循环流化床锅炉排放颗粒物中水溶性离子研究*
2016-03-12吴建会张裕芬孙英明梁丹妮冯银厂
马 咸 吴建会 张裕芬 孙英明 梁丹妮 冯银厂
(南开大学环境科学与工程学院,国家环境保护城市空气颗粒物污染防治重点实验室,天津 300071)
循环流化床锅炉(CFB)广泛应用于发电、供暖和工业生产中[1],是大气颗粒物的重要排放源。近年来,大量CFB加装了脱硫装置以控制SO2的排放[2]。然而,脱硫装置在降低颗粒物和SO2排放的同时,会改变颗粒物构成和模态分布,进而影响其消光作用[3-5]。颗粒物中的硫酸盐和硝酸盐等水溶性物质是使可见光散射的主要成分[6]。但国内外对烟气中颗粒物的水溶性离子特征特别是模态分布研究仍相当有限。本研究利用静电低压撞击器(ELPI+)采集CFB中烟气的颗粒物样品,并分析其水溶性离子组成及模态粒径分布。
1研究方法
1.1 样品采样与分析
选择3台CFB(分别为CFB(a)、CFB(b)、CFB(c))进行烟气中的颗粒物样品采集。3台CFB均采用非选择催化还原(SNCR)脱硝技术,未安装除雾器,其他基本情况见表1。CFB(a)和CFB(c)燃煤掺杂20%(质量分数,下同)石灰石,CFB(b)燃煤掺杂20%~30%污泥。
利用颗粒物采样系统在每台CFB的烟道中采集烟气中粒径为0.006~10.000 μm的颗粒物样品,其中粒径为0.006~0.040 μm的颗粒物未检出。图1为颗粒物采样系统示意图。根据3012H型烟气分析仪测得的烟气流速、烟气温度等参数选取等速采样头[7-8]。ELPI+可装载不同粒径段的铝膜或Teflon膜采集颗粒物样品。烟气经过旋风除尘后进入稀释通道,利用经过过滤、加热的洁净空气按烟气与空气体积比为1∶8稀释烟气。稀释通道可在一定程度上反映污染源排放的真实情况[9]。加热器对稀释通道和旋风除尘管路进行加热,以避免烟气温度在管路中迅速下降而使烟气中的水蒸气凝结,从而影响颗粒物样品的采集。单次采样时间为6~8 h,每次采集不同粒径段的膜样品,共计84组样品。颗粒物浓度均使用基准含氧量6%(体积分数)进行转化[10]。测得的标准化质量浓度(c’,mg/m3)通过式(1)可转换得到颗粒物质量浓度。
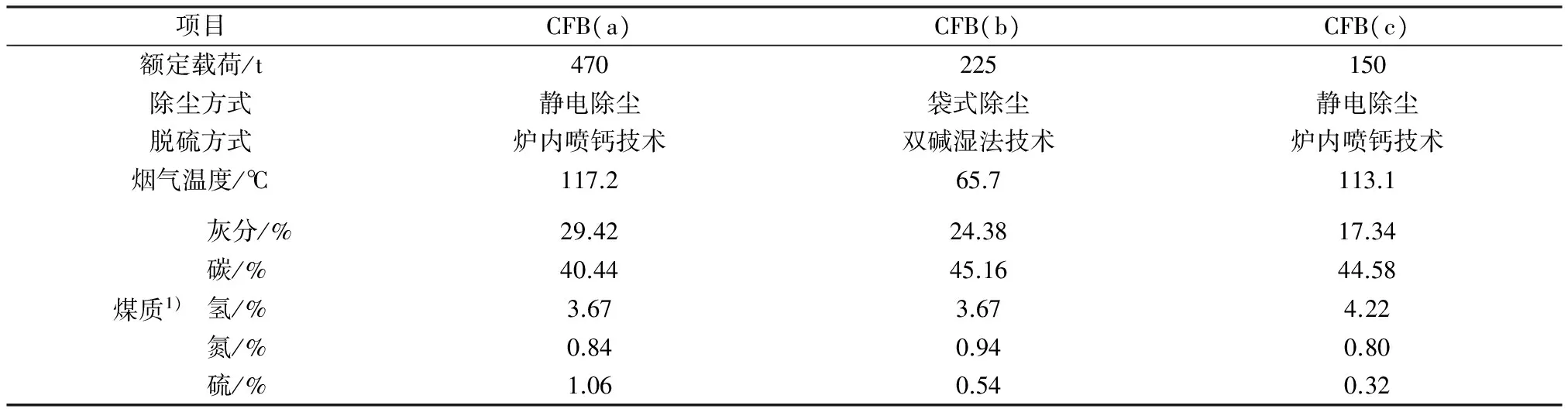
表1 CFB基本情况
注:1)均为质量分数。
c’=dc/d(lgD)
(1)
式中:c为颗粒物质量浓度,mg/m3;D为颗粒物粒径,μm。
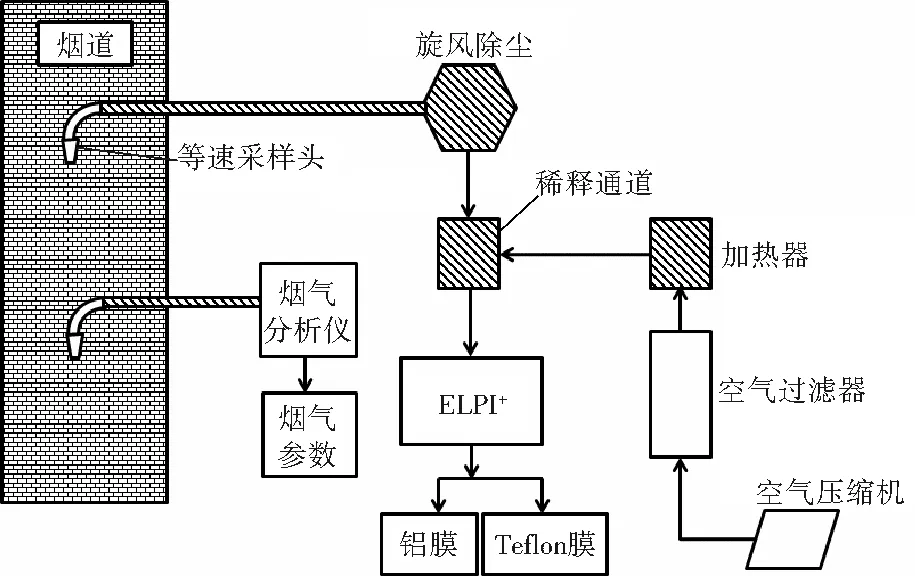
