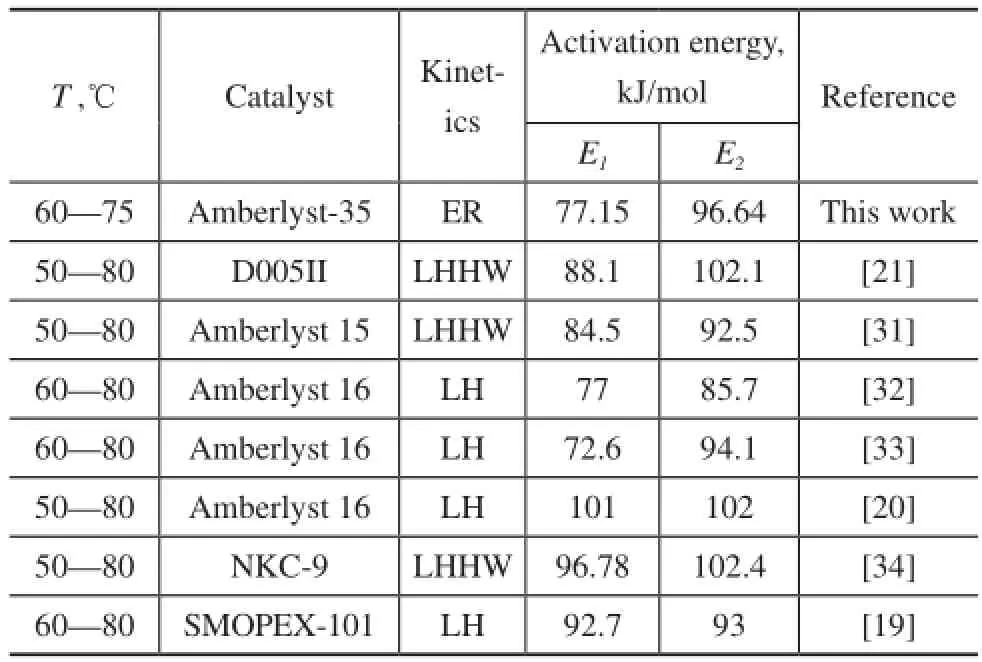
Table 3 Comparison of activation energy with the literature data
Then, the Eley-Rideal kinetic model for catalytic etherifcation reaction over Amberlyst-35 is given in Eq 9—10. Upon using the kinetic model, the predicted methanol conversion is calculated and plotted vs. all actual methanol conversion data in Figure 5. And it is noted that the experimental data have not been used before in the model ftting process. The closeness of the predicted and actual methanol conversion is evident in Figure 5. So it is fully convincing that the kinetic equation is viable for the design of reactor and the IA etherifcation process.
4.3 Design speci fi cations and process design
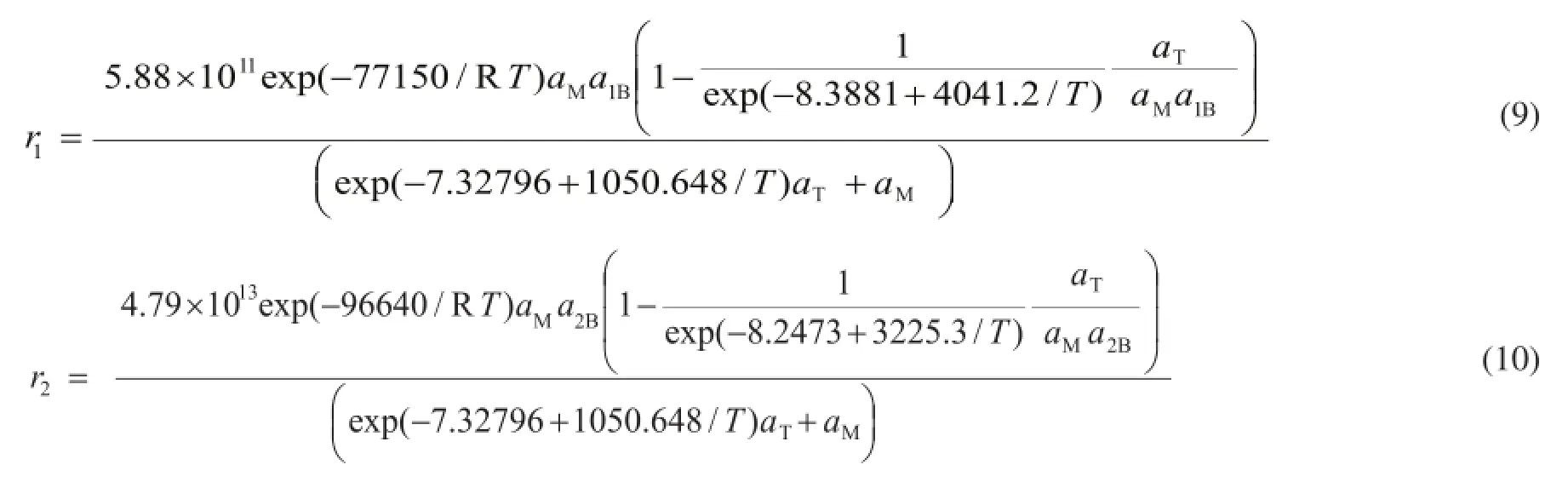

Figure 5 Predicted methanol conversion vs. actual methanol conversion
A brief description, design, and modeling of the process are provided in this section. The FT C5fraction obtained from the upstream pentene skeletal isomerization reactor (operating at 280 ℃ and 17 bar) contains about 57 wt.% of isoamylenes (Table 5). In our study, a minimum IA conversion of 90% and a minimum TAME purity of 95% at the bottom of RD column are simultaneously defned as the target.
The process consists of a pre-reactor and a reactive distillation column that are connected in series as shown in Figure 6, which is proposed by Subawalla[15]and has been further studied[35-36]. The design with a pre-reactor has the advantage over prolonging the lifetime of the catalyst in the RD column, featuring good energy management and economics[8]. The FT C5feed is mixed with methanol and cooled down