间充质干细胞移植治疗糖尿病研究进展
2016-01-25李红敏黄平平
李红敏,黄平平
(中国医学科学院血液学研究所血液病医院,天津300020)
间充质干细胞移植治疗糖尿病研究进展
李红敏,黄平平
(中国医学科学院血液学研究所血液病医院,天津300020)
摘要:近年来,糖尿病发病率不断增高,目前尚无有效治疗方法。间充质干细胞具有诱导分化为胰岛素分泌细胞的潜能,同时具有免疫调控、抗炎及组织损伤修复等功能;其可体外诱导分化为胰岛素分泌细胞,与胰岛共移植治疗糖尿病,具有良好的应用前景。
关键词:间充质干细胞;糖尿病;细胞移植;免疫调控
糖尿病是全球发病率及病死率最高的内分泌疾病。目前其主要治疗方法为口服降糖药及胰岛素替代治疗,虽能改善症状,但现有治疗方案无法精确调节血糖,也无法减轻胰岛β细胞及各种器官功能的进行性损伤。通过各种手段维持或重建胰岛功能一直是糖尿病领域的研究热点。干细胞移植是一种新型糖尿病治疗方法。间充质干细胞(MSCs)是最具代表性的成体干细胞,不仅具有诱导分化为胰岛素分泌细胞的潜能,同时具有免疫调控、抗炎及组织损伤修复等功能。现将近年来MSCs治疗糖尿病的研究进展综述如下。
1MSCs治疗糖尿病的作用机制
1.1MSCs的分化潜能研究发现,MSCs可自发分化形成表达胰岛功能特异性基因或分子标记的胰岛素分泌细胞,且具有来源丰富、易获取、无致畸胎瘤风险、伦理争议小等优点[1]。各种来源的MSCs均可体外扩增并分化形成胰岛素分泌细胞。目前,体外诱导MSCs的方案主要有:①向MSCs中转入胰岛发育相关的转录因子;②模拟体内胚胎发育过程,在培养体系中序贯添加各种生长因子或信号通路激活剂或阻滞剂;③采用胎儿胰岛、胰岛细胞系、发育增殖中的胰腺组织制备的条件培养基或与上述组织的共培养或共移植[3~5]。
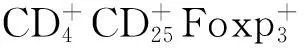
1.3MSCs在抗炎、组织修复中的作用 MSCs具有向不同损伤部位趋化、归巢的能力。Lee等[11]发现,外源性输注BM-MSCs能迁移定植到1型糖尿病小鼠胰岛损伤处,可有效增加糖尿病小鼠胰岛β细胞数量,提高血清胰岛素水平。另有研究发现,胰腺导管外发现新生胰岛细胞团,说明MSCs可促进损伤胰岛细胞的再生。Yeung等[12]研究发现,BM-MSCs共培养条件下的胰岛细胞形态较完整,胰岛素释放反应良好,胰岛细胞凋亡数目较少。此外,MSCs还能分泌血管被生长因子(VEGF)、成纤维细胞生长因子等多种促血管新生的细胞因子,在促进损伤后的胰岛血管新生及外源性胰岛再血管化方面起重要作用[13]。
2MSCs在糖尿病的治疗中的应用前景
2.1MSCs体外诱导分化为胰岛素分泌细胞研究表明,各种来源的MSCs均可体外扩增、分化形成胰岛素分泌细胞,并能逆转糖尿病动物模型的高血糖[2,14]。Vanikar等[15,16]采用AD-MSCs-IPCs联合造血干细胞治疗1型糖尿病患者,结果发现受试者对外源性胰岛素的平均需求量明显下降,血清C肽水平明显升高。但分化后的细胞常表现为对葡萄糖刺激无反应或反应低下,目前尚未用于临床治疗中。
2.2MSCs单独或联合胰岛移植胰岛移植治疗糖尿病效果较好,但常出现受血液介导的急性排斥反应[17~19]。MSCs可抑制胰岛移植后自身免疫系统的激活,抑制淋巴细胞增殖、活化,抑制单核细胞或树突状细胞的分化、成熟,减轻免疫排斥反应[20]。同时,MSCs可旁分泌促血管生成因子,促进移植后胰岛的血管化,提高移植物存活力[21,22]。研究发现,MSCs单独移植对尚残留部分胰岛功能的新发1型糖尿病或2型糖尿病效果较好[23,24]。
综上所述,MSCs具有多向分化潜能,具有免疫调控、抗炎、组织修复等功能,可体外诱导分化为胰岛素分泌细胞,与胰岛共移植途径治疗糖尿病也取得一定进展,具有广阔的应用前景。
参考文献:
[1] Assady S, Maor G, Amit M, et al. Insulin production by human embryonic stem cells[J]. Diabetes, 2001,50(8):1691-1697.
[2] Wu XH, Liu CP, Xu F, et al. Reversal of hyperglycemia in diabetic rats by portal vein transplantation of islet-like cells generated from bone marrow mesenchymal stem cells[J]. WJG, 2007,13(24):3342-3349.
