4种豆粕替代鱼粉对大黄鱼生长、抗氧化及抗菌能力的影响
2016-03-06陈乃松华雪铭黄旭雄陈晓明朱伟星
吴 钊,陈乃松,华雪铭,黄旭雄,陈晓明,王 坛,王 刚,朱伟星,孔 纯
(上海海洋大学农业部水产种质资源与利用重点开放实验室,上海 201306)
4种豆粕替代鱼粉对大黄鱼生长、
抗氧化及抗菌能力的影响
吴 钊,陈乃松,华雪铭,黄旭雄,陈晓明,王 坛,王 刚,朱伟星,孔 纯
(上海海洋大学农业部水产种质资源与利用重点开放实验室,上海 201306)
分别用9种等氮等能的饲料投喂初始体质量为(34.72±0.28)g的大黄鱼(Pseudosciaena crocea)。其中1组投喂对照饲料(含50%鱼粉,不含豆粕),另外8个试验组分别投饲由去皮豆粕(DSM)、酶解豆粕(ESM)、发酵豆粕Ⅰ(FSMⅠ)和发酵豆粕Ⅱ(FSMⅡ)替代20%和40%的鱼粉的饲料,9组分别命名为FM、DSM20、DSM40、ESM20、ESM40、FSMⅠ20、FSMⅠ40、FSMⅡ20、FSMⅡ40。在海水浮式网箱中进行7周的养殖实验后,评定4种豆粕替代鱼粉的可行性及适宜替代水平。结果显示,试验组与对照组鱼存活率和特定生长率无显著差异(P>0.05)。血清生化指标显示,FM组和FSMⅡ20组超氧化物歧化酶(SOD)活性显著高于其它试验组(P<0.05),FM、DSM20、FSMⅠ40、FSMⅡ20组过氧化氢酶(CAT)活性显著高于DSM40、ESM20、ESM40及FSMⅠ20组(P<0.05),不同试验组的丙二醛(MDA)含量均不同程度高于对照组。酶解豆粕替代40%鱼粉导致实验鱼的血清对哈维氏弧菌的抵抗能力下降,去皮豆粕替代20%鱼粉导致血清对溶藻弧菌抵抗能力下降;但发酵豆粕不影响血清及黏液对3种菌的抵抗能力。研究表明,以特定生长率、饲料转化率和抗菌能力为评价指标,发酵豆粕是鱼粉的最佳替代源,发酵豆粕Ⅰ和Ⅱ均能替代20%~40%的鱼粉,但存在抗氧化能力下降的风险,尤其是发酵豆粕Ⅰ40%替代组;去皮豆粕和酶解豆粕替代鱼粉在抗菌能力和抗氧化能力方面无优势。
大黄鱼;鱼粉;豆粕;抗氧化能力;抗菌能力
鱼粉因具有蛋白质含量高、水产动物所必需的氨基酸丰富、易被水产动物消化吸收等优点,在水产饲料中被广泛应用。然而,随着饲料行业的快速发展,导致鱼粉供不应求,价格飚升[1]。目前,世界鱼粉的供应已经不能满足日益增长的饲料业的需求,因此,寻找鱼粉替代源成为国内外研究的热点。
植物性蛋白源因其价格低廉且供应稳定,备受研究者的关注。其中,大豆蛋白源具有消化吸收率高、氨基酸组成较好、价格合理和资源量丰富等特点,是水产饲料应用最多的植物蛋白源之一。但是,大豆蛋白源在鱼类饲料中过量使用,不仅影响鱼类的摄食和生长[2-4],而且影响鱼的健康和免疫功能[5-8]。其主要原因在于适口性不佳[9-11]、抗营养因子多[12-14]和氨基酸不平衡[15]等。通过使用复合植物蛋白源[16]、添加酶制剂[17]、微生物发酵[18-19]及添加晶体氨基酸[20-21]等方法可以缓解以上问题。
