阿折地平对西罗莫司洗脱支架致血管内皮损伤的干预作用及机制探讨
2016-01-12袁玲,聂卫,高萍等
阿折地平对西罗莫司洗脱支架致血管内皮损伤的干预作用及机制探讨
袁玲,聂卫,高萍,李昕,刘伟伟,崔晓雪
(天津市医药科学研究所,天津300020)
摘要:目的观察阿折地平对西罗莫司洗脱支架致血管内皮损伤的干预作用,并探讨其作用机制。方法培养人脐静脉内皮细胞(HUVEC),将HUVEC分为对照组、模型组和实验组。对照组不加药物,模型组给予西罗莫司500 nmol/L,实验组给予阿折地平10 μmol/L+西罗莫司500 nmol/L。各组处理24 h后,HE染色观察细胞形态变化,测定培养液中的NO,Fluo-3/AM 荧光探针标记细胞内Ca2+,JC-1荧光探针检测线粒体膜电位,Annexin V FITC/PI染色法测算细胞凋亡率。结果各组处理24 h后,对照组细胞呈多角形、单层铺路石样排列;模型组细胞肿胀,胞质出现空泡,部分细胞核固缩、碎裂;实验组细胞基本呈多角形、单层铺路石样排列,少见细胞肿胀及胞质空泡。与对照组相比,模型组与实验组细胞培养液中NO水平降低、胞质Ca2+浓度升高、细胞凋亡率升高、线粒体膜电位下降(P均<0.05);与模型组相比,实验组细胞培养液中NO水平升高、胞质Ca2+浓度及细胞凋亡率降低、线粒体膜电位升高(P均<0.05)。结论 阿折地平可在一定程度上抑制西罗莫司洗脱支架导致的血管内皮损伤,其机制可能与提高细胞内NO水平、降低细胞内Ca2+浓度、抑制线粒体膜电位降低、减少细胞凋亡有关。
关键词:钙通道阻滞剂;阿折地平;药物洗脱支架;人脐静脉内皮细胞;血管内皮损伤
doi:10.3969/j.issn.1002-266X.2015.39.003
中图分类号:R543.3;R965.3 文献标志码:A
基金项目:天津市卫生局科技
作者简介:第一袁玲(1979-),女,硕士,助理研究员,主要从事药物毒理、病理及医疗器械安全性评价方面的研究。E-mail: 1128sun@163.com
收稿日期:(2015-07-10)
Protective effect of azelnidipine on sirolimus eluting stent-induced vascular
endothelial injury and its mechanism
YUANLing,NIEWei,GAOPing,LIXin,LIUWei-wei,CUIXiao-xue
(TianjinInstituteofMedicalScience,Tianjin300020,China)
Abstract:ObjectiveTo investigate the protective effect of calcium-channel blockers azelnidipine on sirolimus eluting stent-induced vascular endothelial injury and its mechanism. MethodsHuman umbilical vein endothelial cells (HUVECs) were cultivated and divided into the control group (treated with culture medium), model group (treated with sirolimus 500 nmol/L) and the experimental group (azelnidipine 10 μmol/L + sirolimus 500 nmol/L), respectively. After 24 h of treatment, changes in cell morphology were observed by HE staining. Effects on the production of nitric oxide (NO) were detected by Nitric Oxide Assay Kit. The intracellular calcium ion (Ca2+) concentration was assayed with Fluo-3/AM staining, the changes of mitochondrial membrane potential was detected by JC-1 fluorescence labeling, and the apoptosis rate of HUVECs was analyzed by Annexin V FITC/PI staining. ResultsAfter 24 h of treatment, in the control group, cells were polygonal and in the single cobblestone arrangement; in the model group, cell swelled, cytoplasm had vacuoles, part of the nucleus had pycnosis, and nuclear fragmentation were observed; in the experimental group, cells were substantially polygonal, and in the single cobblestone arrangement, the cell swelling and cytoplasmic vacuoles were rare. Compared with the control group, the levels of NO in the cell culture fluid were reduced, the levels of intracellular free Ca2+ were increased, apoptosis rate was increased and mitochondrial membrane potential was reduced in the model group and experimental group (all P<0.05). Compared with the model group, the levels of NO in the cell culture fluid were increased, the levels of intracellular free Ca2+ were reduced, the apoptosis rate was reduced and the mitochondrial membrane potential was increased in the experimental group (all P<0.05). ConclusionsAzelnidipine has protective effect on sirolimus eluting stent-induced vascular endothelial injury. The possible mechanism might be related to the decrease of intracellular Ca2+ which could alleviate calcium overload and mitochondrial membrane potential and finally reduce apoptosis.