[3] Rahmati S, Alijani N, Kadivar M. In vitro generation of glucose-responsive insulin producing cells using lentiviral based pdx-1 gene transduction of mouse (C57BL/6) mesenchymal stem cells[J]. Bio Res Commun, 2013,437(3):413-419.
[4] Pham P, Nguyen P, Nguyen A, et al. Improved differentiation of umbilical cord blood-derived mesenchymal stem cells into insulin-producing cells by PDX-1 mRNA transfection[J]. Differentiation, 2014,87(5):200-208.
[5] Wang G, Li Y, Wang Y, et al. Roles of the co culture of human umbilical cord Wharton's jelly derived mesenchymal stem cells with rat pancreatic cells in the treatment of rats with diabetes mellitus[J]. Exper Ther Med, 2014, 8(5):1389-1396.

[7] Liu Q, Zheng H, Chen X, et al. Human mesenchymal stromal cells enhance the immunomodulatory function of CD8CD28regulatory T cells[J]. Cell Mole Immun, 2014(12):519-520.
[8] Deng Y, Yi S, Wang G, et al. Umbilical cord-derived mesenchymal stem cells instruct dendritic cells to acquire tolerogenic phenotypes through the IL-6-mediated upregulation of SOCS1[J]. Stem Deve, 2014,23(17):2080-2092.
[9] Hu J, Wang Y, Wang F, et al. Effect and mechanisms of human Wharton's jelly-derived mesenchymal stem cells on type 1 diabetes in NOD model[J]. Endocrine, 2015,48(1):124-134.
[10] Wang Y, Chen X, Cao W, et al. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications[J]. Nat Immun, 2014,15(11):1009-1016.
[11] Lee RH, Seo MJ, Reger RL, et al. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice[J]. Pro Nat Acad Sci, 2006,103(46):17438-17443.
[12] Yeung TY, Seeberger KL, Kin T, et al. Human mesenchymal stem cells protect human islets from pro-inflammatory cytokines[J]. PLoS One, 2012,7(5):38189.
[13] Park KS, Kim YS, Kim JH, et al. Trophic molecules derived from human mesenchymal stem cells enhance survival, function, and angiogenesis of isolated islets after transplantation[J]. Transplantation, 2010,89(5):509-517.
[14] Chen LB, Jiang XB, Yang L. Differentiation of rat marrow mesenchymalstem cells into pancreatic islet beta-cells[J]. WJG, 2004(10):3016-3020.
[15] Trivedi HL, Vanikar AV, Thakker U, et al. Human adipose tissue-derived mesenchymal stem cells combined with hematopoietic stem cell transplantation synthesize insulin[J]. Tran Pro, 2008,40(4):1135-1139.
[16] Vanikar AV, Dave SD, Thakkar UG, et al. Cotransplantation of adipose tissue-derived insulin-secreting mesenchymal stem cells and hematopoietic stem cells: a novel therapy for insulin-dependent diabetes mellitus[J]. Stem Cells Inter, 2010,2010:582382.
[17] Ozmen L, Ekdahl KN, Elgue G, et al. Inhibition of thrombin abrogates the instant blood-mediated inflammatory reaction triggered by isolated human islets: possible application of the thrombin inhibitor melagatran in clinical islet transplantation[J]. Diabetes, 2002,51(6):1779-1784.
[18] Berman A, Pawelec K, Fiedor P. Allogeneic transplantation of isolated islet cells in clinical practice[J]. Pol Arch Med Wew, 2009,119(5):326-332.
[19] Desai NM, Goss JA, Deng S, et al. Elevated portal vein drug levels of sirolimus and tacrolimus in islet transplant recipients: local immunosuppression or islet toxicity[J]. Transplantation, 2003,76(11):1623-1625.
[20] Figliuzzi M, Bonandrini B, Silvani S, et al. Mesenchymal stem cells help pancreatic islet transplantation to control type 1 diabetes[J]. Wor Jour Stem, 2014,6(2):163-172.
[21] Hajizadeh-Saffar E, Tahamtani Y, Aghdami N, et al. Inducible VEGF expression by human embryonic stem cell-derived mesenchymal stromal cells reduces the minimal islet mass required to reverse diabetes[J]. Sci Rep, 2015(5):9322.
[22] Gao X, Song L, Shen K, et al. Bone marrow mesenchymal stem cells promote the repair of islets from diabetic mice through paracrine actions[J]. Mol Cell Endo, 2014,388(1-2):41-50.
[23] Hao H, Liu J, Shen J, et al. Multiple intravenous infusions of bone marrow mesenchymal stem cells reverse hyperglycemia in experimental type 2 diabetes rats[J]. Bio Res Commun, 2013,436(3):418-423.
[24] Lazarus HM, Haynesworth SE, Gerson SL, et al.Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use[J]. Bone Mar Tran, 1995,16(4):557-564.
收稿日期:(2015-06-08)
基金项目:天津市科技计划项目(13ZCZDSY02200)。
中图分类号:R587.1
文献标志码:A
文章编号:1002-266X(2015)46-0096-02
doi:10.3969/j.issn.1002-266X.2015.46.043
