一种新型的烟片复烤机加湿方法
2016-01-08王兆铁
一种新型的烟片复烤机加湿方法*

王兆铁
(湘西鹤盛原烟发展有限责任公司,湖南 吉首 416000)
摘要:传统KG型烟片复烤机加湿效果差,加湿喷嘴容易堵塞与损坏,回潮效率低,回潮区温度超标,严重浪费蒸汽与水.采用新型气水二流体雾化喷嘴,在喷孔外端加装调节帽,实现二次雾化.回潮效果明显提高(达到4%以上),回潮区温度可控制在70℃左右,节约蒸汽30%以上.
关键词:叶片复烤;喷雾加湿;新型加湿
文章编号:1007-2985(2015)06-0049-04
中图分类号:TS43文献标志码:A
DOI:10.3969/j.cnki.jdxb.2015.06.012
收稿日期:*2015-05-20
作者简介:王兆铁(1973—),男,湖南吉首人,湘西鹤盛原烟发展有限责任公司工程师,主要从事机械设备设计及制造研究.
DOI:10.3969/j.cnki.jdxb.2015.06.013

KG型烟片复烤机是打叶复烤生产的主要设备,其功能是将烟叶农产品变为工业原料.烟叶经过复烤机的干燥、冷却和回潮处理,可以调整烟叶水分(一般调到11%~13%),去除烟叶中的杂质和杂气,杀灭烟叶中的害虫和病菌,使烟叶更适合于贮存、醇化和人工发酵,确保烟叶质量规范一致.干燥、冷却均为回潮作准备,回潮性能的高低处决于复烤机的工艺性能与能源消耗水平.目前使用的加湿回潮喷嘴雾化效果差、易堵塞、可靠性差,导致回潮蒸汽浪费多、回潮率低.因此,解决复烤机的加湿问题成为目前研究的热点.
1KG型烟片复烤机加湿存在的问题
1.1 烟片复烤机的吸湿机理
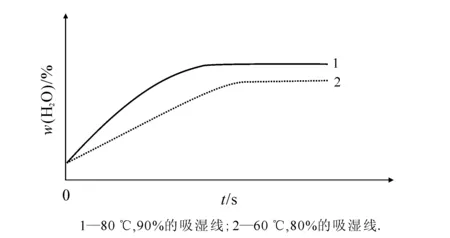
图1 烟叶等温等湿吸湿效果
烟草是一种毛细多孔固体物料,在烘烤干燥过程中,烟草毛细管脱水,空气进入其中.只有当空气中的蒸汽分压力大于烟草表面的蒸汽压力时,烟草才能吸湿.环境温度越高、湿度越大,则空气中的蒸汽分压力越大,烟草吸湿越快.[1-2]在增温增湿初始阶段,提高环境温度或湿度均能增加烟叶的吸湿速率.随着处理时间的延长,环境温度越高,烟叶吸湿变化趋近于0的时间越短(图1).从图1可知,提高烟叶的吸湿能力,其主要途径是提高环境的温度和湿度,但是温度达到一定值时,其吸湿效果并不明显.
1.2 传统加湿方法存在的问题
烟片经复烤机的干燥区干燥后,从冷却区进入回潮区时温度和水分都很低,因此必须将它加热和回潮到打包所需的温度和水分.保证烟片在一定的时间内快速、均匀地吸收水分,就必须有一个较高温度和湿度的环境.KG型烟片复烤机采用的加湿方法为,利用循环风将适当的蒸汽和水雾通过烟片,烟片吸收雾化和蒸汽凝结水,以此连续地加湿加热烟片.传统雾化水的方式为高压喷嘴雾化和汽水混合喷嘴雾化.
高压喷嘴雾化需要的水压高达5~7 MPa,其雾化效果在15 μm以下.该雾化水产生的方式采用变频控制泵的转速,从而控制水分的多少,但存在频率降低、雾化效果下降、控制精度不高等缺陷.另外,高压泵的机械故障率非常高,喷嘴喷孔极小,易堵塞.
汽水混合喷嘴雾化分2种形式.一种为内混式雾化,即汽水在喷嘴内混合后喷射.此种雾化喷嘴为减少内部结垢堵塞,喷孔尺寸往往较大、射程较远,多数喷雾颗粒直径在50 μm以上.另外,汽水比例与各自压力要求较高.水压过大雾化效果达不到要求,汽压过大则水无法喷出.另一种为外混式雾化,即汽水在喷嘴外混合喷射.此种喷嘴利用高速汽流对水进行摩擦产生雾化,具有运行稳定、无需维护等优点,但大多数雾化颗粒直径在50 μm以上,不能被循环风带走,加湿能力不足且水浪费严重.
烟片复烤机回潮区的循环风风速小于0.65 m/s,其目的是防止烟叶被吹起形成风洞,影响均匀性,同时避免15 μm以上的水滴被风带到烟片上形成水渍烟.
由上述分析可知,烟片复烤机加湿能力不足的原因是雾化水颗粒过大,无法被风机带走,从而产生加湿量不足现象.
2烟片复烤机加湿方法的改进
针对传统喷嘴雾化效果差、调节不便等问题,笔者提出一种新型气水二流体压力式喷嘴,选用优质不锈钢材质精密加工而成.采用气水内混压力式雾化,并在喷孔外端加装调节帽实现二次雾化.这种喷嘴雾化后,大多数水滴直径在10 μm以下,最大水流量为12 kg/h.多个喷嘴组合使用后,烟片复烤机的加湿能力达到4%以上,回潮区的温度可控制在70 ℃左右,成品烟箱箱芯温度可控制在30 ℃左右,节约蒸汽达30%以上.
2.1 气水二流体压力式喷嘴的原理
压缩空气经喷嘴内腔沿轴向垂直撞击水流,将水初步雾化,然后喷出,喷射流继续向前运动与喷嘴调节帽的顶针碰撞进行二次雾化.喷嘴利用压缩空气产生的射流与水在喷嘴出口处混合,将水破碎成非常微小的水滴,水滴直径为5~10 μm.小水滴进入空气后蒸发,完成加湿过程.喷嘴的径向装有水量调节阀,可根据压缩空气的流量与压力手动调节水量,达到最佳雾化状态(图2).
二流体主要由空气和液体二部分组成.水雾喷头有2个进口(分别接入气体和水),水进入水雾喷头后,通过气压将水打散形成很细的雾.如果要求雾化粒细小,就必须加大空气的压力;如果要增加雾的流量,就加大液体的压力.在水雾喷嘴中,水与空气之间正常压力比为1∶1.2.
2.2 气水二流体压力式喷嘴的应用
以湖南省湘西鹤盛原烟发展有限责任公司KG型烟片复烤机为例,在烟片复烤机回潮区的每个室中安装2组喷组,每组喷嘴控制方式如图3所示.

