Optimization and Control of Extractive Distillation with Heat Integration for Separating Benzene/Cyclohexane Mixtures
2016-03-22
(State Key Laboratory of Heavy Oil Processing, China University of Petroleum, Qingdao 266580)
Optimization and Control of Extractive Distillation with Heat Integration for Separating Benzene/Cyclohexane Mixtures
Li Lumin; Tu Yangqin; Guo Lianjie; Sun Lanyi; Tian Yuanyu
(State Key Laboratory of Heavy Oil Processing, China University of Petroleum, Qingdao 266580)
In this work, the extractive distillation with heat integration process is extended to separate the pressure-insensitive benzene-cyclohexane azeotrope by using furfural as the entrainer. The optimal design of extractive distillation process is established to achieve minimum energy requirement using the multi-objective genetic algorithm, and the results show that energy saving for this heat integration process is 15.7%. Finally, the control design is performed to investigate the system’s dynamic performance, and three control structures are studied. The pressure-compensated temperature control scheme is proposed based on the frst two control structures, and the dynamic responses reveal that the feed disturbances in both fow rate and benzene composition can be mitigated well.
extractive distillation; heat integration; optimization; genetic algorithm; dynamic simulation
1 Introduction
Benzene is much in demand in modern petroleum and petrochemical operation. High-purity benzene is required as the raw material for manufacture of cyclohexane, toluene, chlorobenzene, styrene and phenol. Additionally, benzene is also the starting material for synthesis of pesticides, dyes and pharmaceuticals. However, benzene easily forms an azeotrope with cyclohexane, which cannot be completely separated in a single distillation column. There are some major distillation techniques applied in industry to separate azeotropes, such as azeotropic distillation, extractive distillation and pressure-swing distillation[1-2]. Since the mixture of benzene and cyclohexane is pressure-insensitive, it is diffcult to separate this mixture into two pure components through the conventional pressure-swing distillation. Azeotropic distillation and extractive distillation are the most commonly used methods to separate aromatic/aliphatic mixtures. In azeotropic distillation or extractive distillation, a third component is added into the system as the entrainer, and the separation system often includes a separation column and an entrainer recovery column. Consequently, the conventional distillation processes for benzene/cyclohexane mixture separation are expensive and cumbersome. To separate the benzene/cyclohexane mixture, Qin, et al.[3]proposed a novel extractive distillation process that operates the entrainer recovery column under vacuum, while the low temperature in the condenser of entrainer recovery column would result in a consequent increase in the operating cost. An extractive dividing wall column was developed to separate the benzene-cyclohexane mixture by Sun, et al., and the results have showed that the energy requirement can be reduced by 22%[4].
Heat integration of distillation process has been widely studied. There are two kinds of heat integration suited to the pressure swing distillation process: one combines the condenser in the high pressure column with the reboiler in the low pressure column, and the other combines the rectifying section in high pressure column with the stripping section in low pressure column[5]. Large energy savings for the separation of close-boiling mixtures can be achieved in the above-mentioned heat integration, and the corresponding steady state and dynamic characteristics have been studied extensively in recent years[6-12].
Luyben[13]compared the pressure-swing distillation and extractive distillation with and without heat integration for acetone-methanol separation system, and the resultsshowed that the total annual cost of extractive distillation system was by 15% lower than that achieved by the pressure-swing distillation. In order to achieve the heat integration of extractive distillation process, the pressure of entrainer recovery column should be increased to obtain the required temperature difference.
The heat integration in extractive distillation is possible for benzene/cyclohexane mixture separation. Therefore, this heat-integrated scheme is introduced to save the energy requirement and reduce the fxed capital investment by operating columns at di ff erent pressures. This paper will focus on the optimization and control of extractive distillation with heat integration for separating benzene/cyclohexane mixtures. In this context, the optimum design of the fowsheet is proposed to achieve the minimum energy requirement by using the multi-objective genetic algorithm method. Then three control structures are proposed to e ff ectively stabilize this optimum design. Finally, some conclusions are presented in the last section.
