磷酸化二氧化硅填充磺化聚醚醚酮质子交换膜的制备及表征
2016-03-19吴洪杨昊赵宇宁李震姜忠义
吴洪,杨昊,赵宇宁,李震,姜忠义
(1天津化学化工协同创新中心,天津 300072;2天津大学化工学院绿色化学合成与转化教育部重点实验室,天津 300072;3天津市膜科学与海水淡化技术重点实验室,天津 300072)
磷酸化二氧化硅填充磺化聚醚醚酮质子交换膜的制备及表征
吴洪1,2,3,杨昊1,2,赵宇宁2,李震1,2,姜忠义1,2
(1天津化学化工协同创新中心,天津 300072;2天津大学化工学院绿色化学合成与转化教育部重点实验室,天津 300072;3天津市膜科学与海水淡化技术重点实验室,天津 300072)
摘要:制备了两种磷酸化改性的介孔二氧化硅亚微米球形颗粒,分别为仅外表面接枝磷酸根基团的颗粒(PMPS-Ⅰ)和内外表面均接枝磷酸根基团的颗粒(PMPS-Ⅱ)。颗粒具有均一的尺寸和规则排布的六面体一维贯通孔道。将制备的二氧化硅颗粒与磺化聚醚醚酮(SPEEK)溶液共混制备杂化膜。与填充PMPS-Ⅰ的杂化膜相比,填充PMPS-Ⅱ的杂化膜显示出较好的质子传导性能。当PMPS-Ⅱ的填充量为5%(质量)时,杂化膜在60℃、100%相对湿度下最高质子传导率为0.241 S·cm-1。研究结果表明,连续贯通的质子传递通道有助于提高杂化膜的质子传导率。
关键词:燃料电池;膜;质子传导率;甲醇渗透率;磷酸化;二氧化硅
2015-04-24收到初稿,2015-06-05收到修改稿。
联系人:姜忠义。第一作者:吴洪(1971—),女,教授。
Received date: 2015-04-24.
Introduction
Direct methanol fuel cells (DMFCs) have become the focus of attention throughout the past decades, owing to the high power density, high efficiency and low greenhouse gas emission. Proton exchange membrane (PEM) serving as the critical component of DMFC plays a key role in the fuel cell performance[1-3]. Organic-inorganic hybrid membranes have been explored extensively to develop high performance PEMs, for the advantages such as the enhanced mechanical, thermal and chemical stability, promoted methanol retention property and the ease of processability[4-6]. The pristine inorganic fillers often serve as proton conducting insulator, thus for the fabrication of high proton conductive PEMs, the immobilizing of proton conducting sites onto the inorganic fillers has been widely studied[7-11].
Ordered mesoporous silica (OMPS) with high specific surface area, controllable nanometer-sized pores or frameworks (pore size range of 2—50 nm) and high structural stability has triggered many investigations since its first discovery in 1992[12]. To be utilized in the PEMs, OMPS was often endowed with proton conducting sites, such as sulfonated acid groups (SO3H), phosphoric acid groups (PO3H2), ionic liquids, heteropoly acids (HPAs) and imidazole groups etc., and then embedded into polymer membrane matrices[2,13-16]. Among them, sulfonation of OMPS was most widely conducted by direct introduction of acid groups into the framework via, for example, co-condensation methods and post-grafting methods[2,17-19]. Recently, phosphorylated PEMs have drawn much research interests as alternatives for conventional sulfonated ones, because of the advantages ofPO3H2groups compared toSO3H groups: the amphoteric property and higher dielectric constant property, higher water binding energy and lower average zero point energy. Thus the phosphorylated PEMs could be conferred with higher proton concentration, higher water retention property and lower proton conduction barrier[13,20-22].
Besides the introduction of more proton conduction sites into PEMs, additional proton conduction channels could be fabricated in the membranes by the controllable pores or frameworks of OMPS[15,17,19,23-24]. For example, the hexagonally ordered mesopores, filled with SO3H groups, rendered effective proton conducting channels in OMPS and improved proton conductivities of the hybrid membranes[19]. In another work, HPAs were assembled into the mesoporous silica matrix with tailor-made pore structures to form continuous and interconnected proton conduction channels in silica additives, which were essential for high proton conductivity of the hybrid membranes. And the body-centered cubic-mesoporous silica impregnated HPAs showed best proton conductivity of 0.061 S·cm-1at 25℃ and 0.14 S·cm-1at 150℃, respectively[24]. As reported by Lin et al.[14], incorporation of appropriate amount of mesoporous silica, hybridized with protic ionic liquids (PILs), significantly increased the proton conductivity of the hybrid membranes, which was probably attributed to the proton conduction channels or networks formed by mesoporous particles in the membranes. The produced hybrid membranes exhibited proton conductivity up to an order of 1×10-2S·cm-1at 160℃ under anhydrous conditions. In a word, the introduction of proton conduction channels into the hybrid membranes seems to be a promising approach for fabrication of PEMs.
