The effect of Mg2+on boron incorporation into carbonate and the infl uence of B/Ca proxies for the deep ocean carbonate system
2016-03-21HEMaoyongXIAOYingkai
HE Maoyong, XIAO Yingkai
(1. State Key Laboratory of Loess and Quaternary Geology, Institute of Earth Environment, Chinese Academy of Sciences, Xi'an 710061, China; 2. Qinghai Institute of Salt Lakes, Chinese Academy of Sciences, Xining 810008, China; 3. Shaanxi Key Laboratory of Accelerator Mass Spectrometry Technology and Application, Xi'an 710061, China)
The effect of Mg2+on boron incorporation into carbonate and the infl uence of B/Ca proxies for the deep ocean carbonate system
HE Maoyong1,3, XIAO Yingkai2
(1. State Key Laboratory of Loess and Quaternary Geology, Institute of Earth Environment, Chinese Academy of Sciences, Xi'an 710061, China; 2. Qinghai Institute of Salt Lakes, Chinese Academy of Sciences, Xining 810008, China; 3. Shaanxi Key Laboratory of Accelerator Mass Spectrometry Technology and Application, Xi'an 710061, China)
Background, aim, and scopeB/Ca proxies for the deep ocean carbonate system is an important proxy to evaluate carbon sink in sea. Mg2+is common element in sea which can effect B/Ca values of marine carbonate.Materials and methodsThe infl uence of Mg2+in a parent solution on boron incorporation in an inorganic carbonate precipitate is studied using a different solubility method technique. The calcium carbonate precipitate is characterized by scanning electron microscopy and X-ray diffraction. The boron concentration of the samplers is analyzed by ICP-AES.ResultsThe calcium carbonate precipitate is confi rmed to be low-Mg calcite. The boron concentrations in the precipitated calcite increased from 63.91 μg·g-1to 582.41 μg·g-1when the artifi cial solution pH values increased from 7.40 ± 0.03 to 8.80 ± 0.03.DiscussionThe results show that boron uptake by low-Mg calcite is greater than boron uptake by free-Mg calcite grown under nearly identical conditions, and the boron concentration in low-Mg calcite is higher than in free-Mg calcite by 2.57 average time (from 1.83 to 3.56). This result suggests that the mechanism for borate-boron co-precipitation with Mg2+present is different than without Mg2+present, and the Mg2+clearly modifies calcite crystal morphology, as identified in SEM images.ConclusionspH has a signifi cant effect on the incorporation of boron into synthetic carbonate, and the Mg2+also infl uence on boron incorporation into calcite precipitate.Recommendations and perspectivesThe data provide a signifi cant scientifi c basis for B/Ca proxies for the deep ocean carbonate system. It is recommended that the Mg2+would be consider when using B/Ca proxies.
effect; magnesium ions; boron; precipitated inorganic carbonate
1 Introduction
Based on the research by Hemming and Hanson (1992), the foraminifera B/Ca ratios have increasingly been used as a paleo-carbonate ion or paleo-pH proxy because borate ions are preferentially incorporated into the calcite lattice relative to boric acid (Foster, 2008; Yu et al, 2010). In recent years, foraminiferal B/Ca ratio analysis, which is a paleoceanographic method with significant potential, has generated significant concern (Hönisch and Allen, 2013). Marine carbonates (e.g., corals and foraminifera) from sediment core or culture experiments reveal that the ratios of boron to calcium (B/Ca) varied with pH, temperature, light, salinity, and seawater boron concentration (Rae et al, 2011; Kender et al, 2014). To apply boron trace element abundance as a geochemical tracer, the factors that influence boron uptake by calcium carbonate must be discerned (Liu et al, 2010; Deng et al, 2012; Xiao et al, 2012). Over the past few decades, several inorganic calcite precipitation experiments have been performed to evaluate the factors that infl uence boron incorporation into marine biocarbonates by comparing such incorporation with that of boron into inorganic calcite, yielding signifi cant achievements. For example, the level of boron coprecipitated into calcite increased by increasing the parent solution pH; the level of boron that coprecipitates with CaCO3is proportional to the concentration of boron in the parent solution; boron is coprecipitated more readily with aragonite than calcite; and the content in aragonite is higher than calcite by 1.5 to 2 (Hemming et al, 1995; Sanyal et al, 2000; Xiao et al, 2006, 2008; Astilleros et al, 2010; Ruiz-Agudo et al, 2010; Dissard et al, 2012).