图2 气水二流体喷嘴示意

1—喷嘴;2—过滤器;3—电控系统;4—气动薄膜阀; 5—气源过滤与减压阀;6—电磁切断阀;7—压力检测装置. 图3 气水二流体喷嘴元件集中控制布置安装
压缩空气经过气源过滤与减压阀、电磁切断阀分别进入每个喷嘴(提供雾化气体),软水经过滤器、气动薄膜阀分别进入每个喷嘴,与压缩空气混合,然后从喷嘴喷出而雾化.软水压力与压缩空气的压力均由压力监测装置实时检测.根据最佳雾化效果所需的气水压差1.0~1.5 kg/cm2,由电气控制系统自动调节气动薄膜阀的开度,从而控制软水的流量,使水压与气压能在任何情况下自然控制,以提供最佳的雾化效果.同时在软水管道上设有自动排污装置,与气动薄膜阀工作时间相反,便于排除管道中的污垢.当水分仪检测出烟片复烤机出口烟叶的水分超出设定值,可由电气控制系统关闭1组或多组喷嘴管路上的电磁切断阀,从而关闭压缩空气.减少回潮区的雾化水量,则可以降低烟叶的水分.当压缩空气压力为0 N时,则自动关闭气动薄膜阀,从而切断软水.
3加湿方法改进效果
3.1 工艺指标的改善
(1)烟箱的包芯温度明显下降.在保持总加湿量不变的情况下,增加雾化水量则必然减少蒸汽量,从而降低回潮区的环境温度,最终降低烟片复烤机出口烟片温度.经过实测回潮区温度,由原来的80 ℃降到70 ℃,烟片复烤机出口烟片温度由原来的55 ℃降到45 ℃,烟箱的包芯温度由40 ℃降到32 ℃.
(2)回潮能力明显增加且水分偏差降低.为了保证烟片复烤机出口烟片的水分质量分数稳定在11%~13%,先将干燥烟片的水分调至临界点(质量分数8%),然后回潮达到要求.若回潮能力未能达到4%以上,则不可能将烟片干燥到质量分数为8%左右.文中用标准偏差σ度量水分的离散程度:
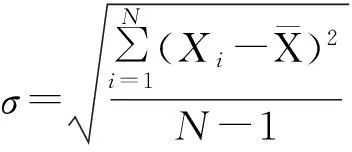
改进前后的实时水分检测结果如图4,5所示.由图4,5可知,改进前打包水分质量分数在11%~12.5%范围内均匀分布.改进后,打包水分质量分数大部分分布在11.5%~12.5%之间.