2 Steady-State Design
2.1 Process design
The extractive distillation is applied to separate the benzene-cyclohexane system, and the proposed fowsheet is presented in Figure 1, which contains two columns, viz.: an extractive distillation column (EDC) operating at atmospheric pressure and an entrainer recovery column (ERC) operating under a pressure of 6 atm. The refluxdrum temperature in HP column is 425 K with the purity of benzene distillate reaching 99.7 mol%, and the base of the extractive column is 409 K, thus there is a reasonable 16 K as the differential temperature driving force to size the reboiler/condenser heat exchanger. The Non-Random Two-Liquid (NRTL) model, which can predict this ternary system very well[14], is used to calculate the related physical properties. In this paper the extractive distillation was simulated using Aspen Plus with the following data: the feed consists of 750 kmol/h of benzene and 250 kmol/h of cyclohexane.
2.2 Sensitivity analysis
The parameters analyzed in this study cover the following items: the operating pressure of high-pressure (HP) column, the refux ratio (RR) of low-pressure (LP) column, the entrainer temperature (ET), and the entrainer to feed ratio (E/F).

Figure 1 Process fl ow diagram for extractive distillation with heat integration
Figure 2 shows the infuence of the operating pressure inside the HP column on the total duty in the process and the temperature difference between the bottom of LP column and the top of HP column. It is observed that the increase of operating pressure in the HP column results in a signifcant rise in the temperature difference during heat transfer, which means a corresponding decrease in the heat transfer area. However, attention should be paid to the increasing total energy requirement in this proposed process. This is because high operating pressure causes more liquid fraction in the feed stream of HP column,which means greater energy requirement at the reboiler. The effect of reflux ratio in the LP column on the cyclohexane composition and the condenser and reboiler duties are presented in Figure 3. It can be seen that the greatest cyclohexane composition is obtained at a refux ratio in the range of between 5.0 and 10.0, with a difference among them being no more than 0.05. It is noticed that the cyclohexane composition in the distillate does not change signifcantly when the refux ratio is greater than 5.0. Indeed, an apparent decrease in the product composition occurs due to the increasing reflux ratio without entrainer in the LP column. It is also possible to observe from Figure 3 the effect of refux ratio on the condenser and reboiler duties.
The energy requirement is significantly effected when the entrainer temperature varies in the range from 280 K to 380 K (see Figure 4). This is because less energy is required to vaporize the liquid at the bottom when the entrainer is fed at higher temperature. As the entrainer feed temperature is higher, more furfural is transferred to the vapor phase and the liquid fow to the bottom is less. However, no apparent infuence on the product purity can be observed until the entrainer feed temperature is higher than 340 K. When the entrainer temperature is higher than 340 K, the cyclohexane and benzene contents in the distillates are lower. This could be ascribed to the lower furfural content in the rectifying section of LP column, resulting in less cyclohexane separated in the distillate.
Figure 5 shows the effect of the entrainer to feed molar ratio (E/F) on the distillate composition and reboiler duty. Based on the above analysis, the refux ratio is maintained at a constant value (3.2). It can be seen that both of the reboiler duties in LP column and HP column show signifcant increase with a rising E/F ratio. Moreover, increasing values of the product purity can be obtained when the E/F ratio is less than 2.0. Nevertheless, when the E/F ratio is higher than 2.0, no signifcant effect on the product purity can be observed.

Figure 2 Effect of pressure in HP column on the total energy requirement and the temperature difference between the reboiler of LP column and the condenser of HP column

Figure 4 Effect of entrainer temperature on the energy requirement and the product composition in the distillate of the extractive distillation

Figure 3 Effect of re fl ux ratio in LP column (R1) on the cyclohexane composition (XD1) in the distillate of the extractive distillation and heat duty of LP column

Figure 5 Effect of entrainer to feed molar ratio (E/F) on the product composition and reboiler duties in the extractive distillation column
2.3 Optimization of extractive distillation process
The application of multi-objective genetic algorithm to optimize the extractive distillation columns has been published in recent papers[4,15-16]. In this paper, the extractive distillation processes with and without heat integration are optimized using multi-objective genetic algorithm to achieve minimum energy requirement. Evaluation of the objective function using multi-objective genetic algorithm with constraints, coupled with Aspen Plus[17]as a design tool, can obtain the expected results. Instead of obtaining one optimal design, a set of optimal designs is obtained through this procedure with integration of the Pareto front. In this way, the engineer can choose a trade-off by picking some points along the Pareto front. For the sake of optimization of multivariable functions, the stochastic optimization methods present a reasonable computational effort and they just need to calculate the objective function without problem reformulation.
