新型2-取代苄基硫代嘧啶衍生物的合成及其细胞毒性*
2016-01-17顾一飞王超杰曹钦坡张孝松陈鹏举蒋腾飞邵坤鹏张秋荣刘宏民郑州大学药学院新药创制与药物安全新评价河南省协同创新中心河南郑州450001
顾一飞,王超杰,曹钦坡,张孝松,陈鹏举,蒋腾飞,邵坤鹏,李 博,可 钰,张秋荣,刘宏民(郑州大学药学院新药创制与药物安全新评价河南省协同创新中心,河南郑州 450001)
新型2-取代苄基硫代嘧啶衍生物的合成及其细胞毒性*
顾一飞,王超杰,曹钦坡,张孝松,陈鹏举,
蒋腾飞,邵坤鹏,李博,可钰,张秋荣,刘宏民
(郑州大学药学院新药创制与药物安全新评价河南省协同创新中心,河南郑州450001)
摘要:以乙酰乙酸乙酯和硫脲为起始原料,经环合、取代、氯代和亲核取代反应合成了16个新型的2-取代苄基硫代嘧啶衍生物(7a~7p),其结构经1H NMR,13C NMR和HR-MS表征。细胞毒性测试结果表明:6-甲基-4-对氯苯胺-2-苄基硫代嘧啶(7b)和6-甲基-4-对溴苯胺-2-苄基硫代嘧啶(7c)对MGC-803(人胃癌细胞)具有较好的抑制活性,其IC50分别为3.126 μg·mL-1和2.197 μg·mL-1,优于5-氟尿嘧啶(IC503.208 μg·mL-1)。
关键词:嘧啶;硫脲嘧啶衍生物;合成;细胞毒性
E-mail:liuhm@ zzu.edu.cn
嘧啶类化合物具有抗微生物[1-3]、抗病毒[4]及抗肿瘤[5-10]等。对该类化合物的分子设计、合成及生物活性的研究是当前新药研究的热点之一[11]。5-氟脲嘧啶(5-FU),氟特嗪和硫鸟嘌呤[12]等作为嘧啶类似物,主要用于治疗肿瘤。通过结构分析发现,硫脲嘧啶是一类非常重要的脲嘧啶核蛋白碱基改性的化学修饰的类似物[13-17]。然而,近年来对甲基硫脲嘧啶衍生物的抗肿瘤活性的研究报告甚少。因此制备一系列新型硫脲嘧啶衍生物并进行其抗癌活性的研究具有重要意义。
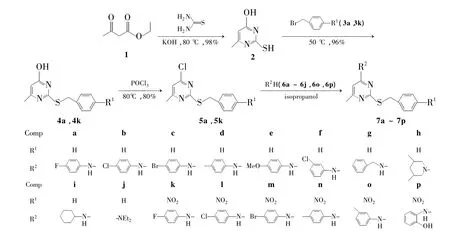
Scheme 1
本文在文献[18]方法的基础上,以乙酰乙酸乙酯(1)和硫脲为起始原料,经环合反应制得4-羟基-6-甲基-硫脲嘧啶(2); 2分别与取代苄基溴(3a,3k)经取代反应制得2-苄基-4-羟基-6-甲基硫脲嘧啶(4a)和2-(4-硝基苯甲基)-4-羟基-6-甲基硫脲嘧啶(4k); 4与POCl3经氯代反应制得2-苄基-4-氯-6-甲基硫脲嘧啶(5a)和2-(4-硝基苯甲基)-4-氯-6-甲基硫脲嘧啶(5k); 5与胺类化合物(6a~6j,6o和6p)经亲核取代反应合成了16个新型的2-取代苄基硫代嘧啶衍生物(7a~7p,Scheme 1),其结构经1H NMR,13C NMR和HRMS表征。测定了7a~7p对4种癌细胞系[MGC-803(人胃癌细胞),EC-9706(人食管癌细胞),PC-3(人前列腺癌细胞),EC-109(人食管癌细胞)]的体外细胞毒性,并对其构效关系进行了研究。
1 实验部分
1.1仪器与试剂
Bruker DPX 400 MHz型核磁共振仪; Waters Micromass Q-T型质谱仪。
所用试剂均为分析纯。
1.2合成
(1)2的合成
在反应瓶中加入1 5.1 mL(40 mmol),硫脲3.044 g(40 mmol)和KOH 2.693 g,搅拌下于80℃反应1 h。冷却,过滤,滤饼干燥得2 5.443 g,产率95.7%。
(2)4的合成(以4a为例)
在反应瓶中加入2 2.8 g(20 mmol),苄基溴(3a)2.4 mL(20 mmol),水15 mL和二噁烷10 mL,搅拌下于50℃反应10 min。用二氯甲烷萃取,合并有机相,减压蒸馏除溶得4a 4.5 g,产率97.6%。
用类似方法制得4k。
(3)5的合成(以5a为例)
在反应瓶中加入4a 1.2 g(5 mmol)和POCl310 mL(110 mmol),搅拌下于80℃反应3 h。静置冷却,逐滴加入冰水中,搅拌下调节pH至中性。过滤,滤饼干燥得5a 1.2 g,产率92.3%。
用类似方法制得5k。
(4)7的合成(以7a为例)
在反应瓶中加入5a 0.3 g(1.2 mmol)和异丙醇7 mL,搅拌使其溶解;加入4-氟苯胺(6a)0.1 mL(1.3 mmol),回流反应2 h(TLC监测)。析出白色固体,抽滤,滤饼用异丙醇洗两次,真空干燥得白色固体7a。
用类似方法合成白色固体7b~7p。