图4 改进前连续4 d的烟箱水分检测结果分布

图5 改进后连续4 d的烟箱水分检测结果分布
打包水分分析如下:2014年10月10—14日,均值为11.7%,出口水分标准偏差为0.38.10月23—27日,均值为11.95%,出口水分标准偏差为0.3.由此可知,改进前后,标准差下降幅度约为21%,均值提升0.25%.
3.2 蒸汽耗量降低
连续实测改造前10 d的平均小时耗汽为5.64 t,连续实测改进后10 d的平均小时耗汽为3.97 t.每小时节约1.67 t蒸汽.实测数据如图6所示.按照复烤厂每年生产180 d计算,节约蒸汽量1.67 t/h×24 h×180 d=7 214.4 t,能量节约30%.

图6 改造前后蒸汽耗量实测数据
4结语
将多个新型气水二流体压力式喷嘴与电气控制组合后,KG型烟片复烤机的水分调节精度与加湿能力得到明显的提升,降低了打包烟箱的水分离散度与包芯温度.改进后的KG型烟片复烤机回潮效果达4%,回潮温度可控制在70 ℃左右,节能效果明显(节约蒸汽30%以上).
参考文献:
[1]顾中铸,吴薇.烤烟烟叶的等温吸湿和解湿特性.南京师范大学学报:工程技术版,2004,4(4):32-34.
[2]增强,李斌,闫亚明,等.增温增湿过程中烟叶吸湿速率的变化.烟草科技,2006(1):15-17;32.
[3]金仁喜,袁江涛,杨立,等.压力喷嘴常温下雾化特性实验研究.海军工程大学学报,2012,24(3):52-56.
[4]郑捷庆,罗惕乾,张军,等.气力雾化喷嘴最佳耗气率的实验.农业机械学报,2007,38(10):195-197;200.
[5]中国烟叶公司.YC/T146—2010烟叶打叶复烤工艺规范.北京:中国标准出版社,2010.
[6]曹显奎,孙志刚,许建良,等.液体射流在同向气流中的破裂.化学工程报,2007,35(5):29-31.
[7]唐虎,成竹,蒋军亮,等.空气助力雾化喷嘴特性实验研究.装备环境工程,2012,9(2):38-41;45.
NewMethodofTobaccoRedryingMachineHumidification
WANGZhaotie
(XiangxiHeshengTobaccoDevelopmentLLC.Jishou416000,HunanChina)
Abstract:The traditional methods of KG-type tobacco redrying machine humidification are not very effective,nozzle gets easily blocked,and the temperature of moisture regaining parts exceeds standards,resulting steam and water waste.The new steam-water atomizing nozzle with adjusting cap can realize re-atomization,thus increasing the moisture regaining effect by over 4%.With this method,the temperature of moisture regaining parts can be controlled within 70 ℃,and the steam can be saved by over 30%.
Keywords:tobaccoredrying;mistspraying;balancedmoisture;energysaving;atomizingnozzlewithsteam-waterfluid;newhumidification
(责任编辑陈炳权)