in the cooler by cooling water. The cooled feed is then fed into a pre-reactor, in which chemical equilibrium conversion is accomplished. The effuent stream containing TAME without separation is then introduced into a reactive distillation column for further transformation. TAME is the bottom product of the CD column, and the unreacted IA and methanol as well as the inert components are obtained in the distillate. The FT-based TAME process was modeled by Aspen Plus. The previous valid ER reaction rate equations are used to calculate the reaction rate in the prereactor and RD column. All the thermodynamic information was computed using the Wilson method.
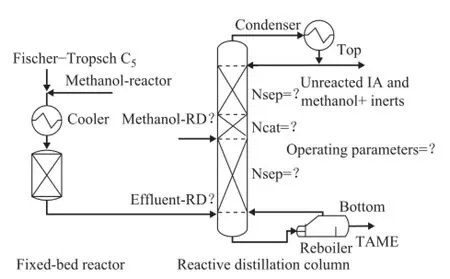
Figure 6 The process fl owsheet for converting Fischer-Tropsch C5 ole fi ns to oxygenate additive
4.4 Application design
The pre-reactor is operated at 0.45 MPa to ensure that the reactants are all in liquid phase at 70 ℃. The methanol to IA mole ratio is 1:1 and the IA conversion is 75.6% in the pre-reactor. The composition of the effuent steam in the pre-reactor can be found in Table 5, which is used as the feed stream to RD column. Hence the design of reactive distillation column is the key to achieve the design specifcations. The IA and TAME are selected respectively as the light and heavy key components, and the percentage of recovery of TAME is defned in the DSTWU module. Under the guide of the design method, the conceptual column design variables and operating parameters of the RD column are obtained only after several searches. The obtained variables and design specifcations are presented together in Table 4.