7a:收率79.5%;1H NMR δ:11.01(s,1H,NH),7.61(dd,J=8.6 Hz,4.9 Hz,2H,ArH),7.38~7.17(m,7H,ArH),6.56(s,1H,CH),4.42(s,2H,CH2),2.34(s,3H,CH3);13C NMRδ:165.72,160.33,137.21,134.46,129.32,128.91,127.78,125.68,124.56,116.99,116.06,102.68~100.49,34.43,20.22; HR-MS m/z:Calcd for C18H16N3FS{[M +H]+}326.112 7,found 326.112 9。
7b:收率92.7%;1H NMR δ:11.40(s,1H,NH),7.64(d,J=8.5 Hz,2H,ArH),7.42(d,J=8.6 Hz,2H,ArH),7.35~7.24(m,5H,ArH),6.67(s,1H,CH),4.47(s,2H,CH2),2.37(s,3H,CH3);13C NMR δ:166.47,160.19,158.56,136.98,136.81,129.30,129.27,128.95,127.88,124.91,124.16,102.18,34.57,20.21; HR-MS m/z:Calcd for C18H16N3ClS{[M + H]+} 342.083 2,found 342.082 6。
7c:收率93.5%;1H NMR δ:11.29(s,1H,NH),7.56(dd,J=18.7 Hz,8.8 Hz,4H,ArH),7.44~7.17(m,5H,ArH),6.66(s,1H,CH),4.46(s,2H,CH2),2.37(s,3H,CH3);13C NMR δ:166.96,164.36,159.02,139.88,136.80,132.00,128.99,128.66,127.57,123.10,115.68,100.25,34.74,24.75; MS m/z:Calcd for C18H16N3BrS{[M +H]+}387.041 3,found 387.041 2。
7d:收率79.5%;1H NMR δ:11.19(s,1H,NH),7.46(d,J=7.8 Hz,2H,Ar),7.24(dd,J=9.9 Hz,4.1 Hz,5H,ArH),7.20(d,J=8.2 Hz,2H,ArH),6.60(s,1H,NH),4.46(s,2H,CH2),2.36(s,3H,CH3),2.29(s,3H,CH3);13C NMR δ:166.39,160.30,158.62,137.93,137.06,130.16,129.41,129.32,128.93,127.82,125.48,123.60,122.71,101.85,34.44,20.21; HR-MS m/z:Calcd for C19H19N3S{[M + H]+} 322.137 8,found 322.138 2。
7e:收率77.5%;1H NMR δ:11.32(s,1H,NH),7.49(d,J=7.2 Hz,2H,ArH),7.28(s,5H,ArH),6.97(d,J=8.6 Hz,2H,ArH),6.59(s,1H,CH2),4.44(s,2H,CH2),3.75(s,3H,OCH3),2.35(s,3H,CH3);13C NMR δ:166.96,164.36,159.02,156.26,136.80,134.82,128.99,128.66,127.57,121.53,115.59,100.25,56.04,34.74,24.75; HR-MS m/z:Calcd for C19H19N3OS{[M +H]+}337.132 7,found 337.132 4。
7f:收率87.8%;1H NMR δ:11.40(s,1H, NH),7.90(s,1H,ArH),7.56(d,J=8.3 Hz,1H,ArH),7.43~7.18(m,7H,ArH),6.69(s,1H,CH),4.49(s,2H,CH2),2.38(s,3H,CH3);13C NMR δ:166.68,160.23,159.10,139.69,136.78,133.58,131.00,129.33,128.99,127.93,124.80,121.91,120.73,102.41,34.62,20.33; HR-MS m/z:Calcd for C19H19ClN3S{[M + H]+}342.083 2,found 342.082 8。
7g:收率74.4%;1H NMR δ:7.91(t,J=5.9 Hz,1H,ArH),7.35~7.19(m,9H,ArH),6.11(s,1H,CH),4.54(s,2H,CH2),4.26(s,2H,CH2),2.18(s,3H,CH3);13C NMR δ:167.91,161.56,139.33,129.25,128.81,128.73,127.60,127.26,127.20,99.88,42.89,34.19,23.68; HR-MS m/z:Calcd for C19H19N3S{[M +H]+}322.137 8,found 322.137 9。
7h:收率69.2%;1H NMR δ:7.45(d,J=7.3 Hz,2H,ArH),7.32~7.27(m,2H,ArH),7.23(dd,J=8.5 Hz,1H,ArH),6.06(d,J=6.2 Hz,1H,CH),4.39(s,2H,CH2),2.30(s,3H,CH3),1.85(d,J=13.0 Hz,1H,CH),1.60(ddd,J=15.2 Hz,11.3 Hz,5.7 Hz,2H,CH2),0.93(dd,J=6.7 Hz,3.6 Hz,6H,CH3),0.82(dd,J=24.7 Hz,11.9 Hz,1H,CH);13C NMR δ:169.81,165.40,161.24,138.70,128.82,128.30,126.78,97.20,51.26,42.56,35.07,30.90,24.11,19.15; HR-MS m/z:Calcd for C19H25N3S{[M +H]+}328.184 7,found 328.184 7。
7i:收率63.2%;1H NMR(CDCl3)δ:7.43(d,J=7.3 Hz,2H,ArH),7.32~7.26(m,2H,ArH),7.22(t,J=7.3 Hz,1H,ArH),5.84(s,1H,CH),4.74(s,1H,NH),4.38(d,J=11.2 Hz,2H,CH2),3.07(s,1H,CH),2.29(d,J=9.7 Hz,3H,CH3),2.03~1.93(m,2H,CH2),1.70(ddd,J=42.6 Hz,9.3 Hz,3.7 Hz,3H,CH2),1.44~1.30(m,2H,CH2),1.28~1.13(m,3H,CH3);13C NMR δ:165.92,165.26,157.84,136.80,128.99,128.66,127.57,98.12,52.57,34.74,33.32,25.92,24.75,24.72; HRMS m/z:Calcd for C18H23N3S{[M +H]+}314.169 1,found 314.169 2。
7j:收率67.6%;1H NMR(CDCl3)δ:7.43(d,J=7.3 Hz,2H,ArH),7.29(t,J=7.3 Hz,2H,ArH),7.21(t,J=7.3 Hz,1H,ArH),5.94(s,1H,CH),4.39(s,2H,CH2),3.46(d,J=5.9 Hz,4H,NCH2),2.29(s,3H,CH3),1.15(t,J=7.1 Hz,6H,2CH3);13C NMR δ:168.23,165.79,165.14,148.08,145.04,129.56,122.95,97.45,44.03,34.74,24.75,13.01; HR-MS m/z:Calcd for C16H21N3S{[M + H]+} 288.153 4,found 288.153 0。
7k:收率75.7%;1H NMR δ:11.15(s,1H),8.10(d,J=8.6 Hz,2H),7.52(ddd,J=34.4 Hz,8.9 Hz,5.0 Hz,4H),7.29(dt,J=47.3 Hz,8.8 Hz,2H),6.58(s,1H),4.53(s,2H),2.35(s,3H);13C NMR δ:166.96,164.36,159.02,158.51,148.08,145.04,137.48,129.56,122.95,121.68,116.89,100.25,34.74,24.75; HR-MS m/z:Calcd for C18H15FN4O2S{[M +H]+}371.097 8,found 371.098 0。
7l:收率82.6%;1H NMR δ:11.33(s,1H),8.14~8.08(m,2H),7.58(dd,J=8.7 Hz,3.0 Hz,4H),7.44~7.38(m,2H),6.65(s,1H),4.57(s,2H),2.37(s,3H);13C NMR δ:166.02,160.28,158.91,147.05,145.56,136.93,130.47,129.30,124.29,123.88,102.23,33.70,20.29; HR-MS m/z:Calcd for C18H15ClN4O2S{[M + H]+} 387.068 2,found 387.068 4。