ArticleID:1007-2985(2015)06-0053-06
CombustionMethodtoSynthesizeLiFePO4and Combustion Mechanism*
Abstract:Many traditional methods used to synthesize LiFePO4 (LFP for short) have the disadvantages of long reaction time or unavoidable oxidation of Fe2+.In this work,the combustion method was developed to facilely synthesize LFP by using ferrous sulfate,diammonium hydrogen phosphate and lithium nitrate as main raw materials.The synthesized LFP was characterized by XRD and TG-DSC.The results showed that the combustion method could facilely synthesize purer LFP at 600 ℃ in a short period without the protection of inert gas.The studies on combustion mechanism indicated that from 120 ℃ to 290 ℃ the organic citric acid in the dry gel was combusted which could lead to a self-propagating combustion reaction and bring in carbon preventing the oxidation of Fe2+ simultaneously.And φ=n(CA)/n(LFP)=2 is the best amount of citric acid to synthesize LFP materials.
Keywords:combustionmethod;synthesize;LiFePO4;battery
CLCnumber:O614.111Documentcode:B
*Received date:2015-04-16 Foundation item:National Natural Science Foundation of China (51374155);National Key Technology R&D Program (2013BAB07B01);Hubei Province Key Technology R&D Program (2014BCB034);National Natural Science Foundation of Hubei Province of China (2014CFB796) Biography:CHEN Juan(1992—),female,was born in Gong’an County,Hubei Province,graduate student for master degree;research area is function materials Corresponding author:HUANG Zhiliang(1964—),male,was born in Wangjiang County,Anhui Province,prorfessor,doctor;research area is inorganic mineral material.

1Introduction
Li-ionbatteryissecondarybatterywithtwodifferentlithiuminsertioncompoundsaspositiveandnegativeelectrodes.AndthestructureofcompoundscouldinsertandemergeLi+reversibly.Li-ionbatteryisoneofthewidelystudiedgreenenergybatteries.TheresearchoncathodematerialsofLi-ionbatteryhasbecomeahottopicsincecommercialization.AmonglotsofthecandidatesforcathodematerialsofLi-ionbattery,olivine-typeLithiumironphosphateisregardedasthemostpotentialoneforapplicationsowingtoitsuniqueadvantages,includingrelativelyhightheoreticalspecificcapacity(170mA·h/g),highandstablecharge-dischargevoltage(about3.4V),goodcyclingperformance,goodthermalstability,wideoperatingtemperaturerange,cheaprawmaterials,highenvironmentalcompatibility,etc.[2-4]
Many methods have been developed to synthesize LFP,mainly including the high-temperature solid-phase method,the sol-gel method,the microwave method,the hydrothermal method and the co-precipitation method.For the high-temperature solid-phase method,high reaction temperature needs to be provided,and great amounts of nonuniform large size particles are unavoidable which limit the electrochemical performances of synthesized LFP.Padhi A K et al used a two-step solid-phase method to successfully synthesize LFP by using Li2CO3,Fe(CH3COO)2andNH4H2PO4asrawmaterials;however,thesynthesizedLFPhadarelativelylowinitialdischargecapacityof110mA·h/g.Asforthesol-gelmethod,thereareremarkableadvantagesinsmallanduniformparticlesize,lowreactiontemperatureandfacilesynthesisprocess,butalongreactiontimeisneededandtheheavyshrinkingeasilyoccursduringdrying.Bythismethod,ScroceFetalsynthesized a kind of Cu/Ag-incorporated LFP cathode materials which enhanced the initial discharge capacity to 140 mA·h/g at room temperature.The reaction of the microwave synthesis is fast and thermally even,but it’s difficult for large-scale production to a certain extent.Despite the advantage of direct synthesis of LFP,easily controlled crystal form and grain size of sample,the hydrothermal method spends much on production facilities which should be heat-resisting and high pressure-resistant.Prosini P P et al used this method to synthesize LFP with a average size of 3 μm and a discharge capacity of 100 mA·h/g at 120 ℃.In the co-precipitation method,Fe2+is oxidized to FePO4solublesaltprecipitationandthenFePO4isreducedtoLFP.Inref. [10],Mg2+is easily incorporated into LFP to improve its conductivity by this method.To sum up,the above-mentioned methods all have the difficulty in fully avoiding the oxidization of Fe2+into Fe3+during reaction despite the protection of inert gases.Therefore,it is urgent to develop a facile,low-cost and fast synthesis method.
Herein,we developed a novel combustion method to synthesize LFP cathode materials,and investigated the combustion mechanism.
2Experiment
2.1ReagentsandInstrumentation
FeSO4·7H2O,(NH4)2HPO4,citric acid and LiNO4were used in this experiment and were of analytical grade.The instruments used were as follows:X-ray powder diffraction (model XD-5A,Japan,Cu target,λ=0.154 056nm,scanningrangeof10°~70°),batterytestsystem(chargeanddischargevoltagerangeof2.5~4.2V,rateof0.2C,1C=170mA·h/g),electrochemicalworkstation(scanningvoltagerangeof2.4~4.2V,thescanningspeedof0.1mV/s).
2.2ExperimentalProcedure
ThesyntheticrouteofLFPisshowninfig. 1.