Both the introduction of proton conducting sites and the construction of proton conducting channels into membranes are essential for achieving high proton conductivity. Base on the amount and location of proton conducting sites as well as the regularity and interconnectivity of the proton conducting channels, sulfonated inorganic materials including zeolites[2], MOFs[25]and OMPS[17], have been widely studied. However, rare research reports the phosphorylated OMPS for the DMFC application. In this work, two kinds of phosphorylated mesoporous silica submicrospheres (PMPS) were prepared and incorporated into sulfonated poly(ether ether ketone) (SPEEK) matrices to fabricate hybrid membranes. Proton conduction sites (PO3H2groups) were anchored on the particle outer surface only or both on the outer surface and on the inner pore walls, thus producing different pore structures and different proton conduction pathways.Membrane performances including proton conductivity, methanol permeability and selectivity were investigated.
1 Experimental
1.1 Materials and chemicals
Cetyltrimethylammonium bromide (CTAB, 99%), TEOS (reagent grade), 3-glycidyloxypropyltrimethoxysilane (GPTMS, AR), phosphorus oxychloride [POCl3, >98% (mass)] and polyether ether ketone (PEEK 381G) were purchased from Aladdin-reagent, Sigma- Aldrich Co. LLC., J&K Scientific Ltd. (Beijing), Shanghai Guangzan Chemical Scientific Ltd. and Victrex England, respectively, and used without further purification. Toluene (AR) was purchased from Tianjin Jiangtian Chemical Technology Co., Ltd. and was distilled prior to use. All the other materials and chemicals were commercially available with analytical pure degree, and used as received. Deionized water was used throughout the work.
1.2 Preparation of the phosphorylated mesoporous silica submicrospheres
1.2.1 Preparation of PMPS with PO3H2groups anchored on the particle outer surface only (PMPS-Ⅰ) Mesoporous silica submicrospheres were prepared according to the procedure in previous work[26]with little modification. CTAB (0.4 g) and 2 mol·L-1NaOH solution (3.5 ml) were dissolved in water (480 ml) with vigorous stirring at 80℃. Then, TEOS (5 ml) was added into the solution. The mixture was stirred afterwards for 3 h. The resulting white precipitates were purified by three cycles of centrifugation and re-suspended in ethanol with ultrasonicbathing, and dried in a vacuum oven at room temperature until constant mass.
The phosphorylated mesoporous silica submicrospheres were synthesized by the method in the literature[21]. To glycidyl-silane modify mesoporous silica submicrospheres, GPTMS (2 ml) was used to react with dry mesoporous silica submicrospheres (2 g). Mesoporous silica submicrospheres were dispersed in 120 ml anhydrous toluene to avoid the silane coupling agents from hydrolyzing before reacting, and then the mixture was refluxed for 24 h. The products were purified by centrifugation and re-suspended in water and ethanol and dried under vacuum freeze-drying.
The resultant glycidyl-silane modified mesoporous silica submicrospheres (1.6 g) were dispersed in 120 ml POCl3and the mixture was maintained at 80 °C under vigorous stirring for 24 h. The final products were purified by centrifugation, water washing and dried under vacuum freeze-drying.
Finally, removing the template of the prepared mesoporous silica submicrospheres according to the procedure reported in previous work[26]with little modification. The prepared silica (1.2 g) was refluxed at 80℃ for 48 h in a solution consisting of HCl [37.4% (mass), 9 ml] and methanol (160 ml). The products were purified by three cycles of centrifugation and re-suspended in ethanol with ultrasonic-bathing (ethanol washing), and dried in a vacuum oven at room temperature until constant mass.
The products were denoted as PMPS-Ⅰ, referred to the phosphorylated mesoporous silica submicrospheres withPO3H2groups anchored on the particle outer surface only.
1.2.2 Preparation of PMPS withPO3H2groups anchored both on the outer surface and on the inner pore walls (PMPS-Ⅱ) Mesoporous silica submicrospheres were prepared as described above, and followed by the removal of the template. The products were denoted as MPS, referred to the unmodified mesoporous silica submicrospheres.
Afterwards, MPS were glycidyl-silane modified and phosphorylated as described above. And the products were denoted as PMPS-Ⅱ, referred to the phosphorylated mesoporous silica submicrospheres with PO3H2groups anchored both on the outer surface and on the inner pore walls.
Above all, by the post grafting of the functional groups without the removal of CTAB template, only the outer surface of MPS was modified (PMPS-Ⅰ)[27]. Contrarily, if the template was removed before the subsequent treatment, the functional groups could be anchored both on the outer surface and on the inner pore walls of MPS (PMPS-Ⅱ)[2,28].
