Effect of NH4F and Nano-SiO2on Morphological Control of α-Al2O3Platelets via Solid-state Reaction
2016-03-22
(Catalyst Workshop, SINOPEC Catalyst (Beijing) Co., Ltd., Beijing 102500)
Effect of NH4F and Nano-SiO2on Morphological Control of α-Al2O3Platelets via Solid-state Reaction
Miao Zhuang; Shi Jiangong; Zhang Wenping; Zhang Minhong
(Catalyst Workshop, SINOPEC Catalyst (Beijing) Co., Ltd., Beijing 102500)
Alpha-alumina (α-Al2O3) platelets were prepared via solid-state reaction using pseudo-boehmite as the starting material, while NH4F and nano-SiO2were used as additives, and nitric acid was used as the binder. The effect of these additives on properties of the alumina platelets was determined via scanning electron microscopy, X-ray diffraction, and measurements of the surface area and silicon content. A mechanism governing their role in the synthesis process was proposed. The results indicated that NH4F could promote the formation of highly crystalline platelets. Addition of nano-SiO2could lead to increase in the diameter and reduction in the thickness of the platelets, and could also prevent their transformation to α-Al2O3. This addition weakened the effect of NH4F because of the reaction of nano-SiO2with F-species and the formation of volatile SiF4.
α-Al2O3, nano-SiO2, NH4F, solid-state reaction
1 Introduction
Alpha-alumina (α-Al2O3) is one of the most important materials in chemical industry, because of its excellent thermal conductivity, chemical corrosion resistance, high mechanical strength and hardness. Meanwhile α-Al2O3platelet not only has the excellent performance of α-alumina, it also has a unique two-dimensional space structure provided with low electrical conductivity and good light reflection ability. These platelets are used in porous ceramics[1-2], in reinforcement of ceramics[3–9]or polymers[10–12], in thermal conductivity improvements[13], as well as in pigments[14], and serve as the catalytic support[15-16]. The morphology of these materials influences their performance in various applications. For example, the ceramic platelets are prone to disperse into a matrix phase, as compared with whiskers or short fbers[17], and can lead to high mechanical strength of composites[10–12]. The platelets are also used as ideal fillers in thermally conductive composites, the sufficiently large size of which can minimize the likelihood of thermal resistance between inter-particle contact[13]. In the case of ceramic applications, the plate-like α-Al2O3particles can increase fracture toughness more significantly than the ball-like particles because the plate-like grains can form crack bridging easily in the ceramic matrix[9]. Moreover, the fracture toughness is increased by reducing the size of the platelets through the introduction of seeds[18]. On the other hand, grains of various shapes, which comprise complexshaped porous bodies such as honeycombs, are critical to the performance of catalysts[15-16]. For these reasons, controlling or having the ability to manipulate the morphology of α-Al2O3plate-like powder is therefore essential.
The solid-state reaction is one of the most important techniques used to prepare α-Al2O3platelets. This highoutput method is simple, low-cost, and can result in only a small amount of pollutants, and is well suited for use in the industry[19]. The platelets may also be prepared via the molten-salt synthesis[20-22]. However, this method entails complex preparation processes, and the materials must be subsequently washed with deionized water (in order to remove inorganic salts) and repeatedly dried. Therefore, in this study, α-Al2O3platelets were obtained via the solidstate synthesis using pseudo-boehmite as the raw material, while adopting NH4F, nano-SiO2, and dilute nitric acid as additives. The effect of the amount of additives used and the nano-SiO2/NH4F mole ratios on the morphology of the platelets was determined. In addition, the mechanismgoverning the Si/F/H in the alumina solid-sate reaction was proposed, based on the results of scanning electron microscopy, X-ray diffraction, elemental analysis, and measurements of the specifc surface area.
2 Experimental
2.1 Raw materials
Pseudo-boehmite (Wenzhou Aluminum Oxide Co. Ltd., China.) was used as the starting material. NH4F (A. R. grade, Longxing Chemical Factory, China) and nano-SiO2(AEROSIL200, Evonik Degussa, Germany) were used as the mineralizer and morphology modifier, respectively. Concentrated nitric acid (65%, Beijing Chemical Works, China) was diluted to 20%.