Fig. 1 Synthetic Route of LFP
FeSO4·7H2O,(NH4)2HPO4and LiNO3(mole ratio 1∶1∶1) were made into saturated solution respectively at room temperature,then mixed,and some turquoise precipitate emerged immediately.Part of the precipitate was washed,filtered,dried and dark green powder was obtained (sample 1).Adding a certain amount of citric acid into the other part of the sediments,the precipitate disappeared.Then the clear liquid was put into a vacuum drying oven (70 ℃) to form dry gel.Finally the dry gel was combusted at 600 ℃ for 6min and some dark green powder was obtained (sample 2).The gases released in the process were discharged into the alkaline solution.In this paper,the amount of citric acid indicated that the φ=n(CA)/n(LFP).Tostudytheeffectthatamountofcitricacidhasonelectrochemicalpropertiesandstructureofthesyntheticmaterials,φ=1,1.5,2,3 (markedas①,②,③,④).
3ResultsandDiscussion
3.1AnalysisofSamples

Fig. 2 XRD Patterns of the Synthetic LFP
Fig. 2showstheXRDpatternsofthesyntheticLFPsamples.Itindicatesthatsample2ismainlycomposedofLFP(JCPDS,No. 401499)whichhasaspacegroupofpnmb[10].Theunitcellparametersarea=0.601 8nm,b=1.034nm,c=0.470 3nm.PeaksofFe3O4and Fe2O3are not found in diffraction pattern of sample 2,indicating that Fe2+wasnotoxidizedtoFe3+incombustionprocess.Therearenoobviouscharacteristicpeaksofcrystallinephaseinsample1,indicatingsample1isamorphous.Itexplainsthatolivine-typeLFPcouldn’tbepreparedwithoutthecomplexationofcitricacid.
XRDpatternsofthesyntheticLFPunderdifferentamountofcitricacidareshowninfig. 3.①ispurephaseLFP,and②stillincludesimpurityphase,③and④arepurephaseLFP,andhaveintensediffractionpeakandenhancedcrystallinity.

Fig. 3 XRD Patterns of the Synthetic LFP Under Different the Amount of Citric Acid

Fig. 4 Cyclic Voltammetry Curves of the Synthetic LFP Under Different the Amount of Citric Acid
Fig. 4isCVcurvesofthesyntheticLFPunderdifferentamountofcitricacid.Thecurveshowsthatallthematerialsemergedapairofredoxpeaksincirculation.Peakcurrentincreasesfirstly,andthendecreaseswiththeincrementoftheamountofcitricacid.①istheminimum,and③themaximum.Peakcurrentsizetosomeextentreflectsthelithiumionsdiffusionrateinthematerial;andthereforein③,Lithiumiondiffusionrateismaximum.Inaddition,thecurveshowsthepotentialdifferenceoftheredoxpeakwiththeamountofcitricacidvaries,thereinto,thepotentialdifferenceofthematerial②istheminimum(approximately0.26V).Thesmallerthepeakpotentialdifference,thesmallerpolarizabilityofthematerial,andthebettertheelectrochemicalreversibility,indicatingthat③hasthecharge-dischargecycleperformance.Experimentalresultsareconsistentwiththis(asshowninfig. 5).Besides,thesizeoftheredoxpeakareareflectsthepolarizedstateofinternalelectrodeandutilizationofactivematerial[11],thehigherthesamplecapacity,thegreaterthepeakarea,indicatingthat③hasthebestmaterialcharge-dischargecapacity.
Fig. 6isthefirstdischargecapacitycurvesofthesyntheticLFPunderdifferenttheamountofcitricacidat0.2C.Itshowsthatwiththeamountofcitricacidincreasing,thedischargecapacityincreases,and③hasmaximumdischargecapacityof121.8mA·h/g.
Insummary,inordertoimprovedynamiccharacteristicsoftheelectrodematerial,andtoenhancethecapacityoftheLFPcathodematerialonthemaximum,undertheexperimentalconditions,φ=2isthebestamountofcitricacidtosynthesizeLFPmaterials.
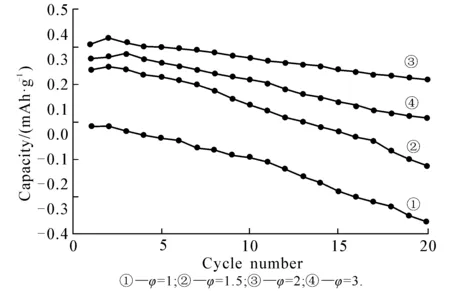
Fig. 5 Cycling Performance Curves of the Synthetic LFP Under Different the Amount of Citric Acid at 0.2 C