1.3 Preparation of SPEEK
SPEEK was prepared via post-sulfonation asdescribed in previous work[21]. The sulfonation degree (DS) of SPEEK was determined to be 62.4% through the titration method described in Section 1.6.
1.4 Preparation of the membranes
Two kinds of phosphorylated mesoporous silica submicrospheres and the unmodified MPS were well dispersed in the homogeneous DMF solutions of SPEEK [the content of SPEEK was 10% (mass)]. Afterwards, the membranes were prepared by solution casting on clean glass plates, followed by drying first at 60℃ for 12 h and then at 80℃ for another 12 h. The pristine SPEEK membrane was prepared by the same method. The as-prepared hybrid membranes including PMPS-Ⅰ (or PMPS-Ⅱ, MPS) were denoted as SPEEK/PMPS-Ⅰ-Z (or SPEEK/PMPS-Ⅱ-Z, SPEEK/MPS-Z), where Z referred to the mass percentage of inorganic particles relative to the SPEEK matrix.
1.5 Characterizations
The size and morphology of the mesoporous silica submicrospheres were characterized by transmission electron microscopy (TEM, JEM100CXII) and high resolution transmission electron microscopy (HRTEM, JEM-2100F).
The Brunauer-Emmett-Teller (BET) specific surface area of the synthesized particles was determined by measuring a nitrogen adsorption/desorption isotherm (TriStar 3000), and the pore size distribution was calculated using the Barrett-Joyner-Halenda (BJH) formula[29]. The phosphorus content in both kinds of PMPSs was measured by Inductively Coupled Plasma Optical Emission Spectrophotometer (ICP, ICP-9000 (N+M), USA Thermo Jarrell-Ash Corp.). The surface elemental composition of the PMPSs was characterized by X-ray photoelectron spectroscopy (XPS) with a PHI 1600 spectrometer and Mg Kαradiation for excitation.
1.6 Sulfonation degree and ion-exchange capacity
The sulfonation degree (DS) of SPEEK was determined by titration method as described in the previous work[21], and was calculated according to Eq. (1)
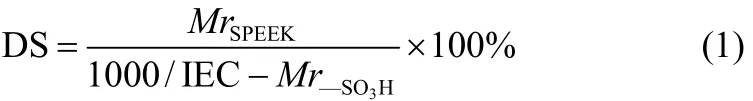
where MrSPEEKand Mr—SO3 Hare the molecular weight of the repeat unit of SPEEK monomer (288) and SO3H (80), respectively.
The ion exchange capacity (IEC, mmol·g-1) value was calculated by Eq. (2)

where m is the mass (g) of the dry SPEEK in H+form, VNaOHand CNaOHare the volume (ml) and concentration (mol·L-1) of the NaOH solution, respectively.
1.7 Water uptake and swelling degree
The water uptake and the swelling degree can be derived from the mass and area of the membranes varied after fully hydration, respectively, in accordance with the previous work[21]. The measurements were repeated three times, and the error was within ±5%. Then the water uptake and swelling were calculated according to the following equations [Eqs. (3) and (4)], respectively.

where Wdryand Adryare the mass (g) and area (cm2) of the dry membranes, respectively. And Wwetand Awetare the mass (g) and area (cm2) of the fully hydrated membranes, respectively.
1.8 Methanol permeability
Methanol permeability (P, cm2·s-1, 2 mol·L-1methanol solution) was measured using a glass diffusion cell as described in the previous work[21], and was calculated from Eq. (5)
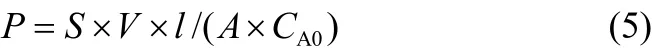
where S is the slope of the straight line of methanol concentration in the compartment B versus time (mol·L-1·s-1); V is the volume of the compartment B (L); l and A are the thickness and the effective area of the membrane, respectively (cm, cm2); CA0is the initial methanol concentration in compartment A (mol·L-1).
1.9 Proton conductivity
A two-point-probe conductivity cell was used to determine the in-plane proton conductivity of the membranes as described in the previous work[21]. The relative humidity was maintained at 100% and thetesting temperature was controlled by the water vapor from the room temperature to 100℃. The proton conductivity σ (S·cm-1) was calculated according to the following Eq. (6)

where l is the distance between the two electrodes (cm); A is the effective cross-sectional area of the membrane sample (cm2); R is the measured membrane resistance (Ω).
1.10 Selectivity
The methanol barrier performance and proton conductivity of proton exchange membrane can be evaluated by selectivity Φ (S·s·cm-3), which was calculated by Eq. (7)

where σ and P are proton conductivity and methanol permeability of membrane, respectively.