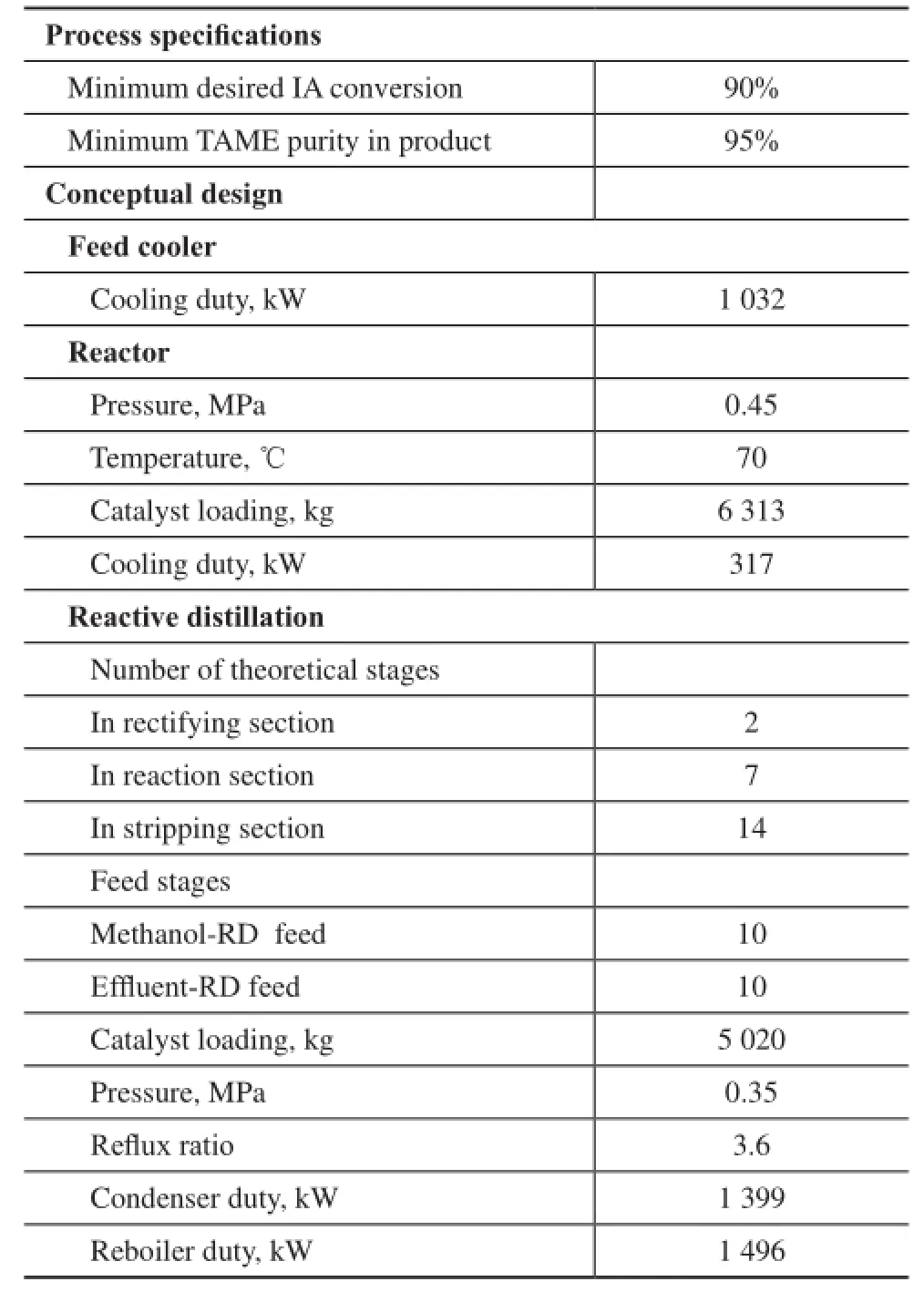
Table 4 Process specification and the conceptual design parameters
At a refux ratio of 3.6, a pressure of 0.35 MPa and a reactive tray number of 7, TAME is enriched in the stripping section represented in the composition profle in Figure 7(a). The mass fraction and mole fraction of TAME in the bottom is 0.95 and 0.9 respectively. The TAME purity is 0.76 only after optimization of operating conditions[9]. It can be indicated that it is better to take the product purity as a target for RD column design. Due to signifcant methanol in the top fow of RD column, the methanol recovery can be further considered. Judging from the temperature profle in Figure 7(b), it is clear that the temperature in the reaction section is around 64—71 ℃, which is in the optimum temperature range for Amberlyst-35 catalyst. The detailed stream composition and mass fow information of the RD process can be found in Table 5. An overall IA conversion of 93.13% and a purity of TAME product of 95.4% are attained, which can meet the design specifcations.