Fig. 6 The First Cycling Performance Curves of the Synthetic LFP Under Different Amount of Citric Acid at 0.2 C
3.2PreliminaryStudiesonCombustionMechanism
3.2.1CombustionMethodThereactionofsynthesizeLFPcathodematerialsbycombustionmethodincludesearlysol-gelreactionandredoxreactionduringthepowdersynthesisprocess.Asolcanbeformedbycoordinationcomplexationbetweencomplexingagentandmetalioninsol-gelreaction.Afterthedryingprocess,thedrygelprecursorisobtained.RedoxreactionisperformedinthecombustionprocessofdrygelgeneratedLFPpowder.Sol-gelmodeofsyntheticLFPbycombustionmethodisshowninfig. 7.
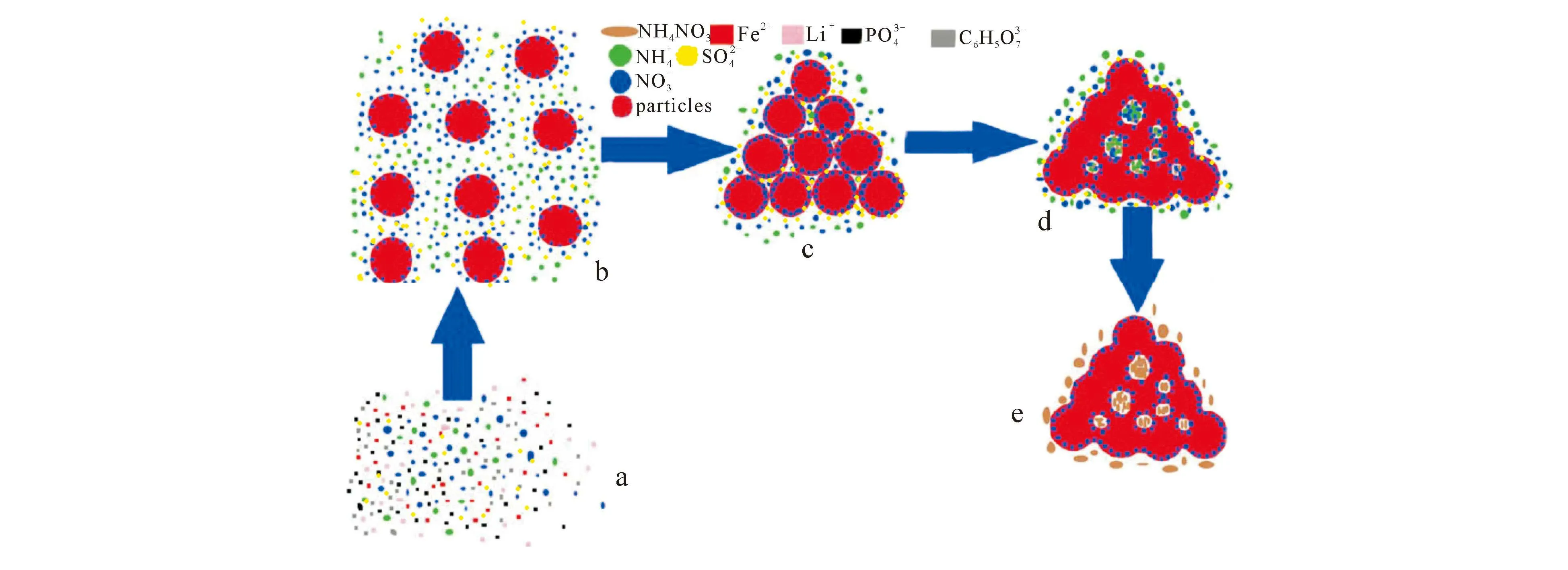
a—solution;b—sol;c—gel;d—aging;e—dry gel. Fig. 7 Sol-Gel Mode of Synthetic LFP by Combustion Method

Fig. 8 TG-DSC Pattern of the Synthetic Sample in Combustion Process
3.2.2ReactionProcessDSC-TGanalysisiscarriedoutonthedrygelinordertostudