Effect of Mixed Oxide Support for Ni/ZnO in Reactive Adsorption Desulfurization
2016-03-22
(The State Key Laboratory of Chemical Engineering, East China University of Science and Technology, Shanghai 200237)
Effect of Mixed Oxide Support for Ni/ZnO in Reactive Adsorption Desulfurization
Chen Weicheng; Yu Xiaoling; Huang Huan; Shi Li; Meng Xuan
(The State Key Laboratory of Chemical Engineering, East China University of Science and Technology, Shanghai 200237)
The effect of mixed oxide support on the performance of Ni/ZnO in the reactive adsorption desulfurization (RADS) reaction was investigated in a fxed bed reactor by using thiophene as the sulfur-containing compound in the model gasoline. A series of oxide supports for Ni/ZnO were synthesized by the co-precipitation method and characterized by XRD, N2-adsorption, TPR and NH3-TPD techniques. It was found that the desulfurization capacity of Ni/ZnO was enhanced greatly when active components were supported on the proper mixed oxide. Ni/ZnO supported on oxides exhibited much higher desulfurization effciency and sulfur adsorption capacity than the unsupported Ni/ZnO and the synthesized Ni/ZnO-SA adsorbent exhibited the highest effciency for thiophene removal. The higher desulfurization activity and sulfur capacity of Ni/ZnO supported on SiO2-Al2O3with small particle size, high specifc surface area and large pore volume could promote the high dispersion of active metal phase and the transfer of sulfur to ZnO with lower mass transfer resistance. γ-Al2O3species could weaken the interaction of active phases and SiO2as well as could increase greatly the amount of weak acids. Therefore, these oxides could impose a great infuence on the structure and chemical properties of the catalyst.
reactive adsorption desulfurization; Ni/ZnO; support effect; mixed oxide
1 Introduction
Sulfur compounds in transportation fuels remain a major source of air pollution. And these pollutant emissions also cause acid rain and decrease the lifetime of any operating systems by deactivating the metal catalyst as well as corroding the internal combustion engine components. Thus, it is of great importance to enhance the effciency of the desulfurization technologies, with strict environmental regulations promulgated to improve the quality of transportation fuels, particularly for the desulfurization of diesel, gasoline and jet fuel. The hydrodesulfurization (HDS) process is highly effcient in removing sulfdes. However, to meet the requirements for ultra-low-sulfur gasoline, the reactor size of HDS process needs to be increased 5—15 times in comparison with those reactors currently used. Several non-HDS-based desulfurization technologies have been proposed recently for removal of sulfur compounds from liquid fuels. Among them, the reactive adsorption desulfurization is considered to be the most promising approach because it combines the advantages of both HDS and the adsorption method. Several literature reports on adsorptive desulfurization are mainly related with the mechanism of reactive adsorption desulfurization (RADS) on Ni/ZnO adsorbent after Tawara, et al.[1]reported that Ni/ZnO could be used as an “autoregenerative catalyst”. Babich and Moulijn have described an overall reaction mechanism[2], indicating that thiophene is decomposed on the nickel surface that is then sulfdized, followed by hydrogenation of NiS sites and transfer of H2S to ZnO. Bezverkhyy, et al.[3-4]proposed a kinetic description and suggested some features of the reaction mechanism. They found that the initial limiting step was the thiophene decomposition on metallic Ni of Ni/ZnO adsorbent; whereas after formation of surface ZnS layer, the thiophene diffusion became the rate control step. As it is known to all, the smaller crystal particles and larger surface area will increase the rate of sulfur transfer in ZnO adsorbent, especially in the succeeding desulfurization process involving thiophene diffusion. Therefore, how to prepare theadsorbent with small particle size, large specifc surface area, high metal dispersion and low diffusion resistance is the key point for the development of RADS process.
The support effect was the principal topic of many studies in catalytic hydrodesulfurization (HDS) process[5-7]. Mixed oxides used as support material have drawn much attention because they can improve the textural properties, enhance the activity, improve the active metal dispersion, eliminate or reduce the coke formation, and prevent thermal sintering[8-10]. SiO2, Al2O3, TiO2, ZrO2and their mixed oxides have been reported to possess an interesting range of textural properties as well as to be capable of conducting different kinds of active metal interaction with the support[11]. Fan, et al.[12]studied reactive adsorption desulfurization over Ni/ZnO-SiO2-Al2O3adsorbent. They examined the effect of reduction and adsorption conditions on the desulfurization performance. Meng, et al.[13]have studied the reaction mechanism and regeneration performance of NiZnO/Al2O3-diatomite adsorbent used in the reactive adsorption desulfurization process. However, little attention has been focused on the support effect on Ni/ZnO in order to achieve a better activity of reactive adsorption desulfurization performance.