Key words: calcium-channel blockers; azelnidipine; drug eluting stent; human umbilical vein endothelial cells; vascular endothelial injury
西罗莫司洗脱支架(SES)能明显减少冠状动脉介入术后血管再狭窄的发生率,但近年研究发现,SES与支架内血栓形成有关[1~3]。钙通道阻滞剂是心血管疾病常用的药物。Kubota等[4]发现钙通道阻滞剂阿折地平能在一定程度上恢复猪冠脉支架植入模型的血管内皮功能并上调内皮型一氧化氮合酶(eNOS)表达。但有关阿折地平对SES致血管内皮损伤的干预作用和机制目前研究较少。为此,我们于2013年6月~2015年5月进行了如下研究。
1材料与方法
1.1西罗莫司及阿折地平作用浓度筛选将HUVEC培养于完全培养液,置于37 ℃、5% CO2及饱和湿度的细胞培养箱中培养,细胞贴壁并生长至融合80%以上时,用0.25%胰酶-EDTA消化传代。取对数生长期细胞用0.25%胰酶-EDTA消化,制成单细胞悬液,按5×103/孔接种于96孔板,分为正常对照组(磷酸盐缓冲液刺激)、西罗莫司组(分别给予西罗莫司62.5、125、250、500 nmol/L处理24 h)、阿折地平组(分别给予阿折地平1、10、20 μmol/L+西罗莫司刺激24 h)。处理后,每孔加入MTT试剂10 μL,继续培养4 h,吸去上清液,加入150 μL DMSO,振荡10 min,酶标仪测定570 nm处的吸光度值(A值)。以仅加入150 μL DMSO的孔作为空白对照。按公式计算细胞存活率:细胞存活率(%)=(A加药-A空白)/(A正常对照-A空白)×100%。每组设置6个复孔,实验重复3次。选择500 nmol/L西罗莫司与10 μmol/L阿折地平进行后续实验。
1.2细胞分组与阿折地平干预方法将HUVEC按5×104/孔接种于6孔板,分为对照组、模型组和实验组。对照组不加药物,模型组给予西罗莫司500 nmol/L,实验组给予阿折地平10 μmol/L+西罗莫司500 nmol/L,各组处理24 h。
1.3观察方法
1.3.1HUVEC形态观察吸弃上清液,PBS洗涤细胞2次,乙醇固定10 min,HE染色,倒置显微镜观察细胞形态并采集图像。
1.3.2HUVEC培养液中NO检测收集各组细胞培养液,按NO检测试剂盒说明操作。
1.3.3HUVEC胞质Ca2+浓度检测消化细胞制成单细胞悬液,于细胞悬液中加入Fluo-3/AM及Pluronic127,37 ℃孵育30 min,以含钙Hank′s平衡盐溶液洗涤3次后,在FACS Calibur流式细胞仪上检测异硫氰酸荧光素绿色荧光,重复测量3次,采用FCS Express4.0软件分析荧光强度,以各组与正常对照的荧光比值代表Ca2+浓度。
1.3.4HUVEC凋亡检测胰酶消化细胞,离心,去上清液,缓冲液重悬,于100 μL细胞悬液中加入5 μL annexin V及5 μL PI室温孵育15 min,加入400 μL 缓冲液,采用流式细胞仪检测细胞凋亡情况。
1.3.5HUVEC线粒体膜电位检测0.25%胰酶-EDTA消化细胞,离心,PBS洗涤,加入0.5 mL JC-1染色工作液。与细胞培养箱中37 ℃孵育20 min。用JC-1染色缓冲液洗涤2次,重悬,采用流式细胞仪检测线粒体膜电位,检测结果以FL2与FL1通道红绿荧光强度比值表示。
1.4统计学方法采用SPSS17.0统计软件。计量资料以±s表示,两组比较用t检验,多组比较用单因素方差分析。P<0.05为差异有统计学意义。
2结果
2.1各组细胞形态比较对照组细胞呈多角形、单层铺路石样排列;模型组细胞肿胀,胞质出现空泡,部分细胞核固缩、核碎裂;实验组细胞基本呈多角形、单层铺路石样排列,少见细胞肿胀及胞质空泡。