As a thermodynamic equivalent of the extractive distillation with heat integration, two coupled RADFRAC units are used in Aspen Plus. The manipulated variables include the heat duty, the refux ratios, the total number of stages, the location of the feed, and the extracting agent fow.
In terms of multi-objective optimization, there are three objectives to minimize, namely: the number of stages of the total columns, the heat duty of the sequences and the extracting agent fow, which are in competition and constrained by the desired purity and recovery in each product stream, therefore the objectives must be optimized simultaneously. This problem can be expressed as follows:

whereNiis the number of stages in the columni,QRis the total heat duty, including the reboiler heat duties of LP column and HP column and the condenser heat duty of the HP column,QR,iis the heat duty of columni,FEAis the extracting agent fow,RRiis the refux ratio of columni,NF,iis the feed stage number of columni.andare the vectors of obtained and required purity and recovery for the componentk, respectively. For the case of extractive distillation with heat integration, 2 000 individuals and 40 generations are chosen as parameters of the genetic algorithm, with crossover and mutation fraction equating to 0.80 and 0.05, respectively. The procedure is carried out as follows. Firstly, a feasible initial design of the extractive column is given as an original solution to the algorithm of each run. The algorithm generates N individuals (i.e., new designs) based on the initial solution to make up the frst population. The manipulated variables of each of the N individuals are sent to Aspen Plus to perform the simulation, and then Aspen Plus gives the values of objective functions and constraints for each individual to the algorithm. The population is divided into subpopulations in terms of the number of satisfed constraints with the retrieved information, and at this time the best individuals can satisfy the c constraints, followed by those individuals that reachc-1 constraints, etc. Inside each subpopulation, the individuals are ranked according to the value of the ftness function. The original objective functions can be optimized through the classifcation of the population, which can also minimize the difference between the required and obtained constraints (recoveries and purity). Finally, a set of optimal designs of the extractive column is obtained. More detailed information about this algorithm and its link to Aspen Plus can be found in the original work[17].
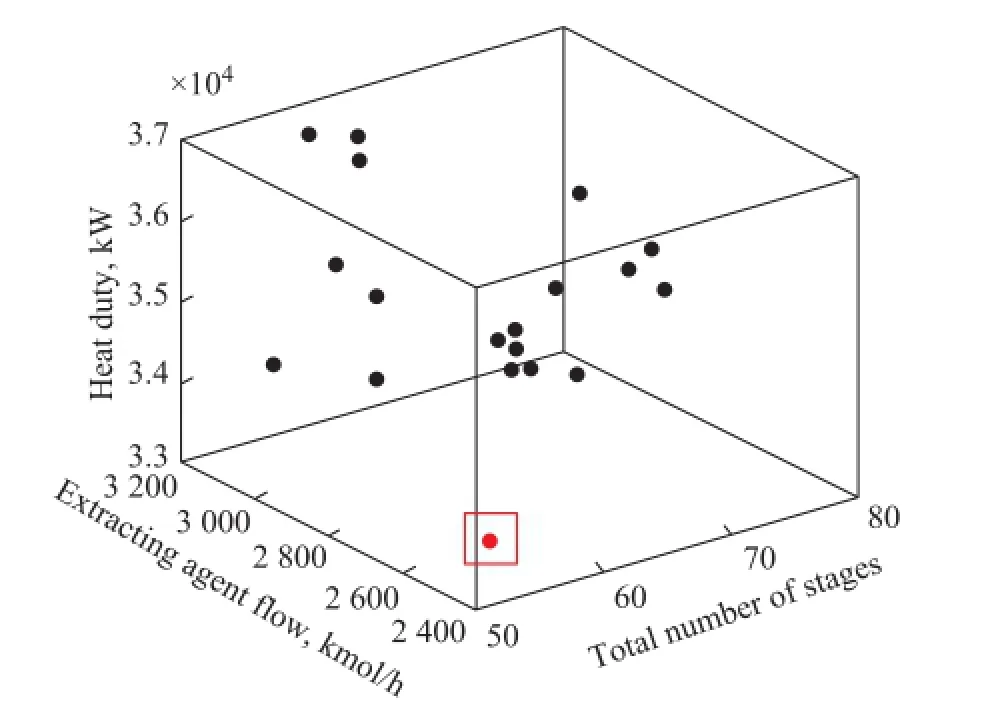
Figure 6 Pareto front of the extractive distillation for benzene/cyclohexane mixture
Figure 6 shows the Pareto front for the benzene/cyclohexane mixture, which includes the objectives to minimize the following, viz.: the heat duty of the sequence, the extracting agent fow and the total number of stages. Finally, 20 optimal designs are observed that make up the Pareto front, indicating that an extractive distillationcolumn with heat integration can perform the extractive separation. These optimal designs can satisfy the specifed purity and recoveries with the lowest energy consumed. In our