7m:收率82.6%;1H NMR δ:11.22(s,1H),8.11(d,J=8.7 Hz,2H),7.63~7.46(m,6H),6.64(s,1H),4.57(s,2H),2.34(d,J=12.7 Hz,3H);13C NMR δ:166.13,160.25,159.40,147.03,145.60,137.43,132.20,130.47,124.51,123.87,117.24,102.24,33.73,20.44; HR-MS m/z:Calcd for C18H15N4O2BrS{[M +H]+}431.017 7,found 431.017 4。
7n:收率72.7%;1H NMR δ:11.16(s,1H),8.09(d,J=8.6 Hz,2H),7.55(s,2H),7.40(d,J=8.0 Hz,2H),7.20(d,J=8.1 Hz,2H),6.59(s,1H),4.55(s,2H),2.35(s,3H),2.29(s,3H);13C NMR δ:166.96,164.36,159.02,148.08,145.04,138.82,132.51,129.56,129.08,122.95,119.60,100.25,34.74,24.75,21.13; HR-MS m/z:Calcd for C19H18N4O2S{[M + H]+} 367.122 9,found 367.122 6。
7o:收率65.9%;1H NMR δ:11.21(s, 1H),8.09(d,J=8.7 Hz,2H),7.54(d,J=7.8 Hz,2H),7.43~7.24(m,3H),7.03(d,J=7.3 Hz,1H),6.63(s,1H),4.57(s,2H),2.36(s,3H),2.25(s,3H);13C NMR δ:165.60,160.35,158.34,147.06,145.67,138.86,137.61,130.57,129.32,126.51,123.87,123.58,120.26,101.76,33.49,21.37,19.96; HR-MS m/z:Calcd for C19H18N4O2S{[M + H]+} 367.122 9,found 367.122 4。
7p:收率47.7%;1H NMR δ:10.67(s,1H),10.22(s,1H),7.46~7.07(m,8H),6.86(t,J=7.5 Hz,1H),6.50(s,1H),4.34(s,2H),2.34(s,3H);13C NMR δ:167.16,164.26,158.10,148.08,147.96,145.04,131.33,129.56,123.93,122.95,122.00,121.94,115.03,101.00,34.74,24.75; HR-MS m/z:Calcd for C18H16N4O3S{[M + H]+}369.102 1,found 369.101 8。
1.3细胞毒性测定
按文献[19]方法测定7a~7p对4种癌细胞系[MGC-803(人胃癌细胞); EC-9706(人食管癌细胞); PC-3(人前列腺癌细胞); EC-109(人食管癌细胞)]的体外细胞毒性。
2 结果与讨论
2.1合成
合成2时,必须待硫脲在氢氧化钾溶液中完全溶解后,才可加入乙酰乙酸乙酯。
2.2细胞毒活性
采用MTT[19]分析法,选用抗癌药物5-FU作为阳性对照药物,评价了7a~7p对4种人癌细胞的细胞毒性。其IC50值见表1。由表1可见,7a~7p对四种人癌细胞显示出较好的的细胞毒性。与5-FU相比,7b(IC503.126 μg·mL-1)和7c(IC502.197 μg·mL-1)对MGC-803的细胞毒性更佳。
由表1还可看出,相对7k~7p来讲,7a~7j的细胞毒性更好。通过结构-活性关系分析可看出:在硫脲嘧啶结构中,R1为H时对提高细胞毒性有促进作用。此外,通过在嘧啶的4-位引入芳香胺可以增强活性,而含对位取代基的芳香胺更有效,其中又以含有Br的取代基效果更加明显。

表1 7a~7p对4种人癌细胞的细胞毒性*Table 1 The cytotoxicities of 7a~7pagainst four human cancer cell lines
3 结论
设计并合成了16个结构新颖的2,4-二取代基-6-甲基硫脲嘧啶衍生物。抗肿瘤活性结果表明:大多数化合物对MGC-803表现出良好的细胞毒性,其中7b和7c的活性最好,IC50分别为3.126 μg·mL-1和2.197 μg·mL-1,优于对照药5-FU。