2 Results and discussion
2.1 Characterizations of mesoporous silica sub-microspheres
The morphology of the PMPS particles was characterized by TEM as shown in Fig.1. The synthesized MPS exhibit a spherical structure with a uniform size of about 135 nm in diameter [Fig.1(a)]. The phosphorylation doesn’t alter the particle morphology compared to the unmodified ones [Fig.1(b) and (c) for PMPS-Ⅰ and PMPS-Ⅱ, respectively]. The detailed information for the pore structure could be obtained by HRTEM characterization, as shown in Fig.2.

Fig.1 TEM images of MPS, PMPS-Ⅰand PMPS-Ⅱmesoporous silica submicrospheres

Fig.2 HRTEM images of MPS, PMPS-Ⅰ and PMPS-Ⅱmesoporous silica submicrospheres
The pore structure of the as-synthesized mesoporous silica submicrospheres is shown in Fig.2. All the mesoporous silica submicrospheres exhibit well-aligned one-dimensional hexahedral pores. It is worthy of pointing out that all the particles were observed in the axial direction in which diffusion takes place, and for the sake of clarity, the image of MPS observed in the direction of perpendicular to the axial is also displayed in Fig.2 (b).
It is accepted that by the post grafting of the functional groups without the removal of CTAB template, only the outer surface of MPS is modified (PMPS-Ⅰ)[27]; on the contrary, if the template is removed before the subsequent treatment, the functional groups can be anchored both on the outer surface and on the inner pore walls of MPS (PMPS-Ⅱ)[2,28]. The specific surface area, pore volume and pore size of mesoporous silica submicrospheres are listed in Table 1. The analysis of the desorption isotherms with the Brunauer-Emmett-Teller (BET) method gave surface areas, pore volumes and pore sizes in the range of 761—477 m2·g-1, 0.840—0.468 cm3·g-1and 2.42—2.90 nm, respectively. The smaller specific surface areas, pore volumes and pore sizes of PMPS-Ⅱ compared to PMPS-Ⅰ might also suggest the differences in the functionalization methods:PO3H2groups were anchored on both the inner pore walls and the outer surfaces of PMPS-Ⅱ, while only the outer surfaces of PMPS-Ⅰ were phosphorylated. In summary, mesoporous silica submicrospheres with different location of phosporylation sites were synthesized successfully as confirmed by the above characterizations.
The content of phosphorus element (P) of all kinds of the PMPS was determined by ICP as shown in Table 1. The P mass percents of both PMPS-Ⅰ and PMPS-Ⅱ are similar (2.1%). The oxidation state of P element of PMPS was analyzed by XPS spectrum (Fig. 3), and after the phosphorylation, all the PMPS exhibit the peak of binding energy at around 134.5 eV, thus indicating the pentavalent-oxidation state of P (P5+) and the presence of P O bond[21]. In addition,-PO3H2groups are distributed on the inner pore walls for PMPS-Ⅱ, and therefore less PO3H2groups are immobilized on the outer surface of the particles, for the little change of the total amounts of the functional groups compared to PMPS-Ⅰ.

Table 1 Structural parameters of synthesized mesoporous silica submicrospheres
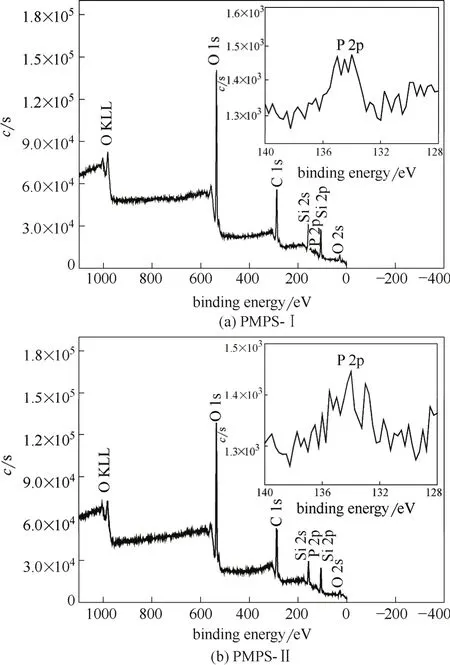
Fig.3 XPS spectra of PMPS-Ⅰ and PMPS-Ⅱ mesoporous silica submicrospheres
2.2 Water stability and mechanical stability of the hybrid membranes
The water stability (including water uptake and swelling properties) is critical to membrane performance such as the proton conductivity (water facilitates proton conduction) and the membrane stability (possible deterioration of the membrane-catalyst interface caused by membrane swelling)[30-31]. For the pristine SPEEK membrane, the water uptake and swelling degree are 33.84% and 18.54%, respectively, and are both in decline tendency after the incorporation of the phosphorylated inorganic particles, further declining with the increased filler content (Fig. 4). Interestingly, the two properties exhibit different behaviors: insignificant change can be observed for the water uptake of the hybrid membranes compared to the pristine SPEEK membrane, while the swelling degree is reduced a lot for the hybrid membranes. There are several competitive factors influencing the water uptake and swelling properties: (1) the introduced inorganic fillers may reduce swelling property, for the fillers do not swell; (2) the mesoporous structure of the inorganic particles is advantageous to the moistureadsorption inside mesopores; (3) the hydrophilic PO3H2groups are beneficial for adsorbing water molecules to the inorganic skeletons. Thus, the swelling properties of the hybrid membranes are inhibited while the water uptake properties decrease slightly, which is favorable for the membrane water stability. Besides, the lower water uptake of the phosphorylated silica hybrid membrane compared with the hybrid membrane doped with unmodified silica also indicates the successful phosphorylation inside the mesopores of the silica submicrospheres, and further confirming the phosphoric acid groups possess favorable water stability.