work, the energy requirement is the criterion for meeting our particular needs, and then a design with the minimum reboiler duty is chosen as the fnal design. The optimum results of the heat integrated extractive distillation are compared with that of the conventional twocolumn process as shown in Table 1. The number of stages for the LP column and the HP column is 35 and 19, respectively. The recycle solvent returns back to the stage 8 and the feed fows to the stage 19. The number 3.80 is chosen as the refux ratio of the LP column. As for the HP column, the value of refux ratio is set at 1.40. In addition, the extracting agent fow rate is 2 500.12 kmol/h. It can be seen that under the above conditions, the total energy requirement of the heat integrated extractive distillation process is 33 461.12 kW, and the corresponding energy saving can reach 15.7% as compared with the conventional extractive distillation process.

Table 1 Optimum results of the conventional two-column design and the extractive distillation column with heat integration design
Figure 7 shows the temperature profles of the LP and HP columns stipulated in the optimum design. There is a rapid rise in the temperature in the stage 15 of the LP column, and it is obvious that the stage 12 displays a fairly steep slope for the temperature of the HP column. The profle distinguished features indicate that the stage 15 and the stage 12 are the proper temperature control points for the LP and HP columns, respectively.

Figure 7 (a) LP column temperature pro fi le; (b) HP column temperature pro fi le
3 Control System Design
3.1 Basic control structure for the extractive distillation (CS1)
The optimized fowsheet is exported to Aspen Dynamics as a pressure-driven simulation after refux drum and base volumes are sized to provide 5 min of holdup when they are half full, pumps and valves are specifed to give adequate pressure drop to handle changes in fow rates, and the pressure is checked.
Control problem mainly comes from the high interaction through the streams connecting the two columns and the heat transfer through the combined condenser and reboiler[18]. The dynamic system must satisfy two conditions, viz.: frstly, the combined condenser/reboiler duty is related to the heat transfer coeffcient, the heat transfer area, and thetemperature difference between the condenser of LP column and the reboiler of HP column; then, the heat input rate of the LP column reboiler is the sum of the heat removal rate of HP column condenser and the heat addition rate in the auxiliary reboiler. The corresponding equations then enter the text editor window as shown in Figure 8.

Figure 8 Flowsheet equations for the partial heat integration
After the flowsheet equations are compiled, simulation is unable to run because of the over-specifcation of two variables. To cope with this problem, the heat duties in the condenser of HP column and the reboiler of LP column must be changed from “fixed” to “free”. Figure 9 shows the basic control structure for extractive distillation process with heat integration, the effectiveness of which will be evaluated later.
(1) The fresh feed to LP column is flow-controlled (reverse acting).
(2) The refux drum level in both columns is held constant by manipulating the distillates fow rate (direct acting).
(3) The base level in LP column is maintained by manipulating the bottom fow rate (direct acting).
(4) The base level in HP column is maintained by manipulating the makeup fow rate (reserve acting).
(5) The pressure in LP column is controlled by manipulating the heat removal rate in the condenser (reverse acting).
(6) The refux ratio in HP column is fxed.
(7) The temperature for the stage 15 in LP column is controlled by manipulating the heat input rate of the auxiliary reboiler (reverse acting).
(8) The temperature for the stage 12 in HP column is controlled by manipulating the heat input rate in the reboiler of HP column (reverse acting).
(9) The total solvent fow rate is controlled using the FC2 controller by manipulating the recycle solvent flow rate (reserve acting).
(10) The solvent feed temperature is controlled by manipulating the cooler heat removal rate (reverse acting).
The PI controllers are used for all flow loops with the normal settings as shown below:KC=0.5 andτI=0.3 min; all level controllers are P-only withKC=2; all pressure loops are proportional-integral with the default values; the relay-feedback tests are used to determine the ultimate gains and periods of the two temperature controllers, using the Tyreus Luyben tuning. Two deadtime elements are inserted in the two temperature control loops with a deadtime of 1 min.

Figure 9 Basic control structure for the extractive distillation process with heat integration
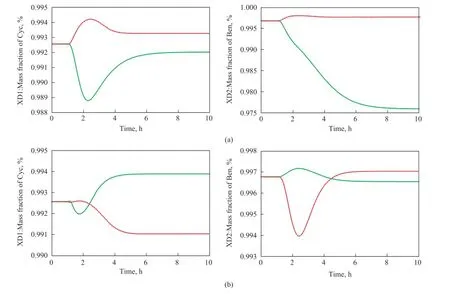
Figure 10 Dynamic responses for the basic control structure CS1: (a)±20% in feed fl ow rate; (b)±10% in benzene composition
Now the dynamic performance of the basic control structure is evaluated by using the disturbances in feed flow rate and composition. Figure 10(a) gives the dynamic responses of the control structure to positive and negative 20% step disturbances in feed fow rate in 1 h, and Figure 10(b) shows the responses to positive and negative 10% disturbances in benzene composition in 1 h.
It is noted that the basic control structure CS1 presents overshootings for the two kinds of disturbances, and the system takes about 6 h to reach a new steady state. It can be seen from Figure 10(b) that for a 10% decrease in benzene concentration of the fresh feed, a relatively large negative offset from the specified purity occurs in the cyclohexane product, leading to an undesired product. Hence, an improved control structure is needed.
3.2 Improved control structure with theQR/Fratio andS/Fratio (CS2)
In the improved control strategy, a multiplier block is added to the basic control structure to make sure that the heat input rate of the HP column reboiler is proportional to the feed fow rate. Moreover, if the fow rate or the concentration of the fresh feed is changed, the solvent flow rate should also be adjusted so that the products can meet the specifed purity. Thus, the S/F ratio is added in the improved control structure. Figure 11 shows the improved control structure CS2 provided with theQR/Fratio andS/Fratio. New relay-feedback tests are run to determine ultimate gains and periods of the two temperature controllers. Figure 12 shows the effectiveness of this improved control strategy. As demonstrated in Figure 12(a), the large transient deviation of cyclohexane purity decreases from 2.6% to 0.5% at a +20% feed flow rate disturbance when the improved control scheme is used. This is because when the feed fow rate increases, the reboiler duty of HP column also increases rapidly, bringing about evaporation of more vapor from the bottom, which would increase the heat removal rate in the HP column condenser and prevent more benzene from escaping from the bottom. However, because of hydraulics lag in the HP column, the effect of this improvement is not signifcant.