In this paper, a series of oxide catalysts were synthesized and characterized, and the effect of support on Ni/ZnO was investigated in order to improve its reactive adsorption desulfurization capacity.
2 Experimental
2.1 Feedstocks and adsorbent preparation
In this study, the model gasoline was prepared by adding thiophene to the sulfur-freen-octane with a sulfur concentration of 2 467.5 mg/L. A mixed solution of Zn(NO3)2·6H2O and Ni(NO3)2·6H2O (with a Zn/Ni molar ratio of 2) was used in the co-precipitation method and sodium carbonate was selected as the precipitating agent. Then, in a round bottom fask, a required amount of silica sol (containing 30% of SiO2) was mixed with deionized water under vigorous stirring. The mixed solution and sodium carbonate solution were simultaneously added dropwise to the suspension of the support materials under vigorous stirring, and the pH value was maintained at 8.0 during dripping process by controlling the flowrate of solutions added. The obtained suspension was stirred for 30 min, filtered, thoroughly washed with deionized water, dried in an oven for 12 h at 120 ℃, and calcined in a muffe furnace at 450 ℃ for 3 h. Then the NiO/ZnO-SiO2adsorbent was prepared. Afterwards, the NiO/ZnO adsorbent was prepared according to the same method without using silica sol. NiO/ZnO-Al2O3was also prepared by the same method except that the support material was replaced by γ-Al2O3. For the mixed oxide samples, γ-Al2O3and silica sol were physically mixed with deionized water under vigorous stirring and the adsorbent was labeled as NiO/ZnO-SA (50% SiO2-50% Al2O3).
2.2 Characterization of adsorbents
2.2.1 X-ray diffraction
X-ray diffraction (XRD) technique was used to characterize the crystal structure of powder adsorbents, using a Siemens D-500 X-ray diffractometer equipped with Nifltrated CuKα radiation (40 kV, 40 mA). The 2θscanning angle range was 10°—80° with the scanning speed equating to 0.02 (°)/s.
2.2.2 N2-adsorption
Nitrogen isotherms were measured at -196 ℃ with an ASAP 2020 instrument (Micromeritrics). Before the experiment, the samples were heated at 300 ℃ for 90 min under a vacuum of 10-5torr to maintain a constant pressure. N2isotherms were obtained in both adsorption and desorption modes.
2.2.3 Temperature programmed reduction
TPR experiments were performed to study the reducibility of the catalysts. The sample was pretreated in a He fow at 250 ℃ for 1 h followed by cooling to 25 ℃. The reducing gas containing 5% H2in the Ar mixture was passed over the samples at a fow rate of 30 mL/min at a heating rate of 10 ℃/min until a temperature of 800 ℃ was reached and maintained for 20 min.
2.2.4 NH3-temperature programmed desorption
NH3-TPD was measured with a Micromeritics AutoChem II chemisorption analyzer. Before adsorption, the sample (ca. 0.15 g) was activated in a fowing He stream (99.999% pure, at a rate of 50 ml/min) at 600 ℃ for 3 h. Adsorption of gas mixture composed of NH3(10%) and He (90%)took place at 50 ℃ until saturation, and then the sample was flushed with flowing He (30 mL/min) at the same temperature until a stable baseline condition was reached. The TPD measurements were carried out from 50 ℃ to 600 ℃ at a heating rate of 10 ℃/min, using He (30 mL/min) as the carrier gas. The amount of desorbed ammonia was detected by a thermal conductivity detector.
2.3 Sulfur adsorption experiments
The RADS experiments were performed at 390 ℃ under a pressure of 1.0 MPa at a pure H2flow rate of 40 mL/ min. The adsorbent (0.5287 g) was placed in a stainless steel column, 6 mm in inside diameter and 250 mm in length. The packed column was placed in a multi-channel convection oven designed in our laboratory for conducting the adsorption experiments. Prior to the reaction, the NiO/ZnO-support adsorbents were reduced to Ni/ZnO-support through the in-situ activation process in the presence of H2gas stream at a fow rate of 40 mL/min under a pressure of 0.5 MPa at 340 ℃ for about 1 h. After the pretreatment of adsorbents, the oven was preheated to the desired adsorption temperature with concurrent increase in the pressure of H2stream. In the course of adsorption experiments, the model fuel was fed into the adsorbent column by a micro-injection pump, and was routed downwards through the adsorbent bed at a weight hourly space velocity (WHSV) of 8 h-1, with the H2/oil volume ratio equating to 400. The liquid products were collected in a cryogenic trap provided with ice water bath and were subjected to analysis periodically. The treated fuel samples were quantitatively analyzed using a TS-3000 fuorescence sulfur analyzer. The desulfurization capacity was calculated based on the analytical results. The sulfur content (a, mg/g) can be calculated according to the following equation:

in whichC0is the initial sulfur concentration (g/g),Cis the fnal sulfur concentration (g/g), WHSV is the weight hourly space velocity (h-1), andtis the liquid fow time (h).