In recent years, the role of magnesium in the growth of calcite was considered. For inorganic calcite precipitation, boron incorporation into Mg(OH)2and boron isotope fractionation during brucite deposition experiments by Xiao et al (2006, 2008) and Xiao et al (2009, 2011) showed that the adsorption capacity for boron with Mg2+was comparatively strong in the presence of Mg2+. Astilleros et al (2002, 2010) systematically studied the role of Mg2+in the growth of calcite by the AFM. They provided an explanation for the development of “dead zones”, which was based on the restrictions that the underlying substrate imposes on the lateral spread of overgrowing layers (the “template effect”).
The main objective of this study was to further assess the influence of magnesium ions on boron incorporation in an inorganic carbonate precipitate. These experiments were conducted (1) to evaluate the systematics of boron coprecipitation in carbonates with Mg2+at different pH, (2) to determine the effect of Mg2+on boron incorporation into precipitated inorganic carbonate, and (3) to improve the understanding of the mechanisms driving the boron and magnesium incorporation into calcium carbonate.
2 Experiment
2.1 Instrumentation
ICP-AES (Perkin Elmer Optima 4300DV) was used to analyze boron concentrations.
The morphologies of the dry precipitates were characterized by Powder X-ray Diffraction (PXRD), and the shapes, particle size distributions, and aspect ratios of the particles were observed by Scanning Electron Microscope (SEM).
2.2 Reagents and materials
All chemical reagents used in this study were purchased from the Tianjin Kermel Reagent Co. Ltd. High-purity NH3and CO2gases (≥99.99%) were usedtoo. MgCO3· 3H2O was synthesized from MgCl2and (NH4)2CO3(Wang et al, 2008).
2.3 Experimental methods
2.3.1 CaCO3precipitation
CaCO3was precipitated via a different solubility method technique. The experimental setup has been described in our previous work (He et al, 2013). Synthesized MgCO3· 3H2O (0.138 g) was added slowly to the artificial magnesium-free seawater instead of Li2CO3. And approximately 100 mg of calcium carbonate was generated. The samples were treated following the method of He et al (2013) too.
2.3.2 Powder X-ray Diffraction (PXRD) and Scanning Electron Microscope (SEM)
X-ray diffraction (XRD) measurements were conducted using an X'Pert PRO MPD with Cu-Kαradiation (40 kV, 200 mA), and 0.02 step and 2θrange of 20° to 60° were selected to analyze the crystal structure and crystal orientation.
The sizes and morphologies of the CaCO3precipitates were characterized using a JEOS JSM-6700F scanning electron microscope at 20 kV after sputter-coating with Au.
3 Results and Discussion
3.1 Characterization of precipitated CaCO3
The solids recovered from the coprecipitation experiments were characterized with XRD to distinguish calcite from the other polymorphs of CaCO3(Fig.1).
Fig.1 shows the representative XRD patterns of the CaCO3obtained in the presence of Mg2+at 15 h with variable pH. All of the relatively sharp peaks are characteristic of low-Mg calcite (LMC) of (Mg0.03Ca0.97) (CO3) (JCPDS file: 01-089-1304). Vousdoukas et al (2007) documented that LMC (a polymorph of CaCO3, containing less than 4% MgCO3with a formula (Mg0.03Ca0.97)(CO3)) is the dominant precipitated carbonate phase as a result of water mixing. This has been confi rmed in the present study.
The crystal structures of carbonate can be further identified by evaluating their SEM. Fig.2 shows representative SEM images of low-Mg-calcite (LMC).