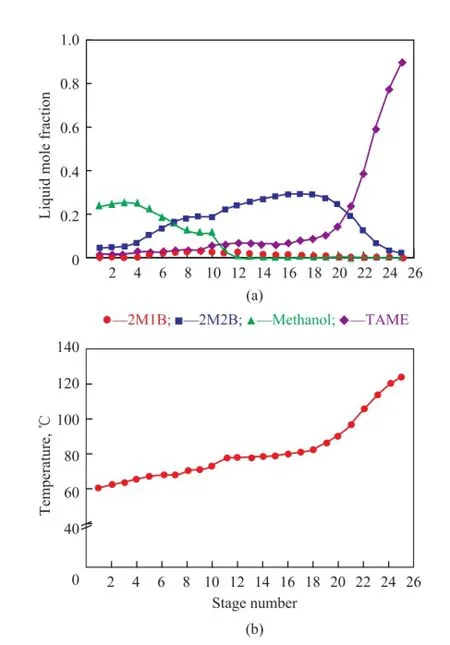
Figure 7 Liquid composition pro fi le (a) and temperature pro fi le (b) in the RD column
5 Conclusions
A proposed equilibrium stage model based design methodology can simultaneously obtain a high reactant conversion and a high product purity by using the reactive distillation column. Based on the proposed design methodology, the isoamylene conversion and the purity of TAME product can reach up to 93.13% and 95.4%, respectively. The kinetic model for isoamylene etherifcation over the high-activity Amberlyst 35 catalyst is presented. The activation energy for 2M1B and 2M2B etherifcation reactions is 77.15 kJ/mol and 96.64 kJ/mol, respectively. Furthermore, the designed etherifcation process is expected to be applied to FT syncrude refnery for high add-value utilization of C5olefns. It is worth noting that the design is at a conceptual level and further detailed work is needed to discuss the methanol recovery system, process dynamics and control system, and energy management.
Acknowledgements: The authors gratefully acknowledge the financial support from the National High Technology Research and Development Program 863 (2011AA05A204) and National Natural Science Foundation of China (U1361202).
Nomenclature
Notation
db—the diameter of the reactor bed, mm
dp—the diameter of the particle diameter, mm
Ej—activation energy for Eq 6, kJ/mol
F0—the total volumetric fow rate of the feed for Eq 3, mL/min
gu(X, θ)—the predicted reaction rates for Eq 7—8
K1—equilibrium constant for Eq 4
K2—equilibrium constant for Eq 5
KT/ KM—adsorption equilibrium constant for Eq 4—5
k1—rate constant for Eq 4, kmol/(kg·h)
k2—rate constant for Eq 5, kmol/(kg·h)
kj0—pre-exponential constants for Eq 6
Lb—the height of the bed, mm
nij— stoichiometric coeffcient of the i component for the j reaction for Eq 3
n—number of responses for Eq 7—8
rj—the rate of the j reaction for Eq 3
W—the weight of the catalyst for Eq 3, g
Xi—the conversion of the i component for Eq 3
X— the vector of independent variables, representing the activity of each component for Eq 7—8
Yu— the vector of random variables representing the observed reaction rates for Eq 7—8
Zu— the vector of errors associated while calculating the response for Eq 7—8
Greek letters
α—activity of a component for Eq 4—5
θ— the parameter vector, representing the kinetic constant for Eq 7—8
Subscripts
1B—2-methyl-1-butene
2B—2-methyl-2-butene
b—reactor bed
i—component
j—reaction
M—methanol
p—particle
T—tert-amyl methyl ether
u—experiment number
[1] García-Peñas A, Cerrada M L, Gómez-Elvira J M, et al. Microstructure and thermal stability in metallocene iPP-materials: 1-pentene and 1-hexene copolymers[J].Polymer Degradation and Stability, 2016, 124: 77-86
[2] Soto R, Fité C, Ramírez E, et al. Equilibrium conversion, selectivity and yield optimization of the simultaneous liquid-phase etherifcation of isobutene and isoamylenes with ethanol over Amberlyst™ 35[J].Fuel Processing Technology, 2016, 142: 201-211
[3] De Klerk A. Etherification of C6Fischer-Tropsch material for linear-olefn recovery[J].Industrial & Engineering Chemistry Research, 2004, 43(20): 6349-6354