3.3 Control structure with pressure-compensated temperature (CS3)

Figure 11 The improved control structure CS2 with theQR/Fratio andS/Fratio

Figure 12 Dynamic responses for the improved control structure: (a)±20% in feed fl ow rate; (b)±10% in benzene content
Luyben has described[13,19]the pressure-compensated temperature control of the partial heat-integrated pressure swing distillation process in detail. In this paper, a pressure-compensated temperature control scheme for extractive distillation with partial heat integration is set up. Implementing the pressure-compensated temperature control in Aspen Dynamics requires the use of a third equation, which will provide a “pressure-compensated”temperature measurement in the HP column. The pressure in the HP column is not controlled in the heat-integrated system, which foats with operating conditions. A larger temperature difference is required when more heat trans-fer is needed in the reboiler/condenser, and consequently the pressure in the HP column increases, which also raises the bubble point temperature in the refux drum. The last equation calculates the signal fed to the deadtime element in the TC2 loop as shown in Figure 13(a), and Figure 13(b) gives the control structure using the pressure-compensated temperature.
The dynamic performance for this pressure-compensated temperature control structure CS3 is demonstrated in Figures 14. It can be seen from Figure 14 that the cyclohexane product purity is maintained to comply with its specifcation after the disturbance in feed fow rate, while a signifcant improvement is obtained in the response expressed in terms of the benzene content. In addition, the cyclohexane content can be easily brought back to its initial level within 4 h as compared with the control structure CS2. The results indicate that the drawbacks of the CS2 can be fairly well rectified by this pressurecompensated temperature control structure.
4 Conclusions
This paper considers the separation of pressure-insensitive benzene/cyclohexane azeotrope via extractive distillation with heat integration. The partially heat-integrated confguration is developed in Aspen Plus, and then the whole process is optimized using the multi-objective genetic algorithm. The simulation results show that the energy requirement for the conventional extractive distillation process and the heat-integrated extractive distillation process is 39 713.91 kW and 33 461.12 kW, respectively,and a 15.7% energy saving can be achieved by the heat integration process. Three control structures are proposed for this extractive distillation process. As for the pressurecompensated temperature control structure, the product purity is maintained to comply with the specifcation with negligible deviations after feed fow rate and composition disturbances, and a robust control can be achieved for this heat-integrated process.
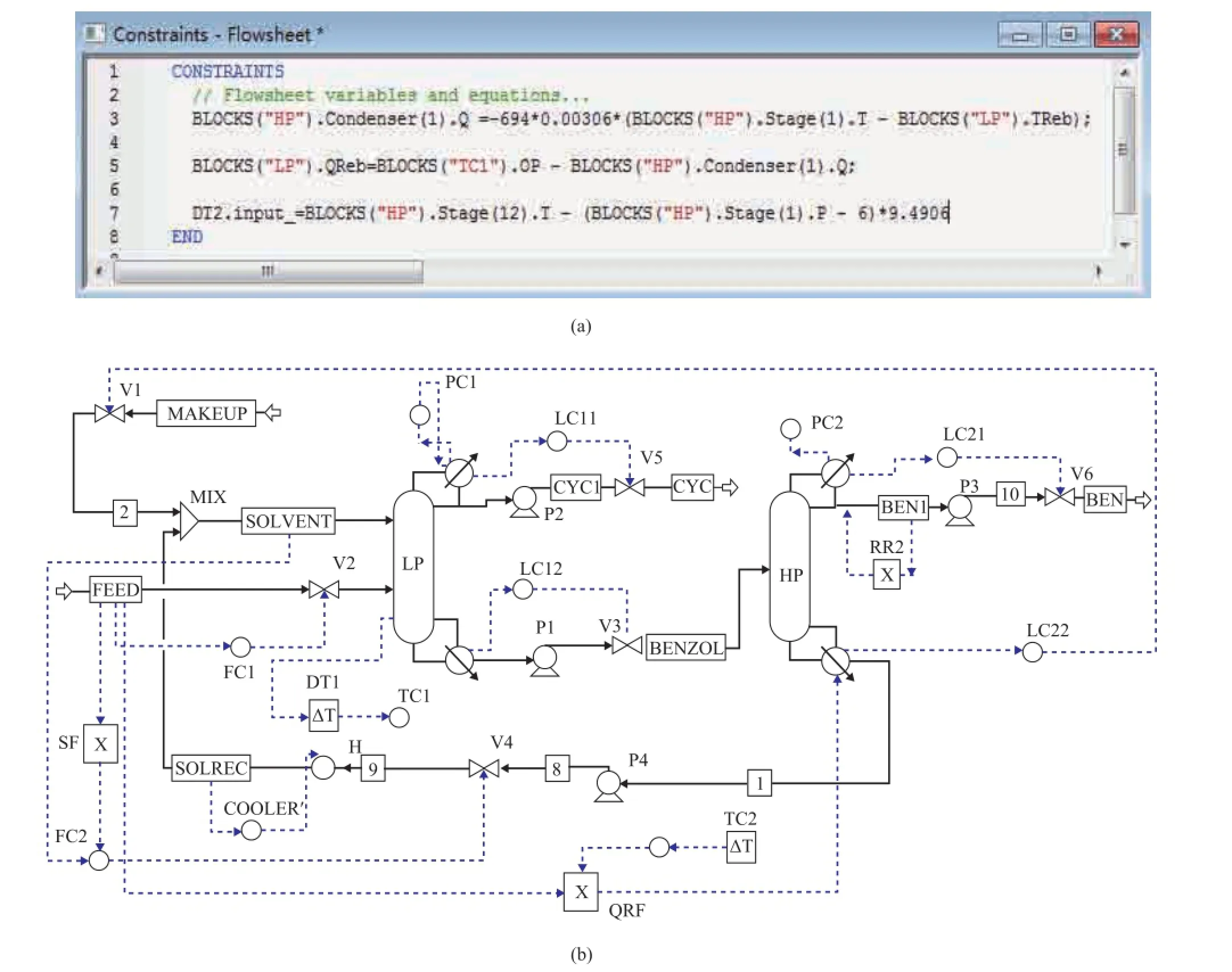
Figure 13 (a) Aspen Dynamics fl owsheet equations for heat integration and pressure-compensated temperature in HP column; (b) The control structure using pressure-compensated temperature CS3

Figure 14 Dynamic responses for the pressure-compensated temperature control structure: (a)±20% in feed fl ow rate; (b)±10% in benzene content
Acknowledgements: This work was supported by the National Natural Science Foundation of China (grant number 21 476 261); the Key Research and Development Plan Project of Shandong Province (grant number 2015GGX107004) Finally the authors are grateful to the editor and the anonymous reviewers.
Nomenclature
Ben—benzene
Cyc—cyclohexane
EDC—extractive distillation column
ERC—entrainer recovery column
F—fresh feed fow rate
FEA—the extracting agent fow
Fur—furfural
KC—controller gain
NF,i—feed stage number of columni
Ni—the number of stages of columni
QR—reboiler heat duty
RRi—the refux ratio of columni
S—recycle solvent fow rate
XD1—cyclohexane composition on the top of the LP column
τI—controller integral time constant
[1] Luyben W L, Chien I L. Design and Control of Distillation Systems for Separating Azeotropes[M]. John Wiley & Sons, 2011
[2] Lladosa E, Montón J B, Burguet M C. Separation of di-npropyl ether and n-propyl alcohol by extractive distillation and pressure-swing distillation: Computer simulation and economic optimization[J]. Chemical Engineering and Processing: Process Intensifcation, 2011, 50(11): 1266-1274
[3] Qin J, Ye Q, Xiong X, et al. Control of benzene–cyclohex-ane separation system via extractive distillation using sulfolane as entrainer[J]. Industrial & Engineering Chemistry Research, 2013, 52(31): 10754-10766
[4] Sun L, Wang Q, Li L, et al. Design and control of extractive dividing wall column for separating benzene/cyclohexane mixtures[J]. Industrial & Engineering Chemistry Research, 2014, 53(19): 8120-8131
[5] Huang K, Shan L, Zhu Q, et al. Adding rectifying/stripping section type heat integration to a pressure-swing distillation (PSD) process[J]. Applied Thermal Engineering, 2008, 28(8): 923-932
[6] Li L, Liu Y, Zhai J, et al. Design and control of self-heat recuperative distillation process for separation of close-boiling mixtures:n-butanol and iso-butanol[J]. China Petroleum Processing & Petrochemical Technology, 2015, 17(4): 111-120
[7] Jana A K. Heat integrated distillation operation[J]. Applied Energy, 2010, 87(5): 1477-1494
[8] Chen Y C, Hung S K, Lee H Y, et al. Energy-saving designs for separation of a close-boiling 1, 2-propanediol and ethylene glycol mixture[J]. Industrial & Engineering Chemistry Research, 2015, 54(15): 3828-3843
[9] Kiss A A, Olujić Ž. A review on process intensifcation in internally heat-integrated distillation columns[J]. Chemical Engineering and Processing: Process Intensifcation, 2014, 86: 125-144
[10] Zhu Z, Wang L, Ma Y, et al. Separating an azeotropic mixture of toluene and ethanol via heat integration pressure swing distillation[J]. Computers & Chemical Engineering, 2015, 76: 137-149
[11] Yu B, Wang Q, Xu C. Design and control of distillation system for methylal/methanol separation. Part 2: pressure swing distillation with full heat integration[J]. Industrial & Engineering Chemistry Research, 2012, 51(3): 1293-1310
[12] Li W, Shi L, Yu B, et al. New pressure-swing distillation for separating pressure-insensitive maximum boiling azeotrope via introducing a heavy entrainer: design and control[J]. Industrial & Engineering Chemistry Research, 2013, 52(23): 7836-7853
[13] Luyben W L. Comparison of extractive distillation and pressure-swing distillation for acetone-methanol separation[J]. Industrial & Engineering Chemistry Research, 2008, 47(8): 2696-2707
[14] Kumar U K A, Mohan R. Quinary and eight-component liquid–liquid equilibria of mixtures of alkanes, aromatics, and solvent (furfural)[J]. Journal of Chemical & Engineering Data, 2013, 58(8): 2194-2201
[15] Modla G, Lang P. Removal and recovery of organic solvents from aqueous waste mixtures by extractive and pressure swing distillation[J]. Industrial & Engineering Chemistry Research, 2012, 51(35): 11473-11481
[16] Modla G. Energy saving methods for the separation of a minimum boiling point azeotrope using an intermediate entrainer[J]. Energy, 2013, 50: 103-109
[17] Gutiérrez-Antonio C, Briones-Ramírez A. Pareto front of ideal Petlyuk sequences using a multiobjective genetic algorithm with constraints[J]. Computers & Chemical Engineering, 2009, 33(2): 454-464
[18] Huang K J, Nakaiwa M, Takamatsu T. Considering process nonlinearity in dual-point composition control of a highpurity ideal heat integrated distillation column, Chinese Journal of Chemical Engineering, 2001, 9(1): 58-64
[19] Luyben W L. Design and control of a fully heat-integrated pressure-swing azeotropic distillation system[J]. Industrial & Engineering Chemistry Research, 2008, 47(8): 2681-2695
Received date: 2016-07-11; Accepted date: 2016-09-09.
Prof. Sun Lanyi, Telephone: +86-13-854208340; Fax: +86-53-286981787; E-mail: sunlanyi@upc. edu.cn.
杂志排行
中国炼油与石油化工的其它文章
- Effect of NH4F and Nano-SiO2on Morphological Control of α-Al2O3Platelets via Solid-state Reaction
- Desulfurization of Petroleum Coke by Calcination in Ammonia Atmosphere below 1 000 ℃
- Synthesis of Nano-ZSM-5 in Ultra-Concentrated System and Its Performance in Diesel Hydrodewaxing
- Effect of Mixed Oxide Support for Ni/ZnO in Reactive Adsorption Desulfurization
- Methodology for Design of Reactive Distillation Column and Kinetics for Isoamylene Etheri fi cation Catalysed by Amberlyst 35
- Effects of Coexisting Substances on Nitrobenzene Degradation with O3/H2O2Process in High-Gravity Fields