3 Results and Discussion
3.1 XRD characterization
Figure 1 compares the XRD patterns of NiO/ZnO, NiO/ ZnO-SiO2, NiO/ZnO-Al2O3, NiO/ZnO-SA, which have the same Zn/Ni molar ratio and same amount of support (20.2%). The XRD patterns in Figure 1 display distinct peaks corresponding to NiO and ZnO. The intensity of different peaks for NiO and ZnO in NiO/ZnO catalyst is much stronger than others, indicating that this NiO/ ZnO catalyst sample has higher crystallinity and larger particle size. As shown in Table 1, for the oxide supported NiO/ZnO adsorbents, the more broadened peaks indicate that the active oxides were highly dispersed on the support and the average crystallite size of NiO and ZnO calculated according to the Scherrer equation (D= 0.89λ/(βcosθ)) was smaller than that in the unsupported catalysts. It is noted that no SiO2and Al2O3species were detected in all samples, which might be ascribed to their amorphous phase or high degree of dispersion. The XRD patterns of NiO/ ZnO supported on oxides show much weaker intensity of diffraction peaks, indicating that the active phases were highly dispersed and the particle size was much smaller than the unsupported NiO/ZnO adsorbent.

Table 1 Crystallite sizes of the calcined samples

Figure 1 XRD patterns of samples of calcined NiO/ZnO supported on different oxide and unsupported NiO/ZnO
3.2 Catalyst texture
The information about the texture of adsorbents is shown in Table 2. As shown in Figure 2, a broad and asymmetric pore distribution was observed for the NiO/ZnO adsor-bent. However, the oxide supported NiO/ZnO adsorbents were prepared to possess an obviously mesoporous structure. As regards the active phases of NiO/ZnO supported on SiO2, a uniform mesoporous structure was observed with its average pore diameter centered at around 15 nm, and the BET surface area of NiO/ZnO-SiO2increased sharply from 29.99 m2/g to 147.74 m2/g. Meanwhile, NiO/ZnO-Al2O3exhibited a relatively broader pore size distribution than NiO/ZnO-SiO2, which was attributed to the pore structure of Al2O3. Comparatively speaking, as for the NiO/ZnO-SA adsorbent, it could be observed that the pore distribution of this adsorbent was much similar to that of NiO/ZnO-SiO2, but the peak was much strongly to be narrowly centered at around 20 nm. In comparison with the pore distribution of NiO/ZnO-Al2O3, the average pore diameter of NiO/ZnO-SA adsorbent was decreased and the BET surface area was enlarged. This fact indicated that silica sol might fill a part of the channels in Al2O3, making the broad and unordered pores become more uniform and irregular small channels, which was in accordance with the BET results.
As illustrated in Table 2, all oxide supported NiO/ZnO adsorbents exhibited relatively large specifc surface area than the unsupported NiO/ZnO adsorbent owing to the property of oxide supports. After hydrogen reduction pretreatment, the specifc surface area, pore volume and pore size of the reduced Ni/ZnO adsorbent became much smaller than those quality items of fresh NiO/ZnO adsorbent, indicating that hydrogen reduction might interfere with the reconstruction of NiO/ZnO adsorbent structure and could lead to agglomeration of active phases. Comparatively, the oxide supported Ni/ZnO adsorbents only showed a slight decrease in specific surface area, while the pore distribution was similar to that of the calcined adsorbents, with the average pore size just slightly increased. The texture property is one of the factors that can enhance the desulfurization performance of the reduced adsorbent. As it has been illustrated above, the mixed oxides SA supported Ni/ZnO showed a relatively high surface area and total volume featuring a narrow and uniform mesopore distribution. Therefore, this novel synthesized mixed-oxides SA has a great infuence on the textural properties of Ni/ZnO adsorbent suitable for reactive adsorption desulfurization.