Fig.1 XRD patterns of CaCO3prepared in different pH conditions: (a) pH = 7.8, (b) pH = 8.2, and (c) pH = 8.6
3.2 The effect of pH on boron incorporation into calcite precipitate with/without Mg2+
Fig.3 shows plots of the boron concentrations measured experimentally in low-Mg calcites. The results indicate that pH has a significant effect on the incorporation of boron into low-Mg calcites. The amount of co-precipitated boron increased with the parent solution pH, from 63.91 μg·g-1at pH = 7.4 to 582.41 μg·g-1at pH = 8.8. The fi ndings of the current study are consistent with previous results of boron incorporation into CaCO3by Sanyal et al (2000), Xiao et al (2008), and He et al (2013). As shown in Fig.3, the primary factor controlling boron incorporation into precipitated synthetic arbonate was the parent solution pH. The level of boron that coprecipitated with carbonate increased with the parent solution pH, with or without Mg2+in the solution. The Mg2+modifies calcite crystal morphology to influence on boron incorporation into calcite precipitate.
At low boron concentrations (B≤0.025 M), boron primarily forms two molecular species in solution: B(OH)3(boric acid; planar, trigonally coordinated) and(borate ion, tetrahedrally coordinated). The following equilibrium is found:

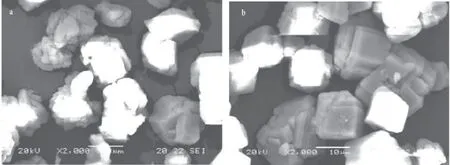
Fig.2 SEM images of CaCO3crystals (a) This study at pH = 8.2 and (b) He et al (2013) at pH = 8.2.

Fig.3 Measured boron content of precipitated carbonates relative to pH
Based on the research by Hemming and Hanson (1992). the incorporation ofinto marine carbonate follows the equilibrium reaction:
CaCO3+↔ Ca(HBO3) ++ H2O (2)
3.3 The effect of Mg2+on boron incorporation into calcite precipitate
Fig.2a is SEM images of LMC in the presence of Mg2+, and Fig.2b is SEM images of calcite precipitated at same condition without Mg2+. Fig.2 indicates that the presence of Mg2+has clearly been shown to modify calcite crystal morphology. It also supports previous research that Mg2+can alter the crystal structure (Paquette and Reede, 1995; Park et al, 2008). Mg2+has a higher affinity for certain sites, and it is likely adsorption or dehydration during incorporation that preferentially reduces growth in specific directions, such as toward the edges and corners. This nonuniform Mg2+adsorption on the calcite surface produces new crystal surfaces, which have a higher Mg2+density and lower growth rate than the original calcite seed surfaces. Under these conditions, boron is coprecipitated more easily with low-Mg calcite than with calcite.
Fig.3 shows that the boron content inprecipitated carbonate was different in the presence of Mg2+andabsent of Mg2+in the solution. The results show that boron is coprecipitated more readily with Mg2+in the solution. The results of our experiments are similar to previous reports by Xiao et al (2008) with Mg2+for a similar pH, but are twice as large as the values reported by Sanyal et al (2000) and He et al (2013) without Mg2+in the solution.
3.4 The infl uence of Mg2+on B/Ca proxies for the deep ocean carbonate system
The surface water B/Ca ratio can be expressed asand was proposed to be a proxy for seawaterThe basic assumption behind this proxy is that the B/Ca ratio in foraminifera is a function of the ratio ofin seawater, with the later being pH dependent.
Compared with the conventional method of analyzing boron isotopes, the B/Ca ratio method is relatively easier and more stable. As a result, it is relatively suitable for high-resolution paleoceanographic studies. Yu et al (2010), Foster (2008), and Palmer et al (2010) used foraminiferal B/Ca ratios to reflect the deep water carbonate saturation state. They suggested that B/Ca ratios vary widely for different foraminifera species and thatKDis not a constant value for the same foraminifera species.
Although B/Ca proxies for the deep ocean carbonates system are widely used, the mechanisms of these proxies are not well accepted. Not only the method is constructed on the basis of an empirical function, but also the B/Ca is impacted of light and temperature (Dissard et al, 2012; Coadic et al, 2013). Mg is an element that is widely distributed in seawater. Mg is not only incorporated into foraminiferal and coral but also impacts the process of boron incorporation. A synthetic calcium carbonate experiment by Hemming et al (1995) showed that boron uptake by aragonite is greater than boron uptake by calcite grown under nearly identical conditions, whereas boron uptake by high-Mg calcite is intermediate. In our study, when all the other parameters are maintained constant, boron concentrations in calcite precipitate increase with increasing Mg2+(Fig.3). It is suggested thatKDperhaps was influenced by Mg2+. Additional work is clearly needed to understand the causes of this behavior, but this is unfortunately beyond the scope of this contribution. These observations complicate the application of B/Ca in planktic foraminifera to trace changes in the carbonate system.