图1 颗粒物采样系统示意图Fig.1 The sampling system for particulate matter

1.2 质量控制
采样前Teflon膜和铝膜均进行60 ℃恒温干燥2 h,以去除表面的有机杂质和水分。采样膜称量前均置于恒温((20.0±2.0) ℃)、恒湿(50%±5%)的室内平衡72 h至恒量。
2 结果与讨论
2.1 烟气中不同粒径颗粒物的浓度分布
3台CFB烟道出口颗粒物标准化质量浓度粒径分布谱如图2所示。PM10、PM2.5和PM1可通过图2积分得到。由于CFB(a)和CFB(c)的除尘方式和脱硫方式相同,因此这两台CFB的颗粒物浓度粒径分布谱基本相似。CFB(a)和CFB(c)的PM10质量浓度分别为29.71、23.40 mg/m3,PM2.5质量浓度分别为4.32、1.78 mg/m3,PM1质量浓度分别为0.05、0.04 mg/m3。CFB(b)的PM10、PM2.5和PM1质量浓度分别为3.38、2.17、0.68 mg/m3。CFB(a)和CFB(c)的PM2.5质量浓度分别占各自PM10的14.54%、7.61%,CFB(b)的PM2.5质量浓度占PM10的64.20%。这可能是由于CFB(b)的袋式除尘器比静电除尘器对大粒径颗粒物有更高的除尘效率[12-13]。

图2 颗粒物标准化质量浓度粒径分布谱Fig.2 Size distribution of particulate matter standard mass concentration
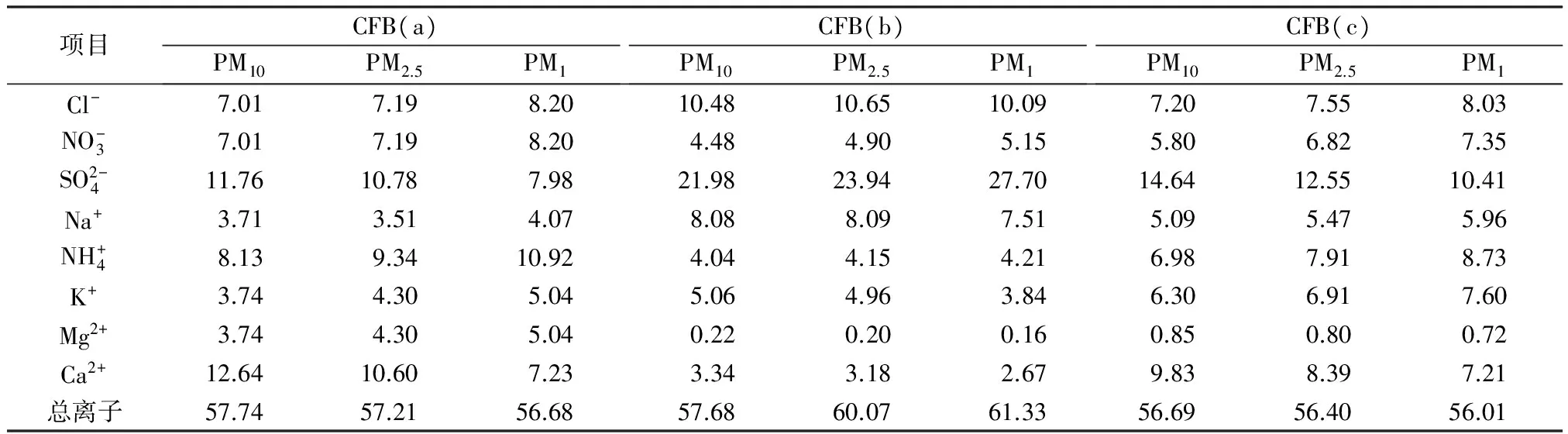
Table 2 Mass percentage of water-soluble ions in particulate matter %
2.2 颗粒物中的水溶性离子分析
颗粒物中水溶性离子的质量分数如表2所示。3台CFB的PM10中水溶性总离子质量分数基本相同。CFB(b)的PM2.5中水溶性总离子质量分数为60.07%,较CFB(a)和CFB(c)中分别高2.86、3.67百分点。CFB(b)的PM1中水溶性总离子质量分数为61.33%,与SIPPULA等[14]2974-2982的研究结果相近,高于CFB(a)和CFB(c),可能是由于烟气经双碱湿法技术脱硫装置后烟气温度较低、湿度较高,易凝结成超细颗粒物[15-18]。
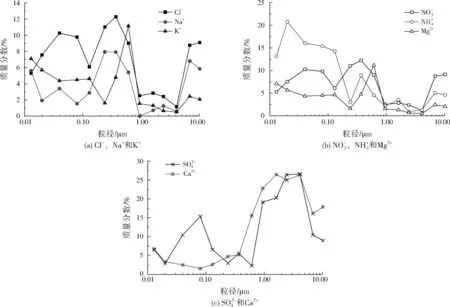
图3 CFB(a)中水溶性离子的模态粒径分布Fig.3 Mode distribution of water-soluble ions in CFB(a)