The mechanical properties of the membranes are shown in Table 2. It can be seen that both the tensile strength and tensile modulus of hybrid membranes are enhanced compared to those of SPEEK control membrane. The enhancement is attributed to the high mechanical strength of silica submicrospheres. Particularly, the tensile strength and tensile modulus of SPEEK/PMPS-Ⅰ-5 are increased by 15% and 23%, respectively. However, the mechanical strength is slightly declined while the filler content up to 15%, which is because of the agglomeration of silica submicrospheres.
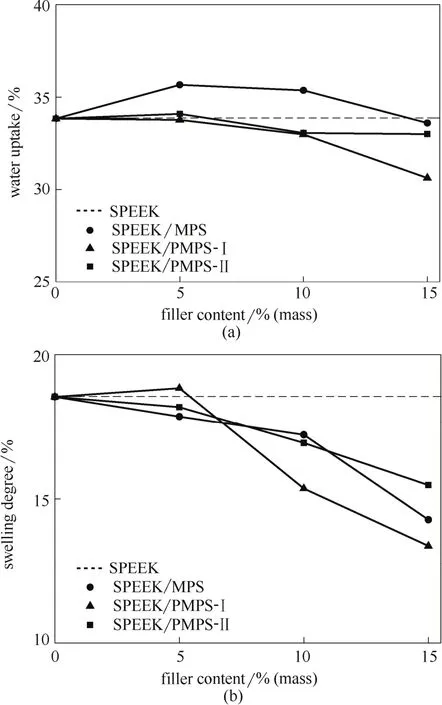
Fig.4 Water uptake and swelling properties of pristine SPEEK and SPEEK hybrid membranes at 25℃

Table 2 Mechanical property of membranes

Fig.5 Methanol permeability of pristine SPEEK and SPEEK hybrid membranes at 25℃
2.3 Methanol permeability of the hybrid membranes
The methanol permeability of the pristine SPEEK membrane is 3.94×10-7cm2·s-1, and is reduced by the introduction of the inorganic particles as shown in Fig.5. On the one hand, the addition of the inorganic fillers into the polymeric matrices contributes to the more tortuous pathways for methanol molecules, thus resulting in high barrier property against methanol molecules[32]. On the other hand, since the methanol molecules transfer across membrane by the solutiondiffusion mechanism, the decreased water uptake [Fig.4(a)] would inhibit methanol permeability of the hybrid membranes. Thus, the methanol permeability significantly decreases by the incorporation of mesoporous silica submicrospheres. The methanol permeabilityis reduced by a maximum of 46% for the SPEEK/ PMPS-Ⅰ-5 membrane. The considerably lower methanol permeability of the hybrid membranes compared to that of Nafion 117 (higher than 10-6cm2·s-1) exhibits their potential in practical applications.

Fig.6 Proton conductivity of pristine SPEEK and hybrid membranes
2.4 Proton conductivity of the membranes
Proton conductivity of both the pristine SPEEK and the hybrid membranes as a function of temperature at 100% RH are shown in Fig.6. For the pristine SPEEK membrane, the proton conductivity is 0.0865 S·cm-1at 29℃, and all the membranes exhibit enhanced conductivity with the increasing temperature. After the incorporation of the inorganic particles, the proton conductivity decreases. It should be mentioned that the proton conductivity of SPEEK/PMPS-Ⅱ-5 is higher than that of the pristine SPEEK membrane. It could also be noted that: (1) the phosphorylated hybrid membranes endure higher temperature before being damaged at 100% RH than both the MPS doped hybrid membranes and the pristine SPEEK membrane (dictated by the ending temperature in Fig.6); (2) the increase in the proton conductivity is accelerated when the temperature increases above 45℃ for the phosphorylated hybrid membranes than the MPS doped hybrid membranes and the pristine SPEEK membrane (evidenced by the slopes of the proton conductivities to the temperature in Fig.6).