2.2 Sample preparation
The α-Al2O3platelet samples were prepared according to the following steps: pseudo-boehmite, and various amounts of NH4F and nano-SiO2were pre-mixed for 15 min in a kneader operating at a rotary speed of 30 r/min. The diluted nitric acid was then added to the mixture, which was then kneaded for another 30 min. During the kneading process, the mixture gradually became damp, thereby leading to the rapid formation of a paste. An extrusion device was then used to shape the plastic feedstock into strips, which were subsequently placed in a drier at 120 ℃ for complete removal of the moisture contained therein. The α-Al2O3particles were obtained by calcining the resulting strips for 4 h at 1 250 ℃ in air.
2.3 Characterization
The morphology of the particles was examined by using a scanning electron microscope (SEM, Quanta 200, FEI Co., USA). Prior to this examination, the samples were placed in copper holders (using conductive carbon tape) and subsequently sputtered with thin conductive layers of gold. The phase composition of the samples was determined via X-ray diffraction (XRD, X’Pert MPD, Philips Co., Netherlands) using Ni-filtered CuKα radiation (λ=0.154 nm); the diffraction patterns were collected at a scanning rate of 4 (°)/min and over a 2θrange of 5°—80°. In addition, the specifc surface area of each sample was determined via N2adsorption (BET method) at -196 ℃, by using a surface area analyzer (NOVA4000e, Quantachrome Instruments). The samples were outgassed at 300 ℃ for 1 h prior to the analysis. The Si content of each sample was determined, in accordance with the Chinese standard GB/T 6609.3—2004.
3 Results and Discussion
3.1 Effect of NH4F on the morphology of α-Al2O3platelets
The effect of NH4F addition on the morphology of α-Al2O3particles was determined via SEM observation (Figure 1). In the absence of NH4F (Figure 1 (a)), the sample obtained via calcination retained the morphology of the starting material thanks to its shape memory effect. Figure 1 (b—d) shows the homogeneous α-Al2O3particles formed with the addition of NH4F. However, various plate-like morphology of alumina platelets was seen after the addition of different amount of NH4F. The block-shaped particles with an average diameter of ~1.95 μm were formed after mixing with 0.8% of NH4F. An addition of 1.60% of NH4F gave rise to the uniform disk-shaped platelets with a grain size of~2.25 μm (diameter)×0.68 μm (thickness). These platelets formed after addition of up to 3.20% of NH4F showed a further growth into particles with a grain size of ~2.95 μm (diameter)×0.53 μm (thickness).
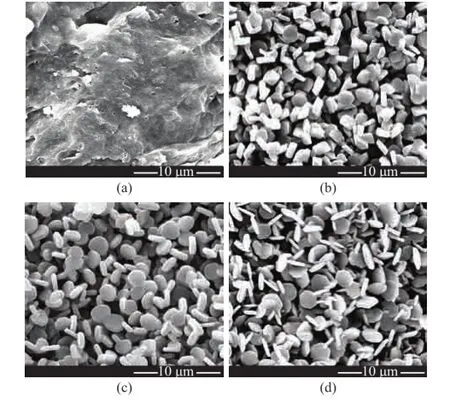
Figure 1 SEM micrographs of α-Al2O3platelets prepared through a 4-h calcination at 1 250 ℃
The specifc surface areas of aggregates of α-Al2O3particles with various contents of NH4F are summarized in Table 1. It can be seen from Table 1 that the surface area of α-Al2O3platelets increased with an increasing addition of NH4F, as evidenced by the increased diameter of the platelets.

Table 1 Specific surface area of aggregates of α-Al2O3platelets obtained after a 4-h calcination at 1 250 ℃ with various contents of NH4F
The XRD results were used to determine the influence of NH4F on the phase transformation of alumina. The XRD patterns shown in Figure 2, which corresponded to platelet samples formed without and with addition of NH4F, were typical of α-Al2O3, which were indicative of a complete transformation of pseudo-boehmite to α-Al2O3. Furthermore, the intensity of the XRD patterns was increased with an increasing amount of NH4F. These results along with the trends observed for the morphology of the sample particles indicated that NH4F could lead to the plate-like morphology and promote the formation of highly crystalline α-Al2O3.
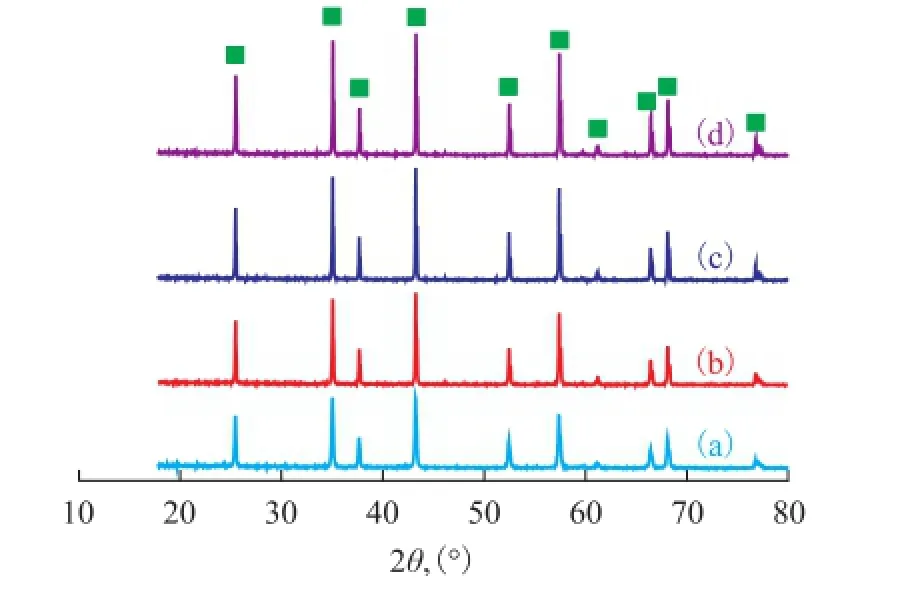
Figure 2 XRD patterns of α-Al2O3platelets prepared through a 4-h calcination at 1 250 ℃
The basis for the NH4F-induced formation of α-Al2O3platelets has been previously investigated[19,23-24]. In brief, at elevated temperature F-ions can disrupt the regular atomic packing and thereby result in Al3+vacancies. These vacancies are conducive to the mass transport and therefore can lead to a decrease in the α-phase transformation temperature and the formation of disk-shaped α-Al2O3particles[25]. Chen[24]used various fluoride additives during the preparation of α-Al2O3platelets, and concluded that NH4F was the most effective in accelerating the phase transformation and promoting the formation of regular plate-like particles, and this effectiveness was stemmed from the low thermal decomposition temperature of NH4F.
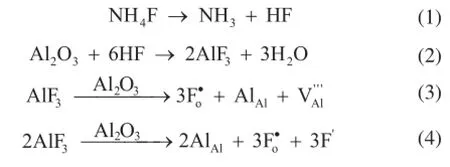
When NH4F is added to the alumina precursor, defects are formed via the following reactions[19,26]:
3.2 Effect of NH4F and nano-SiO2on the morphology of α-Al2O3platelets
The nano-SiO2was used as a morphology modifer during the synthesis. Figure 3 (a–d) shows the SEM micrographs of α-Al2O3platelets obtained when a fxed amount (i.e., 3.20%) of NH4F and various amount of nano-SiO2were used during the reaction. The sizes of these randomly stacked, plate-like particles vary signifcantly. Figure 3 (a and b) shows the platelets formed at nano-SiO2content equating to 0.2% and 0.4%, respectively. These samples are disk-shaped and are similar to the platelets formed without adding nano-SiO2(Figure 1 (d)). Mixing with 0.20% of nano-SiO2could result in homogenous 0.52-μmthick platelets having an average diameter of 3.30 μm. These samples were slightly smaller than the platelets with a particle size of 3.60 μm (diameter)×0.50 μm (thickness) formed (Figure 3 (b)) at a nano-SiO2content of 0.40%. Figure 3 (c) shows much larger hexagonal platelets formed at a nano-SiO2content of 0.60%, with their average size equating to 0.40 μm (thickness)×4.15 μm (diameter). In the case of the sample obtained with addition of up to 0.80% of nano-SiO2(Figure 3 (d)), the platelets exhibited deeply interdigitation structure, which resulted in a further increase in size of platelets to 4.81 μm (diameter)×0.35 μm (thickness). The above results indicated that mixing with nano-SiO2during preparation of α-Al2O3samples resulted in large and thin α-Al2O3platelets.
The specific surface areas of the α-Al2O3platelets pre-pared by adding a fxed 3.2% of NH4F and various contents of nano-SiO2are shown in Table 2. Upon addition of a fxed NH4F dosage, α-Al2O3platelets in the presence of nano-SiO2showed a further increase of surface area, as compared with the case without using nano-SiO2. Moreover, with an increasing addition of nano-SiO2, the surface area of α-Al2O3platelets increased with an increase in the amount of nano-SiO2added thereby.

Table 2 Specific surface area of aggregates of α-Al2O3platelets obtained after 4-h calcination at 1 250 ℃ with 3.2% of NH4F coupled with addition of various contents of nano-SiO2
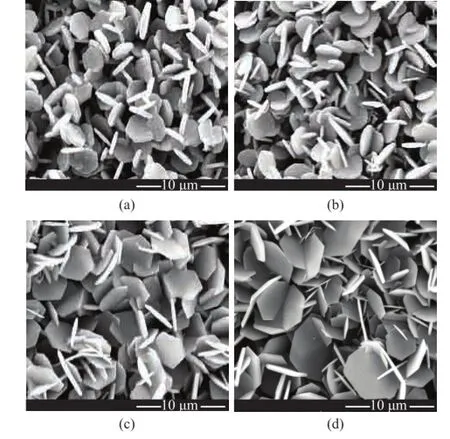
Figure 3 SEM micrographs of α-Al2O3platelets prepared through a 4-h calcination at 1 250 ℃ with 3.2% of NH4F
Figure 4 shows the XRD patterns of the platelets with a fxed 3.2% of NH4F and various contents of nano-SiO2. The diffraction lines shown in Figure 4 are typical of α-Al2O3, which is indicative of complete α phase transformation of all samples. However, the crystallinity of the α-Al2O3decreased significantly with an increasing amount of nano-SiO2added. The previous report [18] gave the same conclusion that addition of nano-SiO2led to a reduction in the crystallinity of the fnal α-Al2O3and inhibited the phase transformation from the transition phase to α-Al2O3.
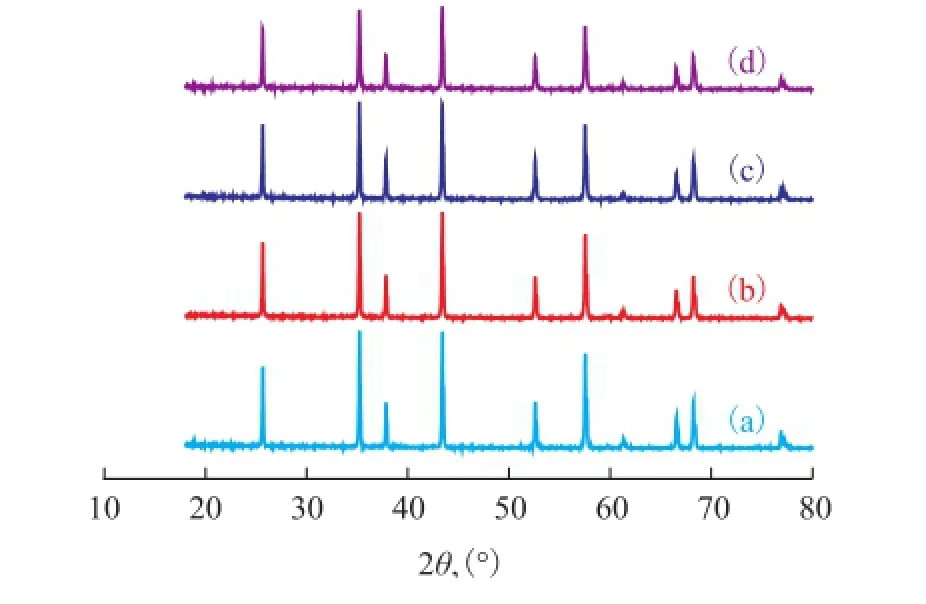
Figure 4 XRD patterns of α-Al2O3platelets prepared through a 4-h calcination at 1 250 ℃ with 3.2% of NH4F
In order to determine the mechanism governing the role of nano-SiO2and NH4F in the solid-sate reaction of alumina, the Si content in the samples obtained upon varying the amount of nano-SiO2and NH4F is listed in Table 3. For the case of α-Al2O3sample prepared without using NH4F, its actual Si content was equal to the theoretical value, which was indicative of no loss of Si when the sample was subject to the calcination process. For the case of samples prepared by using a fxed 3.2% of NH4F, the Si content in the samples increased with the increase of nano-SiO2dosage, but their actual value was obviously lower than the theoretical value. Moreover, the sharply decreased Si content in the samples obtained by using a fxed 0.8% of nano-SiO2coupled with 3.2% and 4.8% of NH4F had indicated that most of the nano-SiO2was consumed during the reaction of nano-SiO2with NH4F.

Table 3 Theoretical and actual Si content of α-Al2O3platelets obtained after 4-h calcination at 1 250 ℃ with various contents of nano-SiO2and NH4F
These results indicate that the large plate morphology and the decrease in the crystallinity of α-Al2O3(Figure 3 and Figure 4) stemmed from the reaction of F-species with nano-SiO2and consequent formation of volatile SiF4. This reaction is presented as follows:
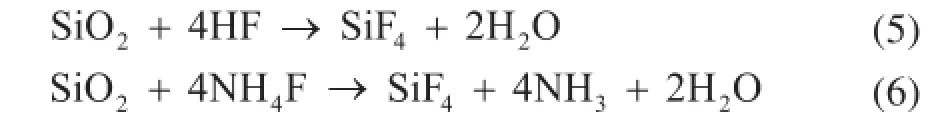
This reaction may proceed via two routes, as shown in Eqs. (5) and (6). It can be learned from Eq. (1) that NH4F is thermally decomposed into HF. This HF then reacts with SiO2, thereby forming volatile SiF4(Eq. (5)). However, as Eq. (6) shows, SiF4can also be formed from the direct reaction of NH4F with SiO2.
Different mole ratios of nano-SiO2and NH4F were mixed homogeneously prior to this calcination process. The residue mass rate was then calculated. During the calcination process, the amount of smoke released at ~350 ℃ was observed. The mixing parameters and the residue mass rate are listed in Table 4. It can be seen from Table 4 that a residue mass rate of higher than zero was obtained when the Si/F mole ratio was higher than 1:4. These rates are indicative of the presence of nano-SiO2. The mass rates were zero when Si/F mole ratios were lower than 1:4, indicating that the nano-SiO2was consumed during the reaction with NH4F to form volatile SiF4. The above results also illustrated that NH4F could react directly with nano-SiO2without nitric acid during the calcination process. The acid could react with pseudoboehmite to form an adhesive alumina gel and provide an environment conducive to the solid-state synthesis of alumina.
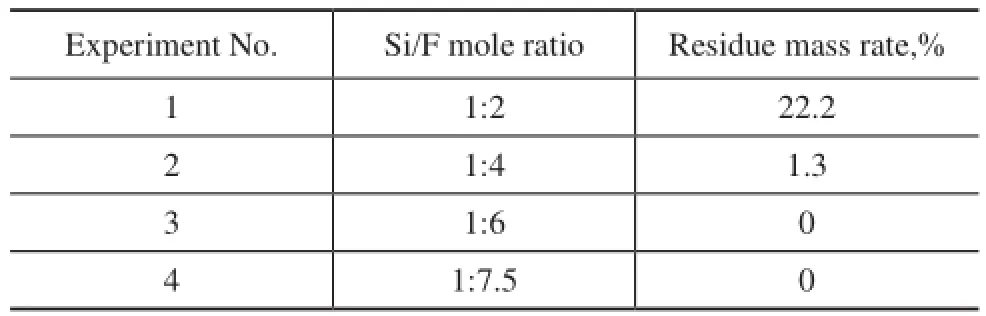
Table 4 Residue mass rate of NH4F and nano-SiO2obtained after 4-h calcination at 1 250 ℃ at various Si/F mole ratios
4 Conclusions
A solid-state reaction route was successfully developed for synthesizing homogeneous α-Al2O3platelets, using pseudo-boehmite as the starting material, while nano-SiO2and NH4F were used as additives. The morphological dependence of the platelets on the amount of NH4F and nano-SiO2used in the reaction was investigated. Furthermore, the mechanism governing the solid-state reaction of alumina was explored. The results revealed that NH4F promoted the formation of highly crystalline α-Al2O3platelets.
Addition of nano-SiO2led to significant increase in the diameter and reduction in the thickness of the platelets, and could prevent the phase transformation to α-Al2O3.
In fact, the addition of nano-SiO2weakened the effect of NH4F, owing to the reaction of nano-SiO2with F-species and subsequent formation of volatile SiF4.
Acknowledgment: This work was supported by the Technology Development (Commission) Project of SINOPEC Catalyst Co. Ltd (Grant No. 14-05-01). The authors would like to express their gratitude to the reviewers whose valuable suggestions improved the quality of the article.
[1] Shinobu H, Nishimura Y, Hirano H, et al. Microtexture control of alumina using anisotropic alumina particles[J]. Advances in Science and Technology, 2010, 70: 82-90
[2] Hashimoto S, Horita S, Ito Y, et al. Synthesis and mechanical properties of porous alumina from anisotropic alumina particles[J]. Journal of European Ceramic Society, 2010, 30 (3): 635-639
[3] Wei Ming, Zhi Dan, Brandon G D. Microstructure and texture evolution in gel-cast α-Al2O3/ alumina platelet ceramic composite[J]. Scripta Materalia, 2005, 53 (12): 1327-1332
[4] Bemardo E, Scarinci G, Hreglich S. Development and mechanical characterization of Al2O3platelet-reinforced glass matrix composites obtained from glasses coming from dismantled cathode ray tubes[J]. Journal of European Ceramic Society, 2005, 25 (9): 1541-1550
[5] Massimiliano G, Mohammad A, Simon R, et al. Strength, fracture toughness and microstructure of a selection of allceramic materials. Part I: Pressable and alumina glassinfltrated ceramics[J]. Dental Materials, 2004, 20 (5): 441-448
[6] Michal K, Jaroslav P, Pavel S, et al. Toughening effects quantifcation in glass matrix composite reinforced by alu-mina platelets[J]. Acta Materialia, 2008, 56 (12): 2908-2918
[7] Lorenz B J, Kirill F, Ludwig G J. Platelet-reinforced polymer matrix composites by combined gel-casting and hotpressing. Part I: polypropylene matrix composites[J]. Composites Science and Technology, 2010, 70 (13): 1958-1965
[8] Lorenz B J, Kirill F, Ludwig G J. Platelet-reinforced polymer matrix composites by combined gel-casting and hot-pressing. Part II: thermoplastic polyurethane matrix composites[J]. Composites Science and Technology, 2010, 70 (13): 1966-1972
[9] Kim J H, Kim G T, Kim J J, et al. Infuence of precursor and additive on the morphology of nanocrystalline α-alumina[J]. Journal of Physics and Chemistry of Solids, 2008, 69: 1521-1524
[10] Dharmendra S K, Rachit S K. Effect of alumina platelet reinforcement on dynamic mechanical properties of epoxy[C]// Proceedings of the World Congress on Engineering, 2011, 3
[11] Dharmendra S K, Venkitanarayanan P. Epoxy composites with 200 nm thick alumina platelets as reinforcements[J]. Journal of Materials Science, 2007, 42 (15): 5964-5972