图4 CFB(b)中水溶性离子的模态粒径分布Fig.4 Mode distribution of water-soluble ions in CFB(b)

图5 CFB(c)中水溶性离子的模态粒径分布Fig.5 Mode distribution of water-soluble ions in CFB(c)
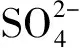
3 结 论
(1) CFB(a)、CFB(b)、CFB(c)的PM10质量浓度分别为29.71、3.38、23.40 mg/m3,PM2.5质量浓度分别为4.32、2.17、1.78 mg/m3,PM1质量浓度分别为0.05、0.68、0.04 mg/m3。
(2) 3台CFB的PM10中水溶性总离子质量分数基本相同。CFB(b)的PM2.5和PM1中水溶性总离子质量分数高于CFB(a)和CFB(c)。
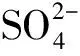
(4) 3台CFB的颗粒物中水溶性离子大都呈现双模态粒径分布,包括超细粒子模态和粗粒子模态。
[1] 程乐鸣,周星龙,郑成航,等.大型循环流化床锅炉的发展[J].动力工程学报,2008,28(6):817-826.
[2] SCHREIFELS J J,FU Yale,WILSON E J.Sulfur dioxide control in China:policy evolution during the 10th and 11th Five-year Plans and lessons for the future[J].Energy Policy,2012,48(3):779-789.
[3] MEIJ R,WINKEL B.The emissions and environmental impact of PM10and trace elements from a modern coal-fired power plant equipped with ESP and wet FGD[J].Fuel Processing Technology,2004,85(6/7):641-656.
[4] WU Xuecheng,ZHAO Huafeng,ZHANG Yongxin,et al.Measurement of slurry droplets in coal-fired flue gas after WFGD[J].Environmental Geochemistry and Health,2015,37(5):1-15.
[5] COSTELLO M J,JOHNSEN S,GILLILAND K O,et al.Predicted light scattering from particles observed in human age-related nuclear cataracts using mie scattering theory[J].Investigative Ophthalmology & Visual Science,2007,48(1):303-312.
[6] IGNATIUS N T.Chemical and size effects of hygroscopic aerosols on light scattering coefficients[J].Journal of Geophysical Research:Atmospheres,1996,1011(D14):19245-19250.
[7] HILLAMO R E,KAUPPINEN E I.On the performance of the Berner low pressure impactor[J].Aerosol Science and Technology,1991,14(1):33-47.
[8] MARJAMAKI M.Performance evaluation of the Electrical Low-Pressure Impactor (ELPI)[J].Journal of Aerosol Science,2000,31(2):249-261.
[9] HILDEMANN L M,CASS G R,MARKOWSKI G R.A dilution stack sampler for collection of organic aerosol emissions:design,characterization and field tests[J].Aerosol Science and Technology,1989,10(1):193-204.
[10] LATVA SOMPPI J,MOISIO M,KAUPPINEN E I,et al.Ash formation during fluidized-bed incineration of paper mill waste sludge[J].Journal of Aerosol Science,1998,29(4):461-480.
[11] SVENNINGSSON B,RISSLER J,SWIETLICKI E,et al.Hygroscopic growth and critical supersaturations for mixed aerosol particles of inorganic and organic compounds of atmospheric relevance[J].Atmospheric Chemistry & Physics,2005,6(7):1937-1952.
[12] YI Honghong,HAO Jiming,DUAN Lei,et al.Fine particle and trace element emissions from an anthracite coal-fired power plant equipped with a bag-house in China[J].Fuel,2008,87(10/11):2050-2057.
[13] YI Honghong,HAO Jiming,DUAN Lei,et al.Characteristics of inhalable particulate matter concentration and size distribution from power plants in China[J].Journal of the Air & Waste Management Association,2006,56(9):1243-1251.
[14] SIPPULA O,HOKKINEN J,PUUSTINEN H,et al.Particle emissions from small wood-fired district heating units[J].Energy & Fuels,2009,23(6).
[15] 王勇,徐晓虎.燃煤电厂除尘器对微细粉尘捕集效率对比试验[J].中国环保产业,2013(6):45-48.
[16] KANG S G,KERSTEIN A R,HELBLE J J,et al.Simulation of residual ash formation during pulverized coal combustion: bimodal ash particle size distribution[J].Aerosol Science and Technology,1990,13(4):401-412.
[17] BUHRE B,HINKLEY J,GUPTA R,et al.Submicron ash formation from coal combustion[J].Fuel,2005,84(10):1206-1214.
[18] 于敦喜,徐明厚,易帆,等.燃煤过程中颗粒物的形成机理研究进展[J].煤炭转化,2004,27(4):7-12.
[19] 胡月琪,马召辉,冯亚君,等.北京市燃煤锅炉烟气中水溶性离子排放特征[J].环境科学,2015,36(6):1966-1974.
[20] 郭兴明,郝吉明,段雷,等.大容量燃煤电站锅炉水溶性离子排放特征[J].清华大学学报(自然科学版),2006,46(12):1991-1994.
[21] 靳晓洁.石灰石—石膏湿法脱硫吸收塔中氯离子问题的探讨[J].电力科技与环保,2013,29(1):46-47.
[22] JONES J M,DARVELL L I,BRIDGEMAN T G,et al.An investigation of the thermal and catalytic behaviour of potassium in biomass combustion[J].Proceedings of the Combustion Institute,2007,31(2):1955-1963.
参考文献:
[23] TISSARI J,SIPPULA O,KOUKI J,et al.Fine particle and gas emissions from the combustion of agricultural fuels fired in a 20 kW burner[J].Energy & Fuels,2008,22(3):2033-2042.
[24] SAROFIM A F,HOWARD J B,PADIA A S.The physical transformation of the mineral matter in pulverized coal under simulated combustion conditions[J].Combustion Science and Technology,1977,16(3/4/5/6):187-204.
[25] MCELROY M W,CARR R C,ENSOR D S,et al.Size distribution of fine particles from coal combustion[J].Science,1982,215(4528):13-19.
[26] MATHIEU Y,TZANIS L,SOULARD M,et al.Adsorption of SOxby oxide materials:a review[J].Fuel Processing Technology,2013,114(3):81-100.
[27] 吴忠标,刘越,谭天恩.双碱法烟气脱硫工艺的研究[J].环境科学学报,2001,21(5):534-537.