[4] Perederic O A, Ple U V, Iancu P, et al. Simulation and process integration fortert-amyl-methyl ether (TAME) synthesis[J].Computers & Chemical Engineering, 2015, 83: 79-96
[5] Jakobsson K, Pyhälahti A, Pakkanen S, et al. Modelling of a side reactor configuration combining reaction and distillation[J].Chemical Engineering Science, 2002, 57(9): 1521-1524
[6] Ojeda Nava J A, Baur R, Krishna R. Combining distillation and heterogeneous catalytic reactors[J].Chemical Engineering Research and Design, 2004, 82(2): 160-166
[7] Masuku C M, Lu X, Hildebrandt D, et al. Reactive distillation in conventional Fischer–Tropsch reactors[J].Fuel Processing Technology, 2015, 130: 54-61
[8] Ferreira M V, Ribeiro A M, Loureiro J M. Experimental and simulation studies of TAME synthesis in a fixed-bed reactor[J].Industrial and Engineering Chemistry Research, 2007, 46(4): 1105-1113
[9] Ramkissoon A, Moonisar R, Maraj D, et al. Multiple analysis in a reactive distillation column for the synthesis of tertamyl methyl ether[J].Chemical Engineering & Technology, 2014, 37(12): 2190-2195
[10] Doherty M, Buzad G. Reactive distillation by design[J].Chemical Engineering Research and Design, 1992, 70(A5): 448-458
[11] Jantharasuk A, Gani R, Górak A, et al. Methodology for design and analysis of reactive distillation involving multielement systems[J].Chemical Engineering Research and Design, 2011, 89(8): 1295-1307
[12] Ciric A R, Gu D. Synthesis of nonequilibrium reactive distillation processes by MINLP optimization[J].AIChE Journal, 1994, 40(9): 1479-1487
[13] Jackson J R, Grossmann I E. A disjunctive programming approach for the optimal design of reactive distillation columns[J].Computers and Chemical Engineering, 2001, 25(11): 1661-1673
[14] González-Rugerio C A, Fuhrmeister R, Sudhoff D, et al.Optimal design of catalytic distillation columns: A case study on synthesis of TAEE[J].Chemical Engineering Research and Design, 2014, 92(3): 391-404
[15] Subawalla H, Fair J R. Design guidelines for solidcatalyzed reactive distillation systems[J].Industrial and Engineering Chemistry Research, 1999, 38(10): 3696-3709
[16] Almeida-Rivera C, Swinkels P, Grievink J. Designing reactive distillation processes: Present and future[J].Computers and Chemical Engineering, 2004, 28(10): 1997-2020
[17] Carrera-Rodríguez M, Segovia-Hernández J G, Hernández-Escoto H, et al. A note on an extended short-cut method for the design of multicomponent reactive distillation columns[J].Chemical Engineering Research and Design, 2014, 92(1): 1-12
[18] Li P, Huang K, Lin Q. A generalized method for the synthesis and design of reactive distillation columns[J].Chemical Engineering Research and Design, 2012, 90(2): 173-184
[19] Pääkkönen P K, Krause A O I. Comparative study of TAME synthesis on ion-exchange resin beads and a fbrous ion-exchange catalyst[J].Reactive and Functional Polymers, 2003, 55(2): 139-150
[20] Pääkkönen P K, Krause A O I. Diffusion and chemical reaction in isoamylene etherification within a cationexchange resin[J].Applied Catalysis A: General, 2003, 245(2): 289-301
[21] Mao W, Wang X, Wang H, et al. Thermodynamic and kinetic study of tert-amyl methyl ether (TAME) synthesis[J].Chemical Engineering and Processing: Process Intensifcation, 2008, 47(5): 761-769