结构-活性关系分析表明:硫脲嘧啶结构中R1为H时对提高细胞毒性有促进作用,R2为对位含有Br的取代基的芳香胺可以增强活性。这对抗癌药物的开发具有重要意义。
参考文献
[1]Bazgir A,Khanaposhtani M M,Soorki A A.Towards novel S-DABOC inhibitors:synthesis,biological investigation,and molecular modeling studies[J].Bioorg Med Chem Lett,2008,18:5800-5803.
[2]Raj T,Singh N,Ishar M P S.Unusual transformation of substituted-3-formylchromones to pyrimidine analogues:synthesis and antimicrobial activities of 5-(ohydroxyaroyl)pyrimidines[J].Bioorg Med Chem Lett,2013,23:6093-6096.
[3]Mai A,Rotili D,Massa S,et al.Discovery of uracilbased histone deacetylase inhibitors able to reduce acquired antifungal resistance and trailing growth in Candida albicans[J].Bioorg Med Chem Lett,2007,17:1221-1225.
[4]Wagner E,Becan L,Nowakowska E.Synthesis and pharmacological assessment of derivatives of isoxazolo [4,5-d]pyrimidine[J].Bioorg Med Chem,2004,12:265-272.
[5]Chai B S,Wang S Y,Yu W Q,et al.Synthesis of novel strobilurin-pyrimidine derivatives and their antiproliferative activity against human cancer cell lines[J].Bioorg Med Chem Lett,2013,23:3505-3510.
[6]Kamal A,Dastagiri D,Ramaiah M J,et al.Synthesis and apoptosis inducing ability of new anilino substituted pyrimidine sulfonamides as potential anticancer agents[J].M Eur J Med Chem,2011,46:5817-5824.
[7]Abbas S E,Gawad N M A,George R F,et al.Synthesis,antitumor and antibacterial activities of some novel tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidine derivatives[J].Eur J Med Chem,2013,65:195-204.
[8]Wang L,Desmoulin S K,Cherian C,et al.Synthesis,benzodiazepine receptor binding and molecular modelling of isochromeno[4,3-c]pyrazol-5(1H)-one derivatives[J].Eur J Med Chem,2012,54:715-720.
[9]Kamal A,Tamboli J R,Nayak V L,et al.Synthesis of pyrazolo[1,5-a]pyrimidine linked aminobenzothiazole conjugates as potential anticancer agents[J].Bioorg Med Chem Lett,2013,23:3208-3215.
[10]Kassab A E,Gedawy E M.Synthesis and anticancer activity of novel 2-pyridylhexahyrocyclooctathieno[2,3-d]pyrimidine derivatives[J].Eur J Med Chem,2013,63:224-230.
[11]钟光祥,陈婷婷,周程程.一锅法合成2-硫脲嘧啶[J].合成化学,2010,18(1):118~120.
[12]Jain K S,Chitre T S,Miniyar P B,et al.Biological and medicinal significance of pyrimidines[J].J Curr Sci,2006,90:793-803.
[13]Fathalla O A,Awad S M,Mohamed M S.Synthesis of new 2-thiouracil-5-sulphonamide derivatives with antibacterial and antifungal activity[J].Arch Pharm Res,2005,28,1205-1212.
[14]Kamalakannan P,Venkappayya D.Synthesis and characterization of cobalt and nickel chelates of 5-dimethylaminomethyl-2-thiouracil and their evaluation as antimicrobial and anticancer agents[J].J Inorg Biochem,2002,90:22-37.
[15]Taher A T,Abou-Seri S M.Synthesis and bioactivity evaluation of new 6-aryl-5-cyano thiouracils as potential antimicrobial and anticancer agents[J].Molecules,2012,17:9868-9886.
[16]Fathalla,A,Zaghary W A,Radwan H H,et al.Synthesis of new 2-thiouracil-5-sulfonamide derivatives with biological activity[J].Arch Pharm Res,2002,25:258-269.
[17]Prachayasittikul S,Worachartcheewan A,Nantasenamat C,et al.Synthesis and structure activity relationship of 2-thiopyrimidine-4-one analogsas antimicrobial and anticancer agents[J].Eur J Med Chem,2011,46:738-742.
[18]Chen P; Yang A,Gu Y,et al.Synthesis,in vitro antimicrobial and cytotoxic activities of novel pyrimidine-benzimidazol combinations[J].Bioorg Med Chem Lett,2014,24:2741-2743.
[19]Zheng Y,Duan Y,Ma J,et al.Triazole-dithiocarbamate based selective Lysine specific demethylase 1(LSD1)inactivators Inhibit gastric cancer cell growth,invasion,and migration[J].J Med Chem,2013,56:8543-8560.
·研究论文·
·研究论文·
Synthesis and Cytotoxicity of Novel
2-Substituted Benzyl Sulfo-pyrimidine Derivatives
GU Yi-fei,WANG Chao-jie,CAO Qin-po,ZHANG Xiao-song,CHEN Peng-ju,JIANG Teng-fei,SHAO Kun-peng,LI Bo,KE Yu,ZHANG Qiu-rong,LIU Hong-min
(School of Pharmaceutical Sciences,Collaborative Innovation Center of
New Drug Research and Saftry Evaluation,Zhengzhou University,Zhengzhou 450001,China)
Abstract:Sixteen novel 2-substituted benzyl sulfo-pyrimidine derivatives(7a~7p)were synthesized by a four-step reactions of cyclization,substitution,chlorination and nucleophilic substitution,using ethyl acetoacetate and thiourea as raw materials.The structures were characterized by1H NMR,13C NMR and HR-MS.Cytotoxicity in vitro tests indicated that 6-methyl-4-parachloroaniline-2-benzyl sulfo-pyrimidine(7b)and 6-methyl-4-parabromoaniline-2-benzyl sulfo-pyrimidine(7c)exhibited the best activities against MGC-803(human gastric cancer cell line)with 3.126 μg·mL-1and 2.197 μg·mL-1of IC50,respectively,which were better than 5-FU(IC503.208 μg·mL-1)
Keywords:pyrimidine; thiouracil derivative; synthesis; cytotoxicity
作者简介:顾一飞(1989-),男,汉族,江苏南通人,硕士研究生,主要从事有机合成的研究。
基金项目:国家自然科学基金资助项目(81172937)
*收稿日期:2014-12-11;
修订日期:2015-05-08
DOI:10.15952/j.cnki.cjsc.1005-1511.2015.06.0461
文献标识码:A
中图分类号:O626.41; O625.52
通信联系人:可钰,硕士生导师,E-mail:ky@ zzu.edu;张秋荣,硕士生导师,E-mail:zqr406@ sina.com;刘宏民,博士生导师,