[12] Lorenz B J, Andre S R, Jorg W, et al. Strong and ductile platelet-reinforced polymer films inspired by nature: microstructure and mechanical properties[J]. Journal of Materials Research, 2009, 24 (9): 2741-2754
[13] Yoshitaka N, Shinobu H, Sawao H, et al. Dielectric breakdown and thermal conductivity of textured alumina from platelets[J]. Journal of Ceramic Society of Japan, 2010, 118 (1383): 1032-1037
[14] Sigrid T, Gerhard P, Katsuhisa N. New effect pigments using innovative substrates[J]. European Coating Journal, 1999, 4: 90-96
[15] Sten W, Juliana S G, Madan B M, et al. Shaped porous bodies of alpha-alumina and methods for the preparation thereof: the United States, US 20100056816[P]. 2010
[16] Li J B, Li X F, Lin W, et al. Carrier for silver catalyst, its preparation, a silver catalyst made from the same and its use: the United States, US 20120172608[P]. 2012
[17] Tan H. B. Preparation of plate-like α-Al2O3particles in Na2SO4fux using coal fy ash as starting materials[J]. Materials Technology, 2011, 26 (2): 87-89
[18] Xie Z P, Lu J W, Gao L C, et al. Influence of different seeds on transformation of aluminum hydroxides and morphology of alumina grains by hot-pressing[J]. Materials and Design, 2003, 24 (3): 209-214
[19] Miao Z, Shi J G, Hao J W, et al. Effect of nano-TiO2and nano-SiO2addition on the morphological control of α-Al2O3platelets via solid-state reaction[J]. Ceramics International, 2016, 42: 1183-1190
[20] Chen H, Wu Q D, Yang T X, et al. The infuence of different titanium sources on faky α-Al2O3prepared by molten salt synthesis[J]. Ceramics International, 2015, 41: 12288-12294
[21] Zhu L H, Huang Q W. Morphology control of α-Al2O3platelets by molten salt synthesis[J]. Ceramics International, 2011, 37: 249-255
[22] Zhu L H, Tu R R, Huang Q W. Molten salt synthesis of α-Al2O3platelets using NaAlO2as raw material[J]. Ceramics International, 2012, 38: 901-908
[23] Yin Z L, Chen W, Li J F. The influence of F-on phase transformation of alumina and microstructure of α-Al2O3[J]. Bulletin of the Chinese Ceramic Society, 2007, 26: 640-644
[24] Chen W. Study on the microstructure evolution of α-Al2O3in the formation process and it’s controlling[D]. Changsha: Central South University, 2010
[25] Wang W L, Bi J Q, Qi Y Q, et al. Preparation of micrometer-sized α-Al2O3platelets by thermal decomposition of AACH[J]. Powder Technology, 2010, 201: 273-276
[26] Fu G F, Wang J, Kang J. Infuence of AlF3and hydrothermal conditions on morphologies of α-Al2O3[J]. Transactions of Nonferrous Metals Society of China, 2008, 18 (3): 743-748
Received date: 2016-07-20; Accepted date: 2016-08-15.
Shi Jiangong, Tel./ Fax: +86-10-6934 6355; E-mail: shijiangong@126.com.
杂志排行
中国炼油与石油化工的其它文章
- Synthesis of Nano-ZSM-5 in Ultra-Concentrated System and Its Performance in Diesel Hydrodewaxing
- Immobilization of Agaricus Bisporus Laccase on Ceramic-Chitosan Composite Support and Their Properties: Potential for Oily Wastewater Treatment
- Effects of Coexisting Substances on Nitrobenzene Degradation with O3/H2O2Process in High-Gravity Fields
- Effect of Mixed Oxide Support for Ni/ZnO in Reactive Adsorption Desulfurization
- Methodology for Design of Reactive Distillation Column and Kinetics for Isoamylene Etheri fi cation Catalysed by Amberlyst 35
- Desulfurization of Petroleum Coke by Calcination in Ammonia Atmosphere below 1 000 ℃