[22] Froment G. Model discrimination and parameter estimation in heterogeneous catalysis[J].AIChE Journal, 1975, 21(6): 1041-1057
[23] Carberry J. Designing laboratory catalytic reactors[J].Industrial and Engineering Chemistry, 1964, 56(11): 39-46
[24] Routray K, Deo G. Kinetic parameter estimation for a multiresponse nonlinear reaction model[J].AIChE Journal, 2005, 51(6): 1733-1746
[25] Rihko L K, Kiviranta-Pääkkönen P K, Krause A O I. Kinetic model for the etherification of isoamylenes with methanol[J].Industrial and Engineering Chemistry Research, 1997, 36(3): 614-621
[26] Wilson G M. Vapor-liquid equilibrium. XI. A new expression for the excess free energy of mixing[J].Journal of the American Chemical Society, 1964, 86(2): 127-130
[27] Lide D. CRC Handbook of Chemistry and Physics[M]. Boca Raton: CRC Press, 2004
[28] Rihko L K, Linnekoski J A, Krause A O I. Reaction equilibria in the synthesis of 2-methoxy-2-methylbutane and 2-ethoxy-2-methylbutane in the liquid phase[J].Journal of Chemical and Engineering Data, 1994, 39(4): 700-704
[29] Oktar N, Mürtezaoǧlu K, Doǧu G, et al. Dynamic analysis of adsorption equilibrium and rate parameters of reactants and products in MTBE, ETBE and TAME production[J].The Canadian Journal of Chemical Engineering, 1999, 77(2): 406-412
[30] Laidler K J. The development of the Arrhenius equation[J].Journal of Chemical Education, 1984, 61(6): 494
[31] Piccoli R L, Lovisi H R. Kinetic and thermodynamics study of the liquid-phase etherification of isoamylenes with methanol[J].Industrial and Engineering Chemistry Research, 1995, 34(2): 510-515
[32] Kiviranta Pääkkönen P, Krause A O I. Simultaneous isomerization and etherification of isoamylenes with methanol[J].Chemical Engineering & Technology, 2003, 26(4): 479-489
[33] Rihko L K, Krause A O I. Kinetics of heterogeneously catalyzed tert-amyl methyl ether reactions in the liquid phase[J].Industrial and Engineering Chemistry Research, 1995, 34(4): 1172-1180
[34] Jin H, Xiao F, Yang C, et al. Micro-kinetics of the liquidphase synthesis of tertiary amyl methyl ether by ionexchange resin with isoamylenes and methanol[J].Journal of Petrochemical Universities, 2002, 15(1): 26-32 (in Chinese)
[35] Baur R, Krishna R. Hardware selection and design aspects for reactive distillation columns. A case study on synthesis of TAME[J].Chemical Engineering and Processing: Process Intensifcation, 2002, 41(5): 445-462
[36] Al-Arfaj M A, Luyben W L. Plantwide control for TAME production using reactive distillation[J].AIChE Journal, 2004, 50(7): 1462-1473
Received date: 2016-06-06; Accepted date: 2016-07-27.
Prof. Li Wenying, Telphone: +86-351-6018453; E-mail: ying@tyut.edu.cn.
杂志排行
中国炼油与石油化工的其它文章
- Effect of NH4F and Nano-SiO2on Morphological Control of α-Al2O3Platelets via Solid-state Reaction
- Desulfurization of Petroleum Coke by Calcination in Ammonia Atmosphere below 1 000 ℃
- Synthesis of Nano-ZSM-5 in Ultra-Concentrated System and Its Performance in Diesel Hydrodewaxing
- Effect of Mixed Oxide Support for Ni/ZnO in Reactive Adsorption Desulfurization
- Optimization and Control of Extractive Distillation with Heat Integration for Separating Benzene/Cyclohexane Mixtures
- Effects of Coexisting Substances on Nitrobenzene Degradation with O3/H2O2Process in High-Gravity Fields