大黄鱼(Pseudosciaena crocea),属鲈形目,石首鱼科,黄鱼属,又名黄鱼、黄瓜鱼,为我国特有的地方性种类,主要分布在福建和浙江沿海地区,因其生长迅速、肉质鲜美而深受消费者的青睐。研究表明,0.57 g的大黄鱼饲料中所需蛋白质水平在47%以上[22],且饲料的蛋白质主要来源于鱼粉。鉴于目前鱼粉的供求矛盾和价格情况,寻找鱼粉的替代源成为大黄鱼配合饲料研制中需要重点解决的问题之一。目前,大黄鱼饲料中鱼粉替代的研究已有报道[23-25],主要集中在蛋白源选择以及替代水平方面,涉及肉骨粉、各种植物蛋白及复合蛋白等,但尚未见几种不同加工工艺的豆粕对鱼粉替代效果的比较研究。另有研究表明,添加晶体氨基酸可以缓解豆粕中氨基酸不平衡;去皮可以消除种皮中的大部分单宁;酶解可以消除豆粕中的植酸以及大豆低聚糖等;微生物发酵可降低豆粕中胰蛋白酶抑制因子、皂甙、植酸等抗营养因子的抗营养效应,从而提高鱼类的摄食及生长[26]。因此,本研究拟在添加包膜晶体氨基酸的条件下,用去皮豆粕、酶解豆粕以及两种不同发酵程度的豆粕分别替代配方中20%和40%的鱼粉,从生长性能、抗氧化以及抗菌能力等方面综合判断4种豆粕替代鱼粉的可行性,以期为豆粕在大黄鱼人工配合饲料中的应用提供参考。
1 材料与方法
1.1 实验饲料
以鱼粉、4种不同豆粕(去皮豆粕、酶解豆粕、发酵豆粕Ⅰ、发酵豆粕Ⅱ)、玉米蛋白粉、喷干血球粉以及谷朊粉为主要蛋白源、鱼油和大豆磷脂油为主要脂肪源,配制9种等氮(48%)等脂(12%)等无氮浸出物(13%)的实验饲料。对照组为50%鱼粉组,试验组以4种不同豆粕分别替代20%和40%的鱼粉,在替代组中添加蛋氨酸、赖氨酸以及苏氨酸以确保此3种氨基酸含量与对照组一致。饲料配方及成分分析见表1。
1.2 养殖实验的设计和饲养管理
养殖实验于上海农好饲料有限公司养殖基地(位于福建省宁德市蕉城区三都澳海区)的网箱中进行。在开始正式实验前,将大黄鱼放入4 m×4 m×3 m的网箱中暂养、驯化2周。经24 h饥饿,挑选体格健壮、大小均匀的大黄鱼(34.72 g ±0.28 g)随机分为9组。每组随机分配3个浮式海水网箱(长×宽×深:1.2 m×1 m×2 m),每个网箱放养40 ind,每天饱食投喂2次(06∶00和16∶00)。养殖周期为49 d,养殖期间每天记录投喂量以及死亡情况。
1.3 样品采集和分析
养殖结束后,将实验鱼饥饿24 h,从网箱中全部捞起,用丁香酚麻醉后计数并称重。分别从每个网箱中随机取12 ind鱼,尾静脉抽血;将血样置于4℃冰箱静置4h后,离心(836×g)10 min,分离取得血清,并于-20℃保存。对抽取血液的6 ind鱼取其肝脏,并称重用于计算肝体比。分离背部侧线上方的肌肉,于-20℃保存用于测定肌肉成分;参考PALAKSHA等[28]的方法,用细胞刮棒在鱼背侧面轻轻刮取体表黏液。剩余的鱼置于-20℃保存用于体组成分析。
饲料、肌肉、全鱼体组成分析参照AOAC(1993)[27]的方法。水分测定在105℃烘箱中烘至恒重;粗灰分在马弗炉中550℃灼烧测定;粗蛋白质采用凯氏定氮法测定;粗脂肪采用氯仿—甲醇法测定。
血清、黏液以及肝脏中超氧化物歧化酶(SOD)、溶菌酶(LZM)、过氧化氢酶(CAT)、丙二醛(MDA)均采用南京建成生物工程研究所试剂盒测定,血清以及组织匀浆中的蛋白浓度采用考马斯亮蓝法测定,血清和肝脏中的谷草转氨酶(AST)、谷丙转氨酶(ALT)采用迈瑞生物医疗电子股份有限公司试剂盒测定。
血清及黏液抗菌活力测定根据SUNYER等[29]方法稍作修改。将100μL稀释的菌液(哈维氏弧菌、溶藻弧菌、嗜水气单孢菌)加入96孔板,并加入等体积的血清或黏液,另取不加菌作阴性对照,不加血清和黏液作阳性对照,将其置于28℃条件下恒温孵育24 h,在620 nm波长条件读取OD值,每2 h读一次,最后结果根据前后两次稳定的OD值来计算。