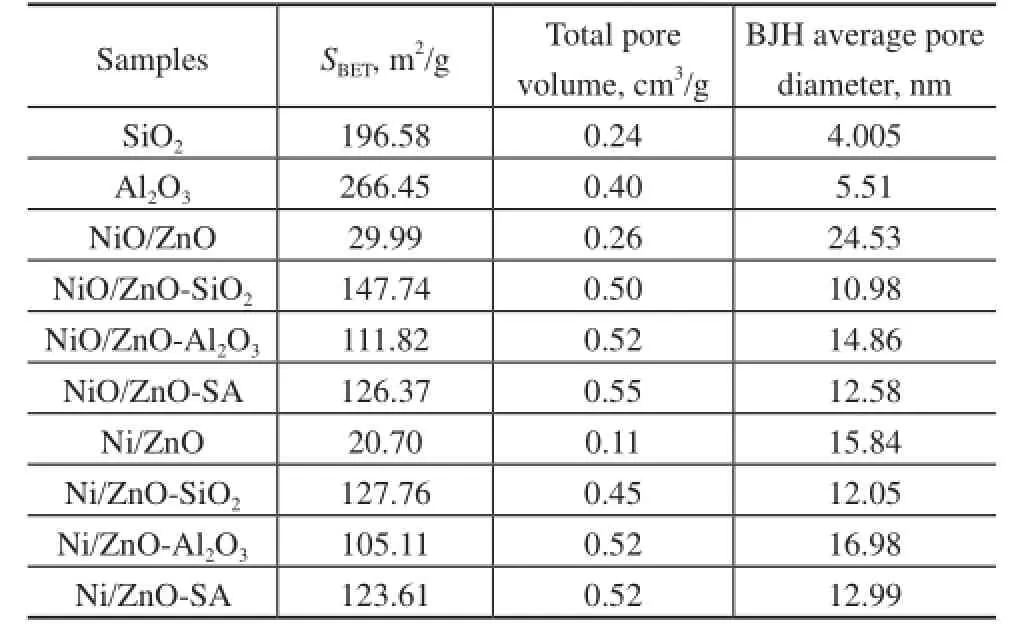
Table 2 Texture of the calcined and reduced samples
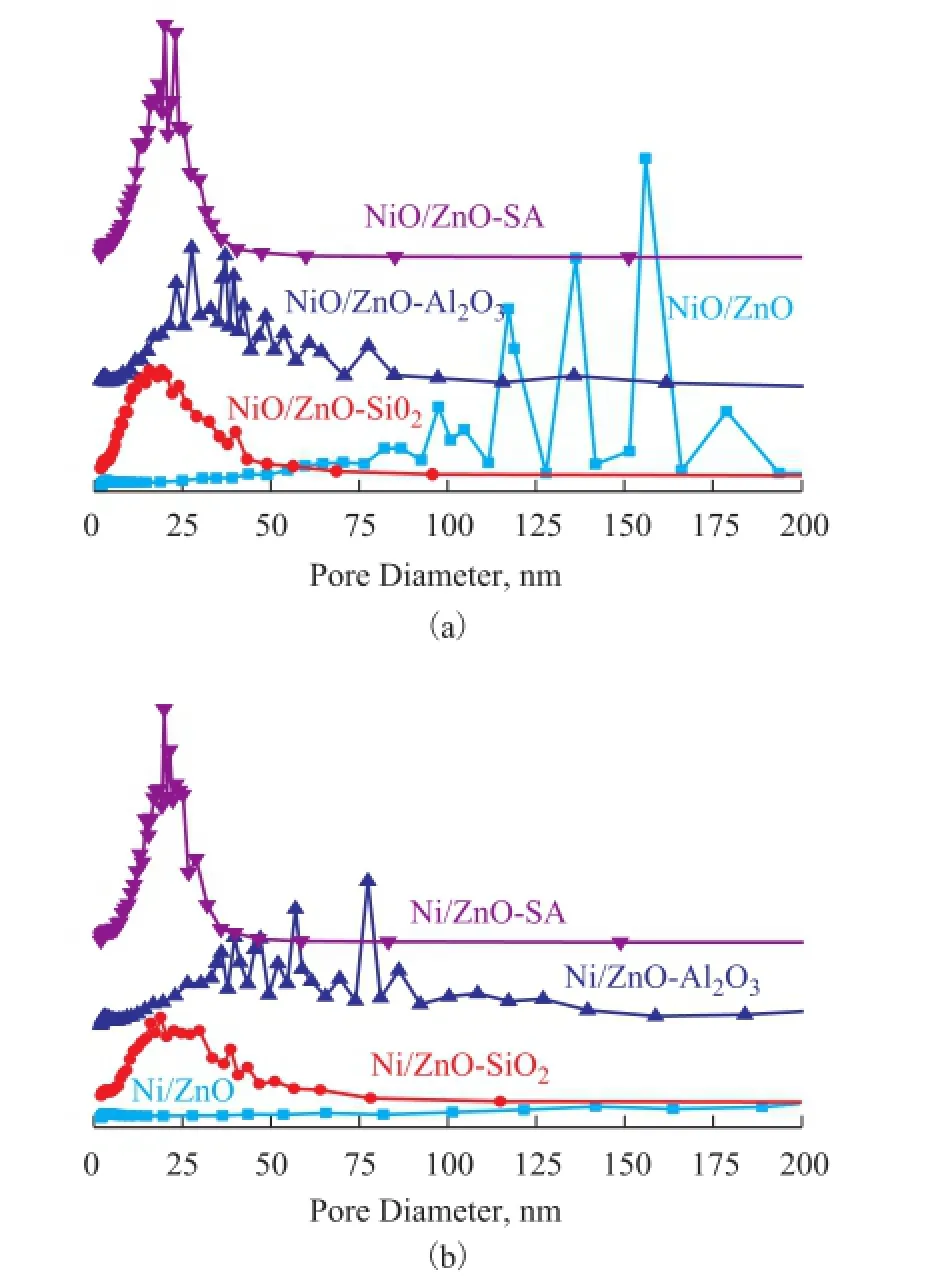
Figure 2 Pore size distribution of calcined (a) and reduced (b) samples
3.3 Temperature programmed reduction (TPR) characterization
The TPR characterization was performed on different samples of NiO/ZnO supported on different oxides to fnd out information about the interaction between the active metal and supports. To evaluate this effect, the TPR patterns of calcined adsorbents are shown in Figure 3. All four samples have an obvious peak withTmaxidentifed at 300—550 ℃, which was attributed to the reductionof NiO particles. The broad peak in the four samples at about 600—700℃ could correspond to the reduction of ZnO species which was consistent with our previous research[14]. The TPR results exhibited that the supported NiO/ZnO samples had much broader temperature profles than the unsupported NiO/ZnO. The reduction temperature of the supported NiO/ZnO samples was much higher than the unsupported NiO/ZnO, suggesting that NiO species located in the cavity of the framework of support were different from those in the unsupported NiO/ ZnO[15]. In addition, a high-temperature broad peak of NiO was observed at about 500 ℃ for the NiO/ZnO-SiO2sample, while theTmaxof NiO in the NiO/ZnO-SA sample was shifted to a lower temperature of 445 ℃, which was similar to the case of NiO/ZnO-Al2O3.This fact indicates that the oxide support could change the interaction between the metal and support and the addition of Al2O3could weaken the interaction of active phases and SiO2, which was in accordance with the report confrming that the mixed oxide supported catalysts could generate a favorable morphology of active phases to improve the metal-support interaction and facilitate the reducibility or sulfdability[10]. Furthermore, the intensity of NiO peak in the NiO/ZnO-SA sample was higher than that of NiO/ZnOAl2O3and was similar to that of NiO/ZnO-SiO2, implying that higher amount of NiO in the NiO/ZnO-SA sample could be readily reduced to the metallic state as compared to that in the NiO/ZnO-Al2O3sample. Mixed oxide supported catalysts are intermediate in terms of the reduction behavior between Si and Al. This fact suggests that both Si and Al ions were involved in binding the supported Ni species.

Figure 3 Temperature programmed reduction pro fi les of NiO/ZnO supported on different oxides and unsupported NiO/ZnO
3.4 NH3-temperature programmed desorption (TPD) characterization