2.2细胞培养液中NO水平、胞质Ca2+浓度、细胞凋亡率、线粒体膜电位比较与对照组相比,模型组与实验组细胞培养液中NO水平降低、胞质Ca2+浓度升高、细胞凋亡率升高、线粒体膜电位下降(P均<0.05);与模型组相比,实验组细胞培养液中NO水平升高、胞质Ca2+浓度及细胞凋亡率降低、线粒体膜电位升高(P均<0.05)。见表1。
表1 各组HUVEC培养液中NO水平、胞质Ca 2+浓度、
细胞凋亡率、线粒体膜电位比较( ± s)
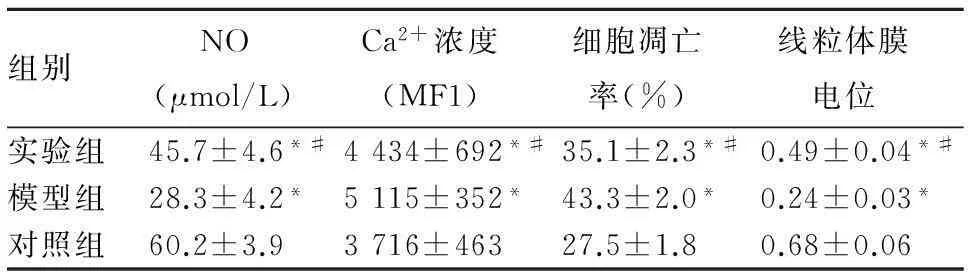
表1 各组HUVEC培养液中NO水平、胞质Ca 2+浓度、
组别NO (μmol/L) Ca2+浓度 (MF1) 细胞凋亡 率(%) 线粒体膜 电位 实验组45.7±4.6*#4434±692*#35.1±2.3*#0.49±0.04*#模型组28.3±4.2*5115±352*43.3±2.0*0.24±0.03*对照组60.2±3.93716±463 27.5±1.80.68±0.06
注:与对照组相比,*P<0.05;与模型组相比,#P<0.05。
3讨论
SES将具有抗再狭窄的药物如西罗莫司等用特殊工艺包被于支架表面的药物载体上,SES置入血管后,药物在病变局部缓慢释放,集中作用于病变部位,发挥抗再狭窄的作用。目前对于西罗莫司是否损害血管内皮仍有争议,其发生机制也尚未明确。有文献[5,6]报道,西罗莫司可阻止细胞周期G1期向S期转化,抑制血管平滑肌细胞增殖、迁移,防止支架置入后再狭窄,但其同时也抑制了内皮细胞增殖,造成支架置入部位内皮化延迟,导致血小板激活和血栓形成,影响内皮细胞功能。也有学者[7]发现,移植血管中西罗莫司给药组一氧化氮合成酶表达增高,局部高浓度NO可能是防止动脉硬化的机制。
我们以不同浓度西罗莫司作用于HUVEC,发现西罗莫司可影响内皮细胞增殖,降低内皮细胞存活率,同时细胞培养基上清液NO水平也减少,提示内皮细胞功能受到了影响,与文献[8,9]结果一致。本实验采用Fluo-3/AM荧光探针特异性标记细胞内Ca2+,观察西罗莫司作用后细胞内游离Ca2+变化,发现西罗莫司可增加内皮细胞内Ca2+水平,同时降低线粒体膜电位,增加细胞凋亡,提示西罗莫司造成的内皮细胞损伤与这些因素有关。
钙通道阻滞剂是心血管疾病常用药物。Ma等[10]报道, 钙通道阻滞剂阿折地平可提高HUVEC基础NO水平并减少MCP-1表达,后者与猕猴支架模型(高胆固醇喂养建模)血管内膜增生不良有关。本研究结果显示,10 μmol/L阿折地平处理HUVEC后细胞存活率升高,细胞凋亡率减少,细胞形态得到改善,表明阿折地平对西罗莫司诱导的HUVEC损伤具有保护作用。我们同时发现,实验组细胞NO水平升高,提示阿折地平不仅能增加内皮细胞存活率,还可改善内皮细胞功能。另外,实验组细胞内Ca2+浓度降低,线粒体膜电位升高,提示胞外Ca2+内流参与了西罗莫司诱导的内皮细胞损伤,而阿折地平可能通过减少细胞外Ca2+内流、减轻钙超载、抑制线粒体膜电位降低、减少细胞凋亡等途径发挥保护内皮细胞的作用,这与相关研究[11,12]结果一致。
综上所述,SES能引起血管内皮细胞损伤,而阿折地平对其诱导的细胞损伤有一定抑制作用,可提高HUVEC存活率,维持正常细胞形态,增加细胞中NO水平,改善细胞功能。
参考文献:
[1] Meier P, Zbinden R, Togni M, et al. Coronary collateral function long after drug-eluting stent implantation[J]. J Am Coll Cardiol, 2007,49(1):15-20.