1.4 计算公式
增重率(WG)(%)=(Wt-W0)/W0×100
特定生长率(SGR)=(LnWt-LnW0)×100/t
摄食量(FI)=Dd/[(N0+Nt)/2]
饲料转化率(FCR)=(Wt-W0)/Dd
肝体比(HSI)(%)=100×WL/Wt
肥满度(CF)(%)=100×Wt/L3
抗菌活力(ATB)=1-(OD2-OD1)/OD0上述各公式中,Wt、W0分别为实验大黄鱼的终末平均个体质量和初始平均个体质量;N0、Nt分别为实验大黄鱼的初始尾数和终末尾数;WL为肝脏湿重;t为实验天数;L是鱼的体长(cm);Dd为总摄食量;OD0、OD1、OD2分别为阳性对照、阴性对照及试验组OD值。
1.5 数据处理与统计分析
采用SPSS 17.0对数据进行单因素方差分析(One-way ANOVA),用Duncan氏法进行多重差异显著性比较,显著水平P<0.05。

表1 实验饲料配方及成分分析(%风干物质)Tab.1 Analysis on composition and proximate of the experimental diets(%air-dry matter)
2 结果与分析
2.1 不同豆粕替代鱼粉对大黄鱼生长和存活率的影响
表2显示,试验组与对照组在存活率、增重率与特定生长率上无显著差异(P>0.05)。由表3可见,DSM20组摄食量显著高于对照组(P<0.05),而其它试验组摄食量与对照组无显著差异(P>0.05),且各组饲料转化率无显著差异(P>0.05)。肝体比以及肥满度在各组之间同样无显著差异(P>0.05)。

表2 不同豆粕替代鱼粉试验组中大黄鱼生长和存活状况Tab.2 Grow th and survival of large yellow croaker in different experimental groups
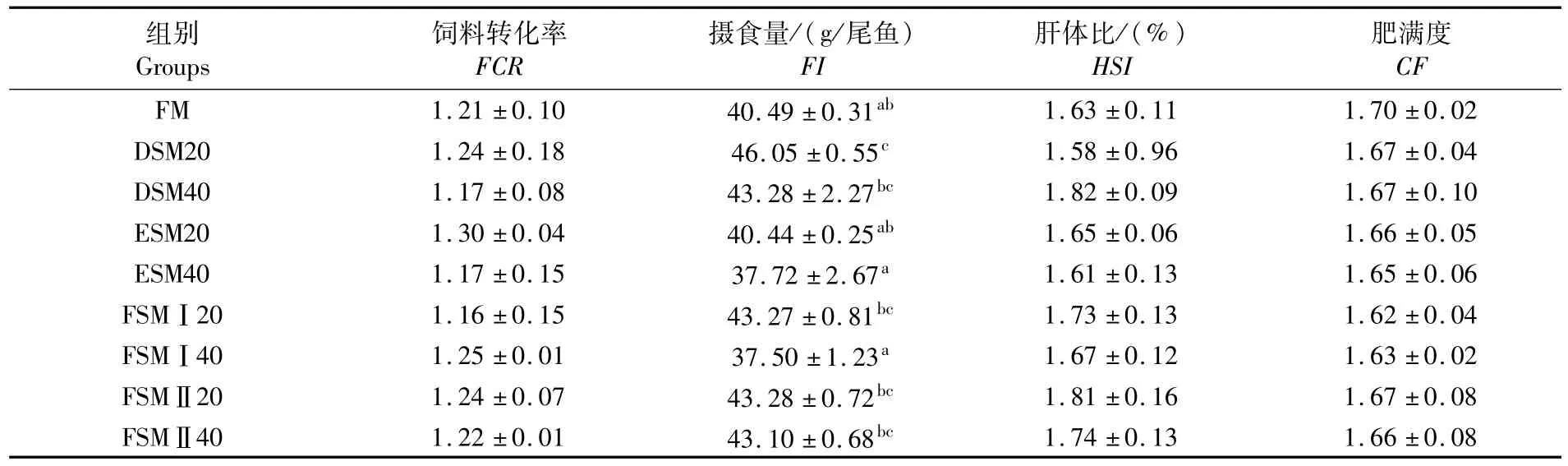
表3 各组的饲料转化率、摄食量、肝体比以及肥满度Tab.3 Feed conversion ratio,feed intake,HSI and CF in experimental groups
2.2 不同豆粕替代鱼粉对大黄鱼体组成的影响
各组鱼全鱼及肌肉组成见表4。不同豆粕替代鱼粉影响大黄鱼全鱼以及肌肉中的蛋白质和脂肪含量,随着替代水平增加,肌肉和全鱼中蛋白含量有下降的趋势。不同豆粕在40%替代水平时,全鱼中蛋白含量显著低于对照组,而脂肪含量呈相反的趋势;肌肉中粗蛋白与粗脂肪亦呈类似的趋势。
2.3 不同豆粕替代鱼粉对大黄鱼生化指标的影响
如表5和表6所示,血清中FM和FSMⅡ20组鱼SOD活性显著高于其它替代组;而肝脏中SOD活性是在DSM20组和FSMⅡ20组最高。血清中CAT活性同样是在FSMⅡ20组最高;而肝脏中CAT活性是在FSMⅠ40组最高。血清中溶菌酶活性在ESM40组最低;而血清中MDA在ESM20组和ESM40组最高,肝脏中MDA水平在ESM40组最高。血清中总蛋白(TP)、AST、ALT以及肝脏中AST、ALT在各组之间没有差异。由表7可知,试验组鱼体表黏液中SOD活性显著高于对照组,黏液中MDA在酶解豆粕20%替代组显著高于对照组。
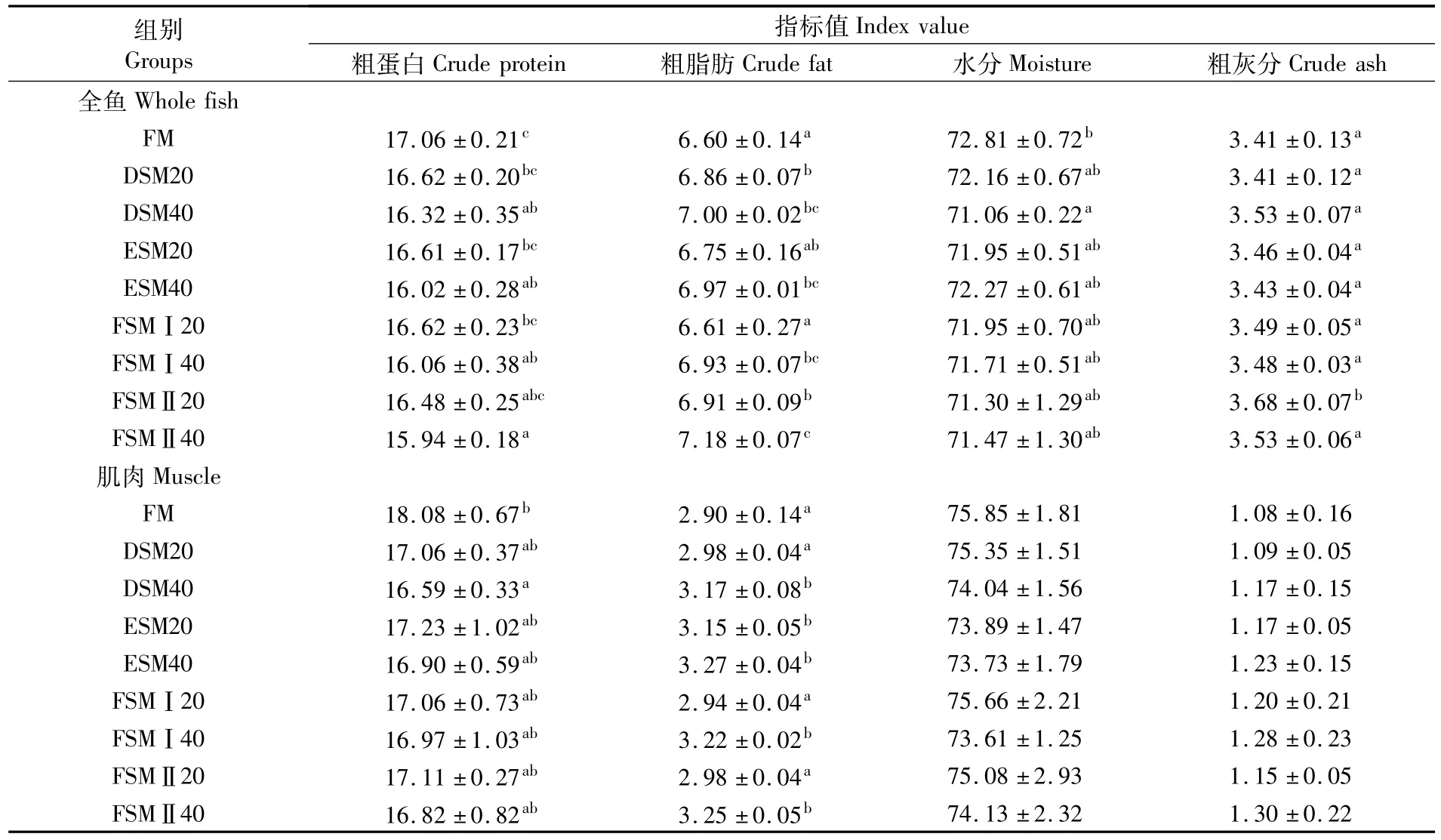