The incorporation of the inorganic particles might render more torturous path ways for proton transportation in the membranes, thus decreasing the proton conductivity of the SPEEK membranes[33]. Comparing the SPEEK/PMPS-Ⅰ membranes with the SPEEK/ PMPS-Ⅱ membranes, it could be found that the proton conductivity is enhanced for the SPEEK/PMPS-Ⅱones in the whole loading range. As shown by HRTEM images [Fig.2 (c) and (d)] and ICP results, both the PMPS-Ⅰ and PMPS-Ⅱ silica submicrospheres exhibit the same mesostructures, namely the well-aligned one-dimensional hexahedral pores, and similar content of phosphorous elements. The differences lie in the location of the proton conduction sites, that is to say, only the outer surface of PMPS-Ⅰ is covered by PO3H2groups, while both the outer surface and the inner pore walls of PMPS-Ⅱ are grafted by proton conducting sites. Inside the regular and continuous mesopores, the acidic groups together with the adsorbed water molecules could facilitate proton conduction through the inorganic particles. As a result, continuous and effective proton conduction channels are formed inside the mesopores of PMPS-Ⅱ, and thus the SPEEK/PMPS-Ⅱ hybrid membranes outperform all the other hybrid membranes. Moreover, forthe phosphorylated hybrid membranes, the high endurance to the temperature elevation and the rapid proton conductivity increment above 45℃ could be attributed to the advantages of PO3H2groups, such as high proton concentration, higher water retention property, and better thermo-oxidative stability.
2.5 Selectivity of the membranes
The potential performance of PEMs can be evaluated by selectivity, which is defined as the radio of proton conductivity to methanol permeability. As shown in Fig.7, the selectivity of the pristine SPEEK membrane is 2.20×105S·s·cm-3. For the SPEEK/MPS membranes, almost all selectivity (except the one with 10% filler content) was lower than that of the pristine SPEEK membrane because of the low proton conductivity. The SPEEK/PMPS-Ⅰ membranes display the highest selectivity of 3.64×105S·s·cm-3when doped with 5% (mass) PMPS-Ⅰ, and the selectivity decreases with the increased filler content. For the SPEEK/PMPS-Ⅱ membranes, all membranes show higher selectivity compared to that of the pristine SPEEK membrane due to their high proton conductivity and methanol barrier property.
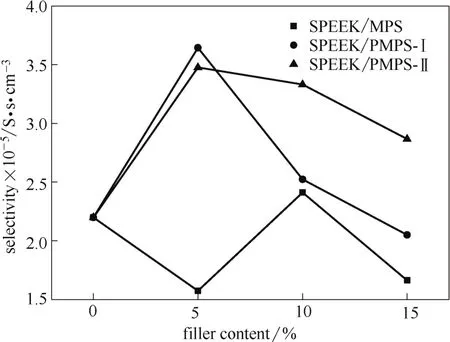
Fig.7 Selectivity of pristine SPEEK and hybrid membranes at 30℃
3 Conclusions
Two kinds of phosphorylated mesoporous silica submicrospheres with well-aligned one-dimensional hexahedral pores and similar amounts of proton conduction sites (i.e., acidic groups) were prepared and incorporated into SPEEK matrices to fabricate phosphorylated hybrid membranes. The location of the proton conduction sites of both kinds of PMPS is different:PO3H2groups are anchored on the particle outer surface only for PMPS-Ⅰ, while are anchored on both the outer surface and the inner pore walls for PMPS-Ⅱ. Continuous proton conduction channels introduced by PMPS-Ⅱ contribute to the high proton conductivity of PEMs. Furthermore, the phosphorylated hybrid membranes show much higher selectivity compared to that of the pristine SPEEK membrane. It is inspired that for the rational design of the hybrid membranes with enhanced proton conductivity, both the introduction of proton conduction sites and the constructing of proton conduction channels are of great importance.
References
[1] KREUER K D. On the development of proton conducting polymer membranes for hydrogen and methanol fuel cells [J]. Journal of Membrane Science, 2001, 185 (1): 29-39.
[2] MCKEEN J C, YAN Y S, DAVIS M E. Proton conductivity in sulfonic acid-functionalized zeolite beta: effect of hydroxyl group [J]. Chemistry of Materials, 2008, 20 (12): 3791-3793.
[3] LUFRANO F, BAGLIO V, STAITI P, et al. Performance analysis of polymer electrolyte membranes for direct methanol fuel cells [J]. Journal of Power Sources, 2013, 243: 519-534.
[4] DUPUIS A C. Proton exchange membranes for fuel cells operated at medium temperatures: materials and experimental techniques [J]. Progress in Materials Science, 2011, 56 (3): 289-327.
[5] LABERTY-ROBERT C, VALLE K, PEREIRA F, et al. Design and properties of functional hybrid organic-inorganic membranes for fuel cells [J]. Chemical Society Reviews, 2011, 40 (2): 961-1005.
[6] 曹先齐, 韩吉田, 陈培培, 等. 阳极和阴极流场组合对直接甲醇燃料电池性能的影响 [J]. 化工学报, 2013, 64 (5): 1780-1788. CAO X Q, HAN J T, CHEN P P, et al. Effect of anode and cathode flow fields on performance of direct methanol fuel cell [J]. CIESC Journal, 2013, 64 (5): 1780-1788.
[7] PARK K T, KIM S G, CHUN J H, et al. Composite membranes based on a sulfonated poly(arylene ether sulfone) and proton-conducting hybrid silica particles for high temperature PEMFCs [J]. International Journal of Hydrogen Energy, 2011, 36 (17):10891-10900.
[8] SU Y H, LIU Y L, WANG D M, et al. Increases in the proton conductivity and selectivity of proton exchange membranes for direct methanol fuel cells by formation of nanocomposites having proton conducting channels [J]. Journal of Power Sources, 2009, 194 (1) : 206-213.
[9] NIEPCERON F, LAFITTE B, GALIANO H, et al. Composite fuel cell membranes based on an inert polymer matrix and proton-conducting hybrid silica particles [J]. Journal of Membrane Science, 2009, 338 (1): 100-110.
[10] KE C C, LI X J, SHEN Q A, et al. Investigation on sulfuric acid sulfonation of in-situ sol-gel derived Nafion/SiO2composite membrane [J]. International Journal of Hydrogen Energy, 2011, 36 (5):3606-3613.
[11] TOKUDA Y, NISHIOKA S, UEDA Y, et al. Preparation of proton-conductive organic-inorganic hybrid titanophosphite membranes [J]. Solid State Ionics, 2012, 225 (SI): 232-235.
[12] BECK J, VARTULI J, ROTH W, et al. A new family of mesoporous molecular sieves prepared with liquid crystal templates [J]. Journal of the American Chemical Society, 1992, 114: 10834-10843.
[13] JIN Y G, QIAO S Z, XU Z P, et al. Phosphonic acid functionalized silicas for intermediate temperature proton conduction [J]. Journal of Materials Chemistry, 2009, 19 (16): 2363-2372.
[14] LIN B, CHENG S, QIU L, et al. Protic ionic liquid-based hybrid proton-conducting membranes for anhydrous proton exchange membrane application [J]. Chemistry of Materials, 2010, 22 (5): 1807-1813.
[15] LU S, WANG D, JIANG S P, et al. HPW/MCM-41 phosphotungstic acid/mesoporous silica composites as novel proton-exchange membranes for elevated-temperature fuel cells [J]. Advanced Materials, 2010, 22 (9): 971-976.
[16] TOELLE P, CAVALCANTI W L, HOFFMANN M, et al. Modelling of proton diffusion in immobilised imidazole systems for application in fuel cells [J]. Fuel Cells, 2008, 8 (3): 236-243.
[17] WON J H, LEE H J, YOON K S, et al. Sulfonated SBA-15 mesoporous silica-incorporated sulfonated poly(phenylsulfone) composite membranes for low-humidity proton exchange membrane fuel cells: anomalous behavior of humidity-dependent proton conductivity [J]. International Journal of Hydrogen Energy, 2012, 37 (11): 9202-9211.
[18] TSAI C H, LIN H J, TSAI H M, et al. Characterization and PEMFC application of a mesoporous sulfonated silica prepared from two precursors, tetraethoxysilane and phenyltriethoxysilane [J]. International Journal of Hydrogen Energy, 2011, 36 (16): 9831-9841.
[19] CHIBA Y, TOMINAGA Y. Poly(ethylene-co-vinyl alcohol)/sulfonated mesoporous organosilicate composites as proton-conductive membranes [J]. Journal of Power Sources, 2012, 203: 42-47.
[20] SCHUSTER M, RAGER T, NODA A, et al. About the choice of the protogenic group in PEM separator materials for intermediate temperature, low humidity operation: a critical comparison of sulfonic acid, phosphonic acid and imidazole functionalized model compounds [J]. Fuel Cells, 2005, 5: 355-365.
[21] ZHAO Y, JIANG Z, LIN D, et al. Enhanced proton conductivity of the proton exchange membranes by the phosphorylated silica submicrospheres [J]. Journal of Power Sources, 2013, 224: 28-36.
[22] JIN Y G, QIAO S Z, XU Z P, et al. Porous silica nanospheres functionalized with phosphonic acid as intermediate- temperature proton conductors [J]. Journal of Physical Chemistry C, 2009, 113 (8): 3157-3163.
[23] ZHANG L, HE H Q, KAMAL R, et al. Fabrication of novel phosphotungstic acid functionalized mesoporous silica composite membrane by alternative gel-casting technique [J]. Journal of Power Sources, 2013, 221: 318-327.
[24] ZENG J, SHEN P K, LU S, et al. Correlation between proton conductivity, thermal stability and structural symmetries in novel HPW-meso-silica nanocomposite membranes and their performance in direct methanol fuel cells [J]. Journal of Membrane Science, 2012, 397: 92-101.
[25] LI Z, HE G W, ZHAO Y N, et al. Enhanced proton conductivity of proton exchange membranes by incorporating sulfonated metal-organic frameworks [J]. Journal of Power Sources, 2014, 262: 372-379.
[26] LIN D, CHENG Q, JIANG Q, et al. Intracellular cleavable poly(2-dimethylaminoethyl methacrylate) functionalized mesoporous silica nanoparticles for efficient siRNA delivery in vitro and in vivo [J]. Nanoscale, 2013, 5 (10): 4291-4301.
[27] VIVERO-ESCOTO J L, SLOWING I I, LIN V S Y. Tuning the cellular uptake and cytotoxicity properties of oligonucleotide intercalator-functionalized mesoporous silica nanoparticles with human cervical cancer cells HeLa [J]. Biomaterials, 2010, 31 (6): 1325-1333.
[28] RADU D R, LAI C Y, JEFTINJIA K, et al. A polyamidoamine dendrimer-capped mesoporous silica nanosphere-based gene transfection reagent [J]. Journal of the American Chemical Society, 2004, 126 (41): 13216-13217.
[29] SHI J, WANG X, JIANG Z, et al. Constructing spatially separated multienzyme system through bioadhesion-assisted bio-inspired mineralization for efficient carbon dioxide conversion [J]. Bioresource Technology, 2012, 118: 359-366.
[30] TRIPATHI B P, SHAHI V K. Organic-inorganic nanocomposite polymer electrolyte membranes for fuel cell applications [J]. Progress in Polymer Science, 2011, 36: 945-979.
[31] LIN Y F, YEN C Y, MA C C M, et al. High proton-conducting Nafion®/ SO3H functionalized mesoporous silica composite membranes [J]. Journal of Power Sources, 2007, 171: 388-395.
[32] ZHAO Y, JIANG Z, XIAO L, et al. Lamellar crystals as proton conductors to enhance the performance of proton exchange membrane for direct methanol fuel cell [J]. Journal of Power Sources, 2011, 196 (15): 6015-6021.
[33] WANG J, ZHANG Y, WU H, et al. Fabrication and performances of solid superacid embedded chitosan hybrid membranes for direct methanol fuel cell [J]. Journal of Power Sources, 2010, 195 (9): 2526-2533.
Foundation item: supported by the National Science Fund for Distinguished Young Scholars (21125627), the Program for New Century Excellent Talents in University (NCET-10-0623) and the Programme of Introducing Talents of Discipline to Universities (B06006).
Preparation and characterization of proton exchange membranes based on sulfonated poly(ether ether ketone) doped with phosphorylated mesoporous silica submicrospheres
WU Hong1,2,3, YANG Hao1,2, ZHAO Yuning2, LI Zhen1,2, JIANG Zhongyi1,2
(1Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Tianjin 300072, China;2Key Laboratory for Green Chemical Technology, School of Chemical Engineering and Technology, Tianjin University, Tianjin 300072, China;3Tianjin Key Laboratory of Membrane Science and Desalination Technology, Tianjin University, Tianjin 300072, China)
Abstract:Two kinds of phosphorylated mesoporous silica submicrospheres were prepared. The phosphoric acid groups (PO3H2) were grafted either only on the particle outer surface (PMPS-Ⅰ) or on both the outer surface and the inner pore walls (PMPS-Ⅱ). All the submicrospheres exhibited a uniform particle size and possessed well-aligned one-dimensional hexahedral pores. Hybrid membranes were fabricated by directly blending silica with the sulfonated poly(ether ether ketone) (SPEEK) solutions. The hybrid membranes doped with PMPS-Ⅱexhibited higher proton conductivity compared to the ones with PMPS-Ⅰ. The highest proton conductivity was 0.241 S·cm-1at 60℃ and 100% relative humidity (RH) for the hybrid membrane with 5% (mass) of PMPS-Ⅱ. The experimental results indicated that continuous proton conduction channels were advantageous for the enhancement of proton conductivity of the hybrid membranes.
Key words:fuel cell; membrane; proton conductivity; methanol permeability; phosphorylated; silica
Corresponding author:Prof. JIANG Zhongyi, zhyjiang@tju.edu.cn
基金项目:国家杰出青年科学基金项目(21125627);新世纪优秀人才支持计划项目(NCET-10-0623);高等学校学科创新引智计划项目(B06006)。
中图分类号:TM 911.4
文献标志码:A
文章编号:0438—1157(2016)01—0358—10
DOI:10.11949/j.issn.0438-1157.20150520