[2] Lüscher TF, Steffel J, Eberli FR, et al. Drug-eluting stent and coronary thrombosis: biological mechanisms and clinical implications[J]. Circulation, 2007,115(8):1051-1058.
[3] Ong ATL, McFadden EP, Regar E, et al. Late angiographic stent thrombosis (LAST) events with drug-eluting stents[J]. J Am Coll Cardiol, 2005,45(12):2088-2092.
[4] Kubota N, Miyauchi K, Kasai T, et al. Synergistic effects of calcium-channel and angiotensin-receptor blockers on endothelial function and inflammatory responses in a porcine drug-eluting stent model[J]. Circ J, 2010,74(8):1704-1710.
[5] Minamino T, Kitakaze M, Papst PJ, et al. Inhibition of nitric oxide synthesis induces coronary vascular remodeling and cardiac hypertrophy associated with the activation of P70 S6 kinase in rats[J]. Cardiovasc Drugs Ther, 2000,14(5):533-542.
[6] Jeanmart H, Malo O, Carrier M, et al. Comparative study of cyclosporine and tacrolimus vs newer irnrnunosuppressants mycophenolate mofetil and rapamycin on coronary endothelial function[J]. J Heart Lung Transplant, 2002,21(9):990-998.
[7] Pham SM, Shears LL, Kawaharada N, et al. High local production of nitric oxide as a possible mechanism by which rapamycin prevents transplant arteriosclerosis[J]. Transplant Proc,1998,30(4):953-954.
[8] Liu HT, Li F, Wang WY, et al. Rapamycin inhibits re-endothelialization after percutaneous coronary intervention by impeding the proliferation and migration of endothelial cells and inducing apoptosis of endothelial progenitor cells[J]. Tex Heart Inst J, 2010,37(2):194-201.
[9] Jin C1, Zhao Y, Yu L, et al. MicroRNA-21 mediates the rapamycin-induced suppression of endothelial proliferation and migration[J]. FEBS Lett, 2013,587(4):378-385.
[10] Ma J, Kishida S, Wang GQ, et al. Comparative effects of azelnidipine and other Ca2+-channel blockers on the induction of inducible nitric oxide synthase in vascular smooth muscle cells[J]. J Cardiovasc Pharmacol, 2006,47(2):314-321.
[11] Kimura Y, Hirooka Y, Sagara Y, et al. Long-acting calcium channel blocker, azelnidipine, increases endothelial nitric oxide synthase in the brain and inhibits sympathetic nerve activity[J]. Clin Exp Hypertens, 2007,29(1):13-21.
[12] Hosoya M, Ohashi J, Sawada A, et al. Combination therapy with olmesartan and azelnidipine improves EDHF-mediated responses in diabetic apolipoprotein E-deficient mice[J]. Circ J, 2010,74(4):798-806.
山东医药第55卷第35期刊发的“血清NGAL、尿KIM-1对拟行CRRT的急性肾损伤患者肾功能转归及预后的预测作用”一文,基金项目为“十二五”国家科技支撑计划(2011BAI10B08)、“十二五”全军重大项目(AWS11J013)。