In order to investigate the acid properties of the synthesized different oxide supported Ni/ZnO adsorbents, the NH3-TPD experiments were carried out, with the profles presented in Figure 4. NH3desorption is temperaturedependent and can be classifed in three stages, viz.: weak (<200 ℃), moderate (200—450℃) and strong (>450℃). It can be seen from Figure 4 that there existed two peaks for the adsorbents corresponding to the desorption of NH3from weak and moderate acid sites, respectively. The intensity of the frst peak increased in the following order: NiO/ZnO ≈ NiO/ZnO-SiO2< NiO/ZnO-Al2O3< NiO/ZnOSA, indicating to the gradual increase in acid amount[16]. The results suggested that the single SiO2had no effect on the acid property of NiO/ZnO, while γ-Al2O3possessed an increased weak acid amount owing to the surface hydroxyl ions of alumina. Moreover, when Ni/ZnO was supported on the mixed oxides SA, the adsorbent exhibited a highest amount of weak acids and moderate acids, indicating that silica sol had interacted with alumina to generate more hydroxyl ions in the adsorbent.

Figure 4 NH3-TPD pro fi les of NiO/ZnO supported on different oxides
3.5 Adsorptive Performance
Figure 5 shows the desulfurization performance of the oxide-supported Ni/ZnO adsorbents used in sulfur adsorption experiments. The sulfur content of thiophene-doped model oil can be reduced to a very low value during the reactive adsorption desulfurization. The desulfurization performance of the studied adsorbents increased in the following order: Ni/ZnO < Ni/ZnO-SiO2< Ni/ZnOAl2O3< Ni/ZnO-SA. The Ni/ZnO-SA adsorbent exhibitedan excellent RADS capacity. It took about one hour to reach a steady-state activity of samples after the feed was pumped into the reactor. The varying trend of sulfur content in the feed is consistent with the result reported by Huang, et al.[17]The sulfur content in the outfow oil after treatment by the Ni/ZnO-SA adsorbent remained lower than 10 ppmw (7.05 mg/L) when the time on stream was in the range of between 1 h to 8.5 h, and it then increased slowly to about 17.0 mg/L in the next 1.5 h of reaction. Moreover, the Ni/ZnO-SiO2adsorbent showed a much better desulfurization capacity than the unsupported Ni/ ZnO adsorbent. According to the BET analysis results, the Ni/ZnO-SiO2adsorbent had a uniform mesoporous structure and much larger surface area to increase the dispersion of active phases. And the Ni/ZnO-Al2O3adsorbent had increased the acid amount, which could contribute to the increase in its desulfurization activity. As for the mixed oxides SA promoted adsorbent, it combined the advantages of pure support SiO2and Al2O3as evidenced by its large mesoporous structure, high surface area and more acid sites. Morphological changes can signifcantly affect the RADS performance. The desulfurization activity and capacity of Ni/ZnO-SA adsorbent were significantly increased. It appears that the mixed oxide support could increase the number of active sites and optimize the proper pore structure.

Figure 5 Effect of different nano-composite oxide support on RADS performance
Furthermore, the theoretical capacity for RADS over Ni/ ZnO-SA adsorbent is calculated to be 287.4 mg-S/g-sorbent, when ZnO and NiO are supposed to be completely sulfdized to ZnS and Ni3S2, respectively. After calculation based on the breakthrough curve, we found out that the total sulfur capacity of Ni/ZnO-SA adsorbent was 292.13 mg-S/g-sorbent at a breakthrough sulfur level of 17.0 mg/L with a trace amount of H2S being detected, the value of which was a bit higher than the calculated value because of the consequence of further HDS reaction. The results indicated that ZnO supported on composite SA could be completely transformed to ZnS. After all ZnO species were sulfdized, a certain amount of sulfur moved from the model oil could not be stored in the adsorbent and was released to the effluent in the form of H2S, as verified through monitoring the effluent with a H2S-detecting tube. This result was in agreement with the report stating that when H2S appeared in the gas phase after complete sulfdation, thiophene conversion in the reactor was possible because the catalytic desulfurization process was similar to the HDS process[18].
3.6 Study of the RADS process
According to the report stating that when ZnO is partially sulfdized, the sulfur diffusion becomes a rate determining step, thus the oxide support with specific physical and chemical properties can affect the performance in the process of desulfurization over the Ni/ZnO adsorbent[3]. Scheme 1 describes a proposed adsorption desulfurization over Ni/ZnO and Ni/ZnO-SA adsorbents. Thiophene in the model fuel is at frst decomposed on the surface of Ni species which can be converted to Ni3S2, while the hydrocarbon portion of the molecule is released back into the process stream. Secondly, Ni3S2is converted to form Ni and H2S in the presence of hydrogen. Then, H2S is stored quickly in the adsorbent along with the conversion of ZnO into ZnS.[17]

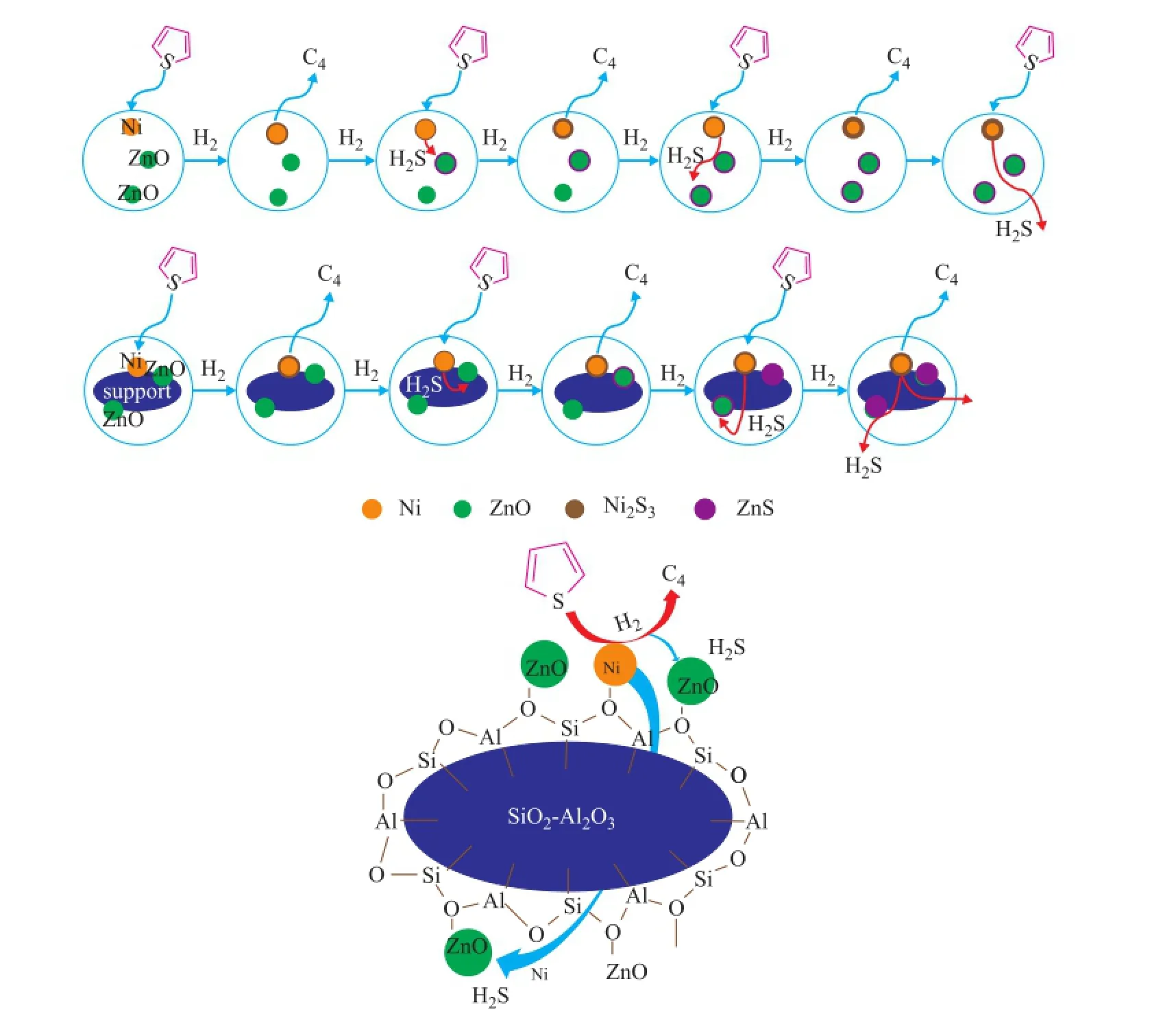
Scheme 1 Proposed scheme for the desulfurization process over Ni/ZnO and the mixed oxides supported Ni/ZnO-SA adsorbents
According to the report, since the transformation of ZnO to ZnS by H2S was accompanied by a strong increase of molar volume and a pronounced agglomeration of particles, it could lead to a collapse of the interparticle voids[17], and then would slow down the thiophene diffusion, making itself the rate determining step other than the thiophene desulfurization reaction rate. The natural texture of mixed oxide support could form a favorable diffusion path to improve the sulfur removal capacity, while promoting the interaction between Ni and ZnO to reduce the resistance on transfer of sulfur from Ni3S2to ZnS. Specifcally, the NiO and ZnO species with a small particle size could be highly dispersed on the mixed oxide support, and the adsorbent with higher specific surface area and more acid sites was characteristic of higher desulfurization activity. After the shell of ZnO particle was sulfdized, the favorable texture structure of support could effectively decrease the mass transfer resistance on sulfur diffusion, leading to a relatively high activity in a longer period of time. As a result, the Ni/ZnO-SA adsorbent with a uniform pore structure and highly dispersed active sites could maintain its high RADS activity and could result in much higher desulfurization capacity. However, it would be really diffcult for H2S to react quickly with the core part of ZnO species in the Ni/ZnO adsorbent and the adsorbent would show a lower adsorption capacity quite deservedly.
4 Conclusions
A series of oxide supports for Ni/ZnO were synthesized by means of the co-precipitation method. The effect of oxide supports on the adsorptive desulfurization performance was investigated by using thiophene as a sulfurcontaining compound of the model gasoline in a fxed bed reactor. The oxide supports for Ni/ZnO adsorbent exhibited strongly positive effect on its ability suitable for the RADS reaction and the synthesized Ni/ZnO-SA adsorbent exhibited a highest effciency for thiophene removal. The extent of this positive effect is related with the texture and chemical properties of supports. Various characterization results have revealed that the support consisting of Al2O3and SiO2with small crystal size, high surface area and mesopore structure can promote the high dispersionof active metal phase and lower mass transfer resistance on sulfur, and the γ-Al2O3species can weaken the interaction of active phases with SiO2and can also increase greatly the amount of weak acids. As a result, the ability of Ni/ZnO adsorbent could be enhanced drastically in the RADS reaction.
Acknowledgments: This work is fnancially supported by the National Natural Science Foundation of China (No.21276086).
[1] Tawara K, Nishimura T, Iwanami H, et al. New hydrodesulfurization catalyst for petroleum-fed fuel cell vehicles and cogenerations[J]. Ind Eng Chem Res, 2001, 40(10): 2367-2370
[2] Babich I V, Moulijn J A. Science and technology of novel processes for deep desulfurization of oil refnery streams: A review[J]. Fuel, 2003, 82(6): 607-631
[3] Bezverkhyy I, Ryzhikov A, Gadacz G, et al. Kinetics of thiophene reactive adsorption on Ni/SiO2and Ni/ZnO[J]. Catal Today, 2008, 130(1): 199-205
[4] Bezverkhyy I, Safonova O V, Afanasiev P, et al. Reaction between thiophene and Ni nanoparticles supported on SiO2or ZnO: In situ synchrotron X-ray diffraction study[J]. J Phys Chem C, 2009, 113(39): 17064-17069
[5] Rana M, Ramirez J, Gutierrezalejandre A, et al. Support effects in CoMo hydrodesulfurization catalysts prepared with EDTA as a chelating agent[J]. J Catal, 2007, 246 (1): 100-108
[6] Yoshida H, Tsuruta T, Yazawa Y, et al. Support effect on oxidation resistance of precious metal catalysts as examined by N2O decomposition[J]. Appl Catal A, 2007, 325 (1): 50-56
[7] Kobayashi M, Morita A, Ikeda M. The support effect in oxidizing atmosphere on propane combustion over platinum supported on TiO2, TiO2-SiO2and TiO2-SiO2-WO3[J]. Appl Catal B, 2007, 71 (1/2): 94-100
[8] Meng X, Huang H, Weng H X, et al. Ni/ZnO-based adsorbents supported on Al2O3, SiO2, TiO2, ZrO2: A comparison for desulfurization of model gasoline by reactive adsorption[J]. Bull Korean Chem Soc, 2012, 33 (10): 3213-3217
[9] Ninh T K T, Massin L, Laurenti D, et al. A new approach in the evaluation of the support effect for NiMo hydrodesulfurization catalysts[J]. Appl Catal A, 2011, 407 (1/2): 29-39
[10] Rana M S, Huidobro M L, Ancheyta J, et al. Effect of support composition on hydrogenolysis of thiophene and Maya crude[J]. Catal Today, 2005, 107-108: 346-354
[11] Breysse M, Afanasiev P, Geantet C, et al. Overview of support effects in hydrotreating catalysts[J]. Catal Today, 2003, 86(1/4): 5-16
[12] Fan J X, Wang G, Sun Y, et al. Research on reactive adsorption desulfurization over Ni/ZnO-SiO2-Al2O3adsorbent in a fxed-fuidized bed reactor[J]. Ind Eng Chem Res, 2010, 49(18): 8450-8460
[13] Meng X, Huang H, Shi L. Reactive mechanism and regeneration performance of NiZnO/Al2O3-diatomite adsorbent by reactive adsorption desulfurization[J]. Ind Eng Chem Res, 2013, 52(18): 6092-6100
[14] Meng X, Weng H X, Shi L. Reactive adsorption of thiophene on ZnNi/diatomite-pseudo-boehmite adsorbents[J]. China Petroleum Processing and Petrochemical Technology, 2012, 14(3): 33-38
[15] Dai W L, Cao Y, Ren L P, et al. Ag-SiO2-Al2O3composite as highly active catalyst for the formation of formaldehyde from the partial oxidation of methanol[J]. J Catal, 2004, 228(1): 80-91
[16] Fan Y, Lu J, Shi G, et al. Effect of synergism between potassium and phosphorus on selective hydrodesulfurization performance of Co-Mo/Al2O3FCC gasoline hydroupgrading catalyst[J]. Catal Today, 2007, 125: 220-228
[17] Huang L C, Wang G F, Qin Z F, et al. A sulfur K-edge XANES study on the transfer of sulfur species in the reactive adsorption desulfurization of diesel oil over Ni/ ZnO[J]. Catal Commun, 2010, 11(7): 592-596
[18] Ryzhikov A, Bezverkhyy I, Bellat J P. Reactive adsorption of thiophene on Ni/ZnO: Role of hydrogen pretreatment and nature of the rate determining step[J]. Appl Catal B, 2008, 84 (3/4): 766-772
Received date: 2016-07-25; Accepted date: 2016-08-25.
Dr. Meng Xuan, Telephone: +86-21-64252383; E-mail: mengxuan@ecust.edu.cn.
杂志排行
中国炼油与石油化工的其它文章
- Synthesis of Nano-ZSM-5 in Ultra-Concentrated System and Its Performance in Diesel Hydrodewaxing
- Immobilization of Agaricus Bisporus Laccase on Ceramic-Chitosan Composite Support and Their Properties: Potential for Oily Wastewater Treatment
- Effects of Coexisting Substances on Nitrobenzene Degradation with O3/H2O2Process in High-Gravity Fields
- Effect of NH4F and Nano-SiO2on Morphological Control of α-Al2O3Platelets via Solid-state Reaction
- Methodology for Design of Reactive Distillation Column and Kinetics for Isoamylene Etheri fi cation Catalysed by Amberlyst 35
- Desulfurization of Petroleum Coke by Calcination in Ammonia Atmosphere below 1 000 ℃