表4 各组全鱼及肌肉组成Tab.4 Proximate composition of fish and muscle in experimental groups (%)
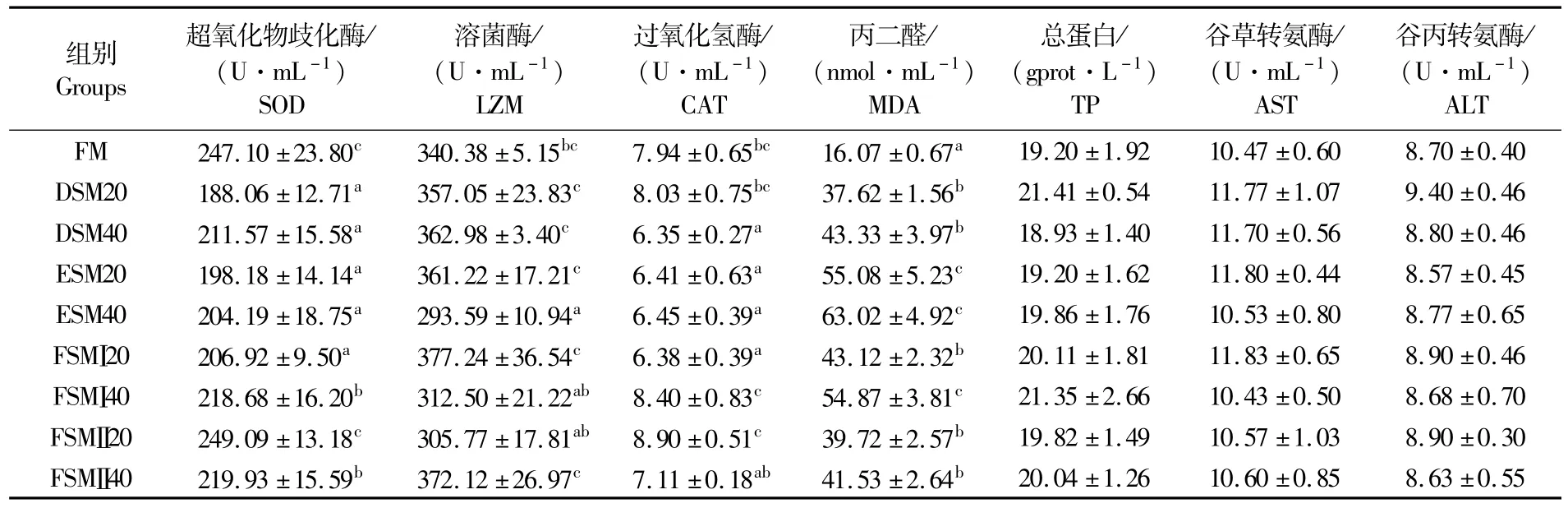
表5 各组鱼血清生化指标含量Tab.5 Contents of biochemical indexs in serum of fish in experimental groups
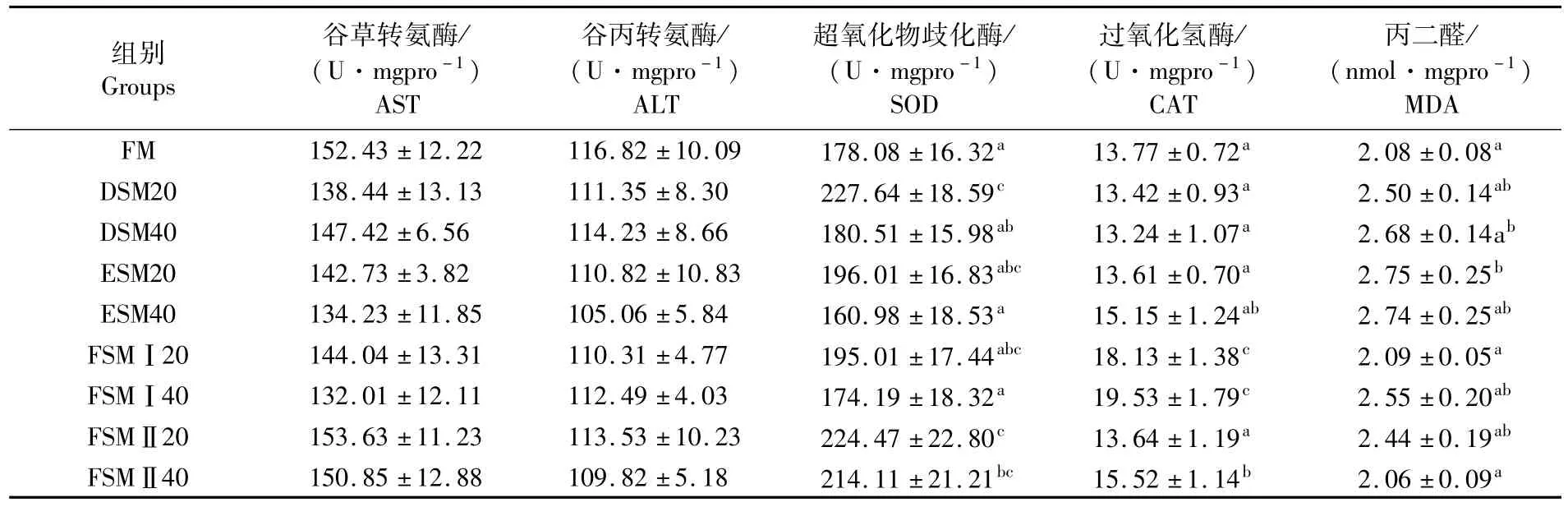
表6 各组鱼肝脏生化指标含量Tab.6 Contents of biochemical indexs in liver of fish in experimental groups
2.4 不同豆粕替代鱼粉对大黄鱼血清及黏液抗菌能力的影响
血清及体表黏液对哈维氏弧菌、溶藻弧菌以及嗜水气单孢菌的抵抗能力如表8。ESM40组血清对哈维氏弧菌抵抗能力显著低于FM组,其它试验组与对照组无显著差异;而DSM20组血清对溶藻弧菌抵抗能力显著低于FM组,黏液对溶藻弧菌抵抗能力在ESM20组和ESM40组最低。不同组鱼血清及黏液对嗜水气单孢菌的抵抗能力没有明显差异。
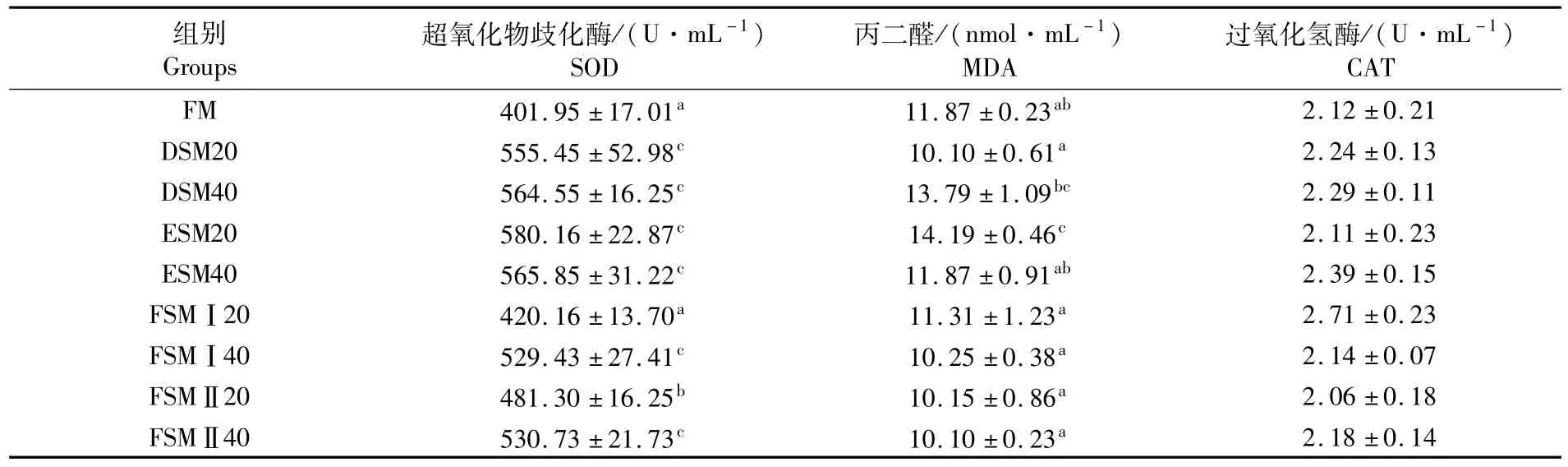
表7 各组鱼黏液-生化指标含量Tab.7 Contents of biochemical mucus in liver of fish in experimental groups

表8 血清及黏液抗菌活力Tab.8 Antimicrobial activity of serum and mucus
3 讨论
3.1 不同豆粕替代鱼粉对大黄鱼生长和存活率的影响
本实验中试验组与对照组相比,存活率、增重率与特定生长率均无显著差异,表明在一定的范围内豆粕对鱼粉的替代效果不受替代水平的影响[26]。同样的实验在虹鳟(Oncorhynchus mykiss)[30-31]、奥尼罗非鱼(Oreochromis niloticus ×O.aureus)[32]上也均显示用适量的豆粕替代鱼粉不会对鱼生长造成负面影响。LI等[33]在大黄鱼的研究中发现,大黄鱼幼鱼饲料中豆粕替代鱼粉的最适替代比例为30%,而本研究在40%的替代水平下依然不会对大黄鱼生长造成负面影响,这可能与本研究所使用豆粕的抗营养因子减少[26,34-35]以及添加复合包膜晶体氨基酸有关。在虹鳟[36]、红拟石首鱼(Sciaenops ocellatus)[37]的研究中已证实添加晶体氨基酸可以改善豆粕替代鱼粉的效果。
3.2 不同豆粕替代鱼粉对大黄鱼体组成的影响
ZHANG等[24]在大黄鱼饲料中用植物蛋白替代鱼粉的研究中发现,随着替代水平的增加,鱼体粗蛋白质含量出现降低趋势,而本实验中也出现相同的趋势,这可能是由于植物蛋白替代鱼粉虽能满足大黄鱼生长所需的蛋白质量,但仍存在包膜晶体氨基酸用于合成鱼体蛋白质的利用率不如蛋白质来源的结合态氨基酸,游离氨基酸的不同步吸收导致鱼体内蛋白质的合成下降的可能[24,38]。随着替代比例的增加,鱼体肌肉中蛋白含量出现下降,脂肪含量出现上升的趋势,这与陈乃松等[38]在大口黑鲈(Micropterus salmoides)中的研究结果一致。
3.3 不同豆粕替代鱼粉对大黄鱼健康的影响