4 Conclusions
Boron is coprecipitated more readily with low-Mg calcite than with calcite under the same pH and temperature conditions, and the levels of low-Mg calcite are higher than those of calcite by 1.83 to 3.56, with a 2.57 average value. The presence of Mg2+clearly modifies the calcite crystal morphology and may influence on B/Ca proxies for the deep ocean carbonate system.
Astilleros J M, Fernández-Díaz L, Putnis A. 2010. The role of magnesium in the growth of calcite: An AFM study [J].Chemical Geology, 271: 52 – 58.
Astilleros J M, Pina C M, Fernández-Díaz L, et al. 2002. Molecular scale surface processes during the growth of calcite in the presence of manganese [J].Geochimica et Cosmochimica Acta, 66: 3177 – 3189.
Coadic R, Bassinot F, Dissard D, et al. 2013. A core-top study of dissolution effect on B/Ca in Globigerinoides sacculifer from the tropical Atlantic: Potential bias for paleo-reconstruction of seawater carbonate chemistry [J].Geochemistry, Geophysics, Geosystems, 14: 1053 – 1068.
Deng W F, Wei G J, Yu K F. 2012. Variations of midlate Holocene rainy season reconstructed from coral geochemical proxies in the Leizhou Peninsula, northern coast of South China Sea [J].Journal of Earth Environment, 3(2): 826 – 834.
Dickson A G. 1990. Thermo dynamics of dissociation of boric acid in synthetic seawater from 273.15 to 318.15 K [J].Deep-Sea Research, 37: 755 – 766.
Dissard D, Douville E, Reynaud S, et al. 2012. Light and temperature effect onδ11B and B/Ca ratios of the zooxanthellate coral Acropora sp.: results from culturing experiments [J].Biogeosciences, 9: 5969 – 6014.
Foster G L. 2008. Seawater pH,pCO2and [] variations inthe Caribbean Sea over the last 130 kyr: a boron isotope and B/Ca study of planktic foraminifera [J].Earth and Planetary Science Letters, 271: 254 – 266.
He M Y, Xiao Y K, Jin Z D, et al. 2013. Quantifi cation of boron incorporation into synthetic calcite under controlled pH and temperature conditions using a differential solubility technique [J].Chemical Geology, 337 / 338: 67 – 74.
Hemming N G, Hanson G N. 1992. Boron isotopic composition and concentration in modern marine carbonates [J].Geochimica et Cosmochimica Acta, 56: 537 – 543.
Hemming N G, Reeder R J, Hanson G N. 1995. Mineral-fl uid partitioning and isotopic fractionation of boron in synthetic calcium carbonate [J].Geochimica et Cosmochimica Acta, 59: 371 – 379.
Hönisch B, Allen K A. 2013. Carbon Cycle Proxies (δ11B,δ13Ccalcite,δ13Corganic, shell weights, B/Ca, U/Ca, Zn/Ca, Ba/Ca) [J].Encyclopedia of Quaternary Science, 2: 849 – 858.
Kender S, Yu J, Peck V L. 2014. Deep ocean carbonate ion increase during mid Miocene CO2decline [J].Scientific Reports, 4: PA4219, doi: 10.1038/srep04187.
Liu W G, Xiao J, Hemming N G, et al. 2010. Boron isotopic evidence for contribution of melting ice to ocean pH value and sea level during global warming [J].Journal of Earth Environment, 1(1): 43 – 47.
Palmer M R, Brummer G J, Cooper M J, et al. 2010. Multiproxy reconstruction of surface waterpCO2in the northern Arabian Sea since 29 ka [J].Earth and Planetary Science Letters, 295: 49 – 57.
Paquette J, Reeder R J. 1995. Relationship between surface structure, growth mechanism, and trace element incorporation in calcite [J].Geochimica et Cosmochimica Acta, 59: 735 – 749.
Park W K, Ko S J, Lee S W. 2008. Effects of magnesium chloride and organic additives on the synthesis of aragonite precipitated calcium carbonate [J].Journal of Crystal Growth, 310: 2593 – 2601.
Rae J W B, Foster G L, Schmidt D N, et al. 2011. Boron isotopes and B/Ca in benthic foraminifera: Proxies for the deep ocean carbonate system [J].Earth and Planetary Science Letters, 302: 403 – 413.
Ruiz-Agudo E, Kowacz M, Putnis C V, et al. 2010. The role of background electrolytes on the kinetics and mechanismof calcite dissolution [J].Geochimica et Cosmochimica Acta, 74: 1256 – 1267.
Sanyal A, Nugent M, Reeder R J, et al. 2000. Seawater pH control on the boron isotopic composition of calcite: evidence from inorganic calcite precipitation experiments [J].Geochimica et Cosmochimica Acta, 64: 1551 – 1555.
Vousdoukas M J, Velegrakis A F, Plomaritis T A. 2007. Beach rock occurrence, characteristics, formation mechanisms and impacts [J].Earth Science Reviews, 85: 23 – 46.
Wang Y, Li Z B, Demopoulos G P. 2008. Controlled precipitation of nesquehonite (MgCO3·3H2O) by the reaction of MgCl2with (NH4)2CO3[J].Journal of Crystal Growth, 310: 1220 – 1227.
Xiao J, Xiao Y K, Liu C Q, et al. 2009. Boron isotopic fractionation during incorporation of boron into Mg(OH)2[J].Chinese Science Bulletin, 54: 3090 – 3100.
Xiao J, Xiao Y K, Liu C Q, et al. 2011. Boron isotope fractionation during brucite deposition from artificial seawater [J].Climate of the Past, 7: 693 – 706.
Xiao Y K, Li H L, Liu W G, et al. 2008. Boron isotopic fractionation in laboratory inorganic carbonate precipitation: evidence for the incorporation of B(OH)3into carbonate [J].Science in China Series D, 51: 1776 – 1785.
Xiao Y K, Li S Z, Wei H Z, et al. 2006. An unusual isotopic fractionation of boron in synthetic calcium carbonate precipitated from seawater and saline water [J].Science in China Series B, 49: 454 – 465.
Xiao Y K, Zhang Y L, Liu W G, et al. 2012. Study on correlation betweenδ18Ocarbof reef coral and sea surface salinity — Coral culture experiments at different salinities [J].Journal of Earth Environment, 3(4): 969 – 981.
Yu J M, Broecker W S, Elderfi eld H, et al. 2010. Loss of carbon from the deep sea since the Last Glacial Maximum [J].Science, 330(6007): 1084 – 1087.
Mg2+离子对深海碳酸盐中硼的掺入和B/Ca指标的影响
贺茂勇1,3,肖应凯2
(1.中国科学院地球环境研究所 黄土与第四纪地质国家重点实验室,西安 710061;2.中国科学院青海盐湖研究所,西宁 810008;3.陕西省加速器质谱技术及应用重点实验室,西安 710061)
对母液中Mg2+离子对硼掺入无机碳酸盐沉积的影响进行了研究。通过扫描电子显微镜和X射线衍射确定在Mg存在时生成的无机碳酸盐是低镁方解石。实验发现:溶液的pH值是硼进入碳酸盐的主要控制因素,低Mg2+方解石中硼的浓度从63.91 μg·g-1(pH = 7.40 ± 0.03)增加到582.41 μg·g-1(pH = 8.80 ± 0.03)。Mg2+离子严重影响硼进入碳酸盐中的量,在相同实验条件下,硼在低镁方解石中的含量高于无Mg2+方解石中的含量,平均为2.57倍(1.83 — 3.56倍)。这一结果表明:有Mg2+离子时,硼掺入无机碳酸盐的机制和无Mg2+离子的是不同的。Mg2+离子的存在改变了晶体的形貌。这对利用B/Ca指标恢复深海碳酸盐系统研究有重要影响。
作用;镁离子;硼;无机碳酸盐沉积
10.7515/JEE201603009
Received Date:2015-11-30;Accepted Date:2016-01-08
Foundation Item:National Natural Science Foundation of China (41573013, U1407109); “Key Program” of the West Light Foundation of Chinese Academy of Sciences (42904101) ; Natural Science Fund of Shaanxi Province (2015JM4143)
HE Maoyong, E-mail: hemy@ieecas.cn