生物有机体在正常的生理生化反应中需要氧自由基的参与,并通过生物体内酶和非酶性抗氧化系统清除过量自由基,维持机体内氧化与抗氧化的动态平衡,这种动态平衡向氧化移动,则会导致生物有机体的氧化应激损伤[39]。抗氧化系统中,SOD是生物体内唯一以自由基为底物的酶,负责抑制超氧化物自由基的生成,催化超氧阴离子发生歧化反应,转变为H2O2和O2[40],随后由CAT催化H2O2分解为H2O和O2[41],从而使细胞免于遭受H2O2的毒害。若多余的活性氧自由基不能被及时清除,则产生大量的脂质过氧化产物MDA,引起组织和细胞氧化损伤[42]。本研究中血清SOD和CAT在各组之间有显著差异,表明大黄鱼的抗氧化系统受到豆粕种类和使用量的影响;而肝脏作为机体的代谢中心,粘液作为非特异免疫系统的第一道防线,SOD和CAT在这两个组织的活性在各组间也有不同程度的变化,说明肝脏和粘液的抗氧化作用也受到豆粕种类和使用量的影响,但平衡氧自由基的表现方式与血液稍有不同。MDA在对照组血清中的含量显著低于试验组,说明不同试验组的鱼体内抗氧化能力相对弱于对照组。同样,酶解豆粕20%替代组、酶解豆粕40%替代组及发酵豆粕Ⅰ40%替代组血清MDA显著高于其它试验组,这也说明不同豆粕对大黄鱼体内抗氧化能力的影响也是有差异的,而这可能与不同工艺豆粕中的抗营养因子的差异相关,具体原因有待进一步分析。由ALT和AST结果表明,不同豆粕替代鱼粉不影响鱼体肝脏氨基酸代谢活动,肝功能未受到影响,这可能是由于鱼体内有天然的抗氧化系统得以维持正常的生理功能[43],但一旦超过鱼体自身调节的范围,就会对机体造成损伤。
鱼体血清及黏液含有一些抵御病菌的分子[44-45],其抗菌能力是鱼体免疫机能强弱最直接的一种表现。本实验中不同豆粕替代鱼粉对大黄鱼血清及黏液抵抗嗜水气单孢菌的能力没有影响,酶解豆粕替代40%的鱼粉会导致大黄鱼血清对哈维氏弧菌抵抗力下降,酶解豆粕替代20%或40%鱼粉会导致大黄鱼体表黏液对溶藻弧菌的抵抗力下降,而两种发酵豆粕分别替代20%及40%鱼粉均没有导致大黄鱼血清及黏液对3种菌的抵抗能力显著下降,这可能是由于在发酵过程中,大豆异黄酮中的葡萄糖苷会转化为葡萄糖苷元,从而使得苷元成分大增,因此抗菌性能提高[46]。以上结果说明,大黄鱼血清及黏液的抗菌能力因病原菌种类而异,SANGEETHA等[47]发现河鳟(Salvelinus alpinus)、黑线鳕(Melanogrammus aeglefinus)、黏液鳗(Myxineglutinosa)的酸性黏液提取物对鱼和人类的病原具有较强的特殊抗菌活性,这与鱼体表黏液中的丝氨酸和半胱氨酸蛋白水解酶类溶解寄生物有关[48-49]。
4 小结
本研究表明,以特定生长率、饲料转化率和抗菌能力为评价指标,发酵豆粕Ⅰ和Ⅱ能替代20%~40%的鱼粉,但存在抗氧化能力下降的风险,尤其是发酵豆粕Ⅰ40%替代组;去皮豆粕和酶解豆粕替代鱼粉在抗菌能力和抗氧化能力方面均无优势;4种豆粕替代鱼粉,全鱼和肌肉的蛋白质含量、脂肪含量分别呈下降和上升趋势。
[1] TACON A G J,METIAN M.Global overview on the use of fish meal and fish oil in industrially compounded aquafeeds:trends and future prospects[J].Aquaculture,2008,285(1-4):146-158.
[2] OLLI J J,KROGDAHL Å,VAN DEN LNGH T S,etal.Nutritive value of four soybean products in diets for Atlantic salmon(Salmo salarL.)[J].Acta Agriculturae Scandinavica,Section A-Animal Science,1994,44(1):50-60.
[3] OLLI J J,KROGDAHLÅ,VAN DEN LNGH T S.Dehulled solvent-extracted soybean meal as a protein source in diets for Atlantic salmon,Salmo salarL.[J].Aquaculture Research,1995,26(3):167-174.
[4] 仲维玮,文 华,蒋 明,等.混合植物蛋白源对罗非鱼幼鱼生长、体组成及表观消化率的影响[J].华中农业大学学报,2010,29(3):356-362.
ZHONGW W,WEN H,JIANG M,et al.Effect of plant protein sources on growth performance,body composition and apparent digestibility in juvenile Nile tilapia(Oreochromis niloticus)[J].Journal of Huazhong Agricultural University,2010,29(3):356-362.
[5] 刘 勇,冷向军,李小勤,等.豆粕替代鱼粉对奥尼罗非鱼生长、表观消化率及血清非特异性免疫的影响[J].中国粮油学报,2009(12):95-100.
LIU Y,LENG X J,LIX Q,et al.Effects of replacing fish meal with soybean meal on growth,digestibility and immunity of tilapia(Oreochromis niloticus×O.aureus)[J].Journal of the Chinese Cereals and Oils Association,2009(12):95-100.
[6] KROGDAHL A,BAKKE-MCKELLEP A M,RODE K H,et al.Feeding Atlantic salmonSalmo salarL.soybean products:effects on disease resistance(furunculosis),and lysozyme and IgM levels in the intestinal mucosa[J].Aquaculture Nutrition,2000,6(2):77-84.
[7] REFSTIE S,STOREBAKKEN T,BAEVERFJORD G,et al.Long-term protein and lipid growth of Atlantic salmon(Salmo salar)fed diets with partial replacement of fish meal by soy protein products at medium or high lipid level[J].Aquaculture,2001,193(1):91-106.
[8] 张锦秀,周小秋,刘 扬.去皮豆粕对幼建鲤生长性能和肠道的影响[J].中国水产科学,2007,14(2):315-320.
ZHANG JX,ZHOU X Q,LIU Y.Effects of dehulled soybean meal on growth performance and intestine of juvenileCyprinus carpiovar.jian[J].Journal of Fishery Sciences of China,2007,14(2):315-320.
[9] BURRELLS C,WILLIAMSP D,SOUTHGATE P J,et al.Immunological,physiological and pathological responses of rainbow trout(Oncorhynchus mykiss)to increasing dietary concentrations of soybean proteins[J].Veterinary Immunology and Immunopathology,1999,72(3):277-288.
[10] BUREAU D P,HARRIS A M,YOUNG C C.The effects of purified alcohol extracts from soy products on feed intake and growth of chinook salmon(Oncorhynchus tshawytscha)and rainbow trout(Oncorhynchus mykiss)[J].Aquaculture,1998,161(1):27-43.
[11] GOMESE F,KAUSHILK S.Effect of replacement of dietary inorganic zinc by zinc-methionine on vegetable and animal protein utilization by rainbow trout[C]//Kaushik S J,Luquet P.Fish Nutrition in Practice.France:INRA,1993:897-902.
[12] GOMESE F,REMA P,KAUSHIK S J.Replacement of fish meal by plant proteins in the diet of rainbow trout(Oncorhynchus mykiss):digestibility and growth performance[J].Aquaculture,1995,130(2):177-186.
[13] FRANCIS G,MAKKAR H P,BECKER K.Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish[J].Aquaculture,2001,199(3):197-227.
[14] HEIKKINEN J,VIELMA J,KEMILAINEN O,et al.Effects of soybean meal based diet on growth performance,gut histopathology and intestinal microbiota of juvenile rainbow trout(Oncorhynchusmykiss)[J].Aquaculture,2006,261(1):259-268.
[15] CHOU R L,HER B Y,SU M S,et al.Substituting fish meal with soybean meal in diets of juvenile cobia,Rachycentron canadum[J].Aquaculture,2004,229(1):325-333.
[16] VIOLA S,MOKADY S,ARIELIY.Effects of soybean processing methods on the growth of carp(Cyprinus carpio)[J].Aquaculture,1983,32(1):27-38.
[17] BISWAS A K,KAKU H,JI S C,et al.Use of soybean meal and phytase for partial replacement of fishmeal in the diet of red sea bream,Pagrus major[J].Aquaculture,2007,267(1-4):284-291.
[18] SANNI A I,ONILUDE A A,IBIDAPO O T.Biochemical composition of infant weaning food fabricated from fermented blends of cereal and soybean[J].Food Chemistry,1999,65(1):35-39.
[19] SUZUKI Y,KONDO K,ICHISE H,et al.Dietarysupplementation with fermented soybeans suppresses intimal thickening[J].Nutrition,2003,19(3):261-264.
[20] CHENG Z J,HARDY RW.Effects of extrusion and expelling processing,and microbial phytase supplementation on apparent digestibility coefficients of nutrients in full-fat soybeans for rainbow trout(Oncorhynchus mykiss)[J].Aquaculture,2003,218(1):501-514.
[21] FURUYA W M,PEZZATO L E,BARROSM M,etal.Use of ideal protein concept for precision formulation of amino acid levels in fish meal free diets for juvenile Nile tilapia(Oreochromis niloticus L.)[J].Aquaculture Research,2004,35(12):1110-1116.
[22] DUAN Q Y,MAIK S,ZHONG H Y,etal.Studies on the nutrition of the large yellow croaker,Pseudosciaena croceaR.I:growth response to graded levels of dietary protein and lipid[J].Aquaculture Research,2001,32(s1):46-52.
[23] AIQ H,MAIK S,TAN B P,et al.Replacement of fish meal by meat and bone meal in diets for large yellow croaker,Pseudosciaena crocea[J].Aquaculture,2006,260(1-4):255-263.
[24] ZHANG L,MAI K S,AI Q H,et al.Use of a compound protein source as a replacement for fish meal in diets of large yellow croaker,Pseudosciaena croceaR.[J].Journal of the World Aquaculture Society,2008,39(1):83-90.
[25] 张 帆.大黄鱼(Pseudosciaena croceaR.)脂类营养生理和饲料替代蛋白源的研究[D].青岛:中国海洋大学,2012.
ZHANG F.Lipid requirement and fish meal replacement in diets of large yellow croaker,Pseudosciaena croceaR.[D].Qingdao:Ocean University of China,2012.
[26] 艾庆辉,谢小军.水生动物对植物蛋白源利用的研究进展[J].中国海洋大学学报,2005,35(6):925-935.
AIQ H,XIE X J.Advance in utilization of plant proteins by aquatic animals[J].Periodical of Ocean University of China,2005,35(6):925-935.
[27] AOAC.Official methods of the analysis of the association of official analytical chemists[S].
[28] PALAKSHA K J,GEE W S,YOUNG R M,et al.Evaluation of non-specific immune components from the skin mucus of olive flounder(Paralichthysolivaceus)[J].Fish&Shellfish Immunology, 2008,24(4):479-488.
[29] SUNYER J O,TORT L.Natural hemolytic and bactericidal activities of sea bream(Sparus aurata)serum are effected by the alternative complement pathway[J].Vet Immunol Immunopathol,1995(45):333-345.
[30] KAUSHIK S J,CRAVEDIJP,LALLES JP,et al.Partial or total replacement of fish meal by soybean protein on growth,protein utilization,potential estrogenic or antigenic effects,cholesterolemia and flesh quality in rainbow trout,Oncorhynchus mykiss[J].Aquaculture,1995,133(3):257-274.
[31] YAMAMOTO T,AKIYAMA T.Substitution of soybean meal for white fish meal in a diet for fingerling rainbow troutOncorhynchus mykiss[J].Bulletin of National Research Institute of Aquaculture(Japan),1991(20):25-32.
[32] SHIAU S,LIN S,YU S,et al.Defatted and full-fat soybean meal as partial replacements for fishmeal in tilapia(Oreochromis niloticus×O.aureus)diets at low protein level[J].Aquaculture,1990,86(4):401-407.
[33] LIJ,ZHANG L,MAIK S,et al.Potential of several protein sources as fish meal substitutes in diets for large yellow croaker,Pseudosciaena crocea R.[J].Journal of the World Aquaculture society,2010,41(s2):278-283.
[34] 易中华,计 成,马秋刚,等.去皮豆粕和普通豆粕对肉鸡生产性能及营养素利用率的影响[J].中国饲料,2006(1):15-17.
YIZ H,JIC,MA Q G,et al.The effect of dehulled soybean meal and regular soybean meal on growth and utilization rate of nutrients of broiler chicken[J].China Feed,2006(1):15-17.
[35] 陈乃松,杨志刚,崔惟东,等.酶制剂体外酶解豆粕中抗营养因子的研究[J].大豆科学,2008,27(4):663-668.
CHEN N S,YANG Z G,CUIW D,et al.Enzymes hydrolyzing in vitro anti-nutritional factors in soybean meal[J].Soybean Science,2008,27(4):663-668.
[36] MEDALE F,BOUJARD T,VALLEE F,et al.Voluntary feed intake,nitrogen and phosphorus losses in rainbow trout(Oncorhynchus mykiss)fed increasing dietary levels of soy protein concentrate[J].Aquatic Living Resources,1998,11(4):239-246.
[37] MCGOOGAN B B,GATLIN D M.Effects of replacing fish meal with soybean meal in diets for red drumSciaenops ocellatus and potential for palatability enhancement[J].Journal of the World Aquaculture Society,1997,28(4):374-385.
[38] 陈乃松,马秀丽,赵 明,等.大口黑鲈幼鱼饲料中白鱼粉与两种豆粕的适宜配比[J].水产学报,2013,37(9):1389-1400.
CHEN N S,MA X L,ZHAO Met al.Suitable inclusion levels of white fish meal and two kinds of soybean meal in diets for juvenile largemouth bass(Micropterus salmoides)[J].Journal of Fisheries of China,2013,37(9):1389-1400.
[39] BAI S C,LEE K.Different levels of dietary dl-αtocopheryl acetate affect the vitamin E status of juvenile Korean rockfish,Sebastes schlegeli[J].Aquaculture,1998,161(1-4):405-414.
[40] MCCORD J M,FRIDOVICH I.Superoxide dismutase:An enzymeatic function for erythrocuprein(hemocuprein)[J].Journal of Experimental Biology,1969,244(6):49-55.
[41] FILHO D W.Fish antioxidant defenses:a comparative approach[J].Brazilian Journal of Medical and Biological Research,1996,29(12):1735-1742.
[42] MARTINEZ ALVAREZ RM,MORALES A E,SANZ A.Antioxidant defenses in fish:biotic and abiotic factors[J].Reviews in Fish Biology and Fisheries,2005,15(1-2):75-88.
[43] DIAS J,ALVAREZM J,DIZE A,et al.Regulation of hepatic lipogenesis by dietary protein/energy in juvenile European seabass(Dicentrarchus labrax)[J].Aquaculture,1998,161(1-4):169-186.
[44] FAST M D,SIMS D E,BURKA J F,et al.Skin morphology and humoral non-specific defence parameters of mucus and plasma in rainbow trout,coho and Atlantic salmon[J].Comparative Biochemistry and Physiology Part A:Molecular&Integrative Physiology,2002,132(3):645-657.
[45] HUANG Z H,MA A J,WANG X A.The immune response of turbot,Scophthalmus maximus(L.),skin to high water temperature[J].Journal of Fish Diseases,2011,34(8):619-627.
[46] 胡梦红,王有基,熊邦喜,等.发酵豆粕在水产饲料中的应用研究[J].饲料工业,2007(12):60-62.
HU M H,WANG Y J,XIONG B X,et al.The application research of fermented soybean meal in aquatic feed[J].Feed Industry,2007(12):60-62.
[47] SUBRAMANIAN S,ROSSNW,MACKINNON S L.Comparison of antimicrobial activity in the epidermal mucus extracts of fish[J].Comparative Biochemistry and Physiology Part B:Biochemistry and Molecular Biology,2008,150(1):85-92.
[48] KOWALSKI R,WOJTCZAK M,GLOGOWSKJ J,et al.Gelatinolytic and anti-trypsin.activities in seminal plasma of common carp:relationship to blood,skin mucus and spermatozoa[J].Aquatic Living Resources,2003,16(5):438-444.
[49] SALLES C M C,GAGLIANO P,LEITAO S A T,etal.Identification and characterization of proteases from skin mucus of tambacu,a Neotropical hybrid fish[J].Fish Physiol Biochem,2007(33):173-179.
Effects of replacement of dietary fish meal by four kinds of soybean meal on grow th,antioxidant and antibacterial ability of Pseudosciaena crocea R.
WU Zhao,CHEN Nai-song,HUA Xue-ming,HUANG Xu-xiong,CHEN Xiao-ming,WANG Tan,WANG Gang,ZHU Wei-xing,KONG Chun
(Key Laboratory of Freshwater Fishery Germplasm Resources,Ministry of Agriculture,Shanghai Ocean University,Shanghai 201306)
Due to the high protein content and rich essential amino acids for aquatic animals,fish meal has been widely used in aquaculture feed industry.However the worldwide fish meal supply can not meet thegrowing demand for aquaculture,it is necessary to look for alternative sources for fish meal.Soybean meal is easily digested and absorbed by aquatic animals,so it is one of the most widely used plant protein sources in aquatic feed.While excessive use of soybean meal with amino acid imbalance and many antinutritional factors in feed,affects not only feeding and growth of fish,but also the health and immune function.It has been demonstrated that the problem of antinutritional factors and amino acid imbalance can be eased by enzyme additives,microbial fermentation and crystalline amino acids additive.Large yellow croakerPseudosciaena croceaR.is an important commercial marine fish species in China.It is favored by consumers due to its high nutritional value.As other carnivores,the high protein requirement of large yellow croaker is mainly from fish meal,so substitution of fish meal in the feed is also a key problem in their culture.In this study,a total of1080Pseudosciaena croceaR.with an average body weight of(34.72g±0.28g)were randomly divided into 9 groups with 3 replicates each,and each replicate contained 40 fish.Fish were fed with 9 isonitrogenous and isoenergetic diets,the control group(50%fishmeal,without soybean meal)and 8 experimental groups formulated by replacing 20%or 40%fish meal with dehulled,enzyme-treated,fermented soybean mealⅠor fermented soybean mealⅡwith crystalline amino acids,respectively.The 9 groups was respectively named as FM,DSM20,DSM40,ESM20,ESM40,FSMⅠ20,FSMⅠ40,FSMⅡ20,FSMⅡ40.A 7-week trial was conducted in floating nets to evaluate the feasibility of the replacement of fishmeal by four different kinds of soybean meal,and to measure the appropriate replacement level.Results showed that there was no significant difference in the survival rate,special growth rate between the control group and experimental groups.And there was no significant difference on feed conversion ratio,hepatosmatic index and condition factor among all groups.The superoxide dismutase activity and catalase in serum,liver and mucus were significantly different among groups.The superoxide dismutase activity of serum in FM and FSMⅡ20 was higher than other experimental groups.The catalase of serum in FM,DSM20,FSMⅠ40 and FSMⅡ20 was higher than that in DSM40,ESM20,ESM40 and FSMⅠ20.The malondialdehyde in serum and liver of replacement groups were higher than that in the control group.Serum showed significantly lower capacity to killV.harveyiin the group of 40%fish meal replaced by enzyme-treated soybean meal and lower capacity to killV.alginolyticusin the group of 20%fish meal replaced by dehulled soybean meal.Serum and mucus showed no significant difference on the capacity to killA.hydrophilaamong all groups.Fermented soybean meal did not affect serum and mucus to kill the three kinds of bacteria.In conculsion,among the tested soybean meal,fermented soybean meal was the best substitution for fish meal,it could replace 20%-40%fish meal based on the specific growth rate,feed conversion ratio and antibacterial ability,but there was a risk of reduced antioxidation ability especially in the group of40%fish meal replaced by fermented soybean meal I.While dehulled and enzyme-treated soybean meal had inferior position on antibacterial ability as well as antioxidation ability.
Pseudosciaena crocea;fish meal;soybean meal;antioxidant ability;antibacterial ability
S 963.31
A
1004-2490(2016)05-0495-12
2015-11-19
上海市科技兴农重点攻关项目(2013第5-8号);水产动物遗传育种中心上海市协同创新中心(ZF1206)
吴 钊,男,硕士研究生,研究方向为水产动物营养学。
华雪铭,副教授,主要研究方向为水产动物营养。E-mail:xmhua@shou.edu.cn,Tel:021-61900416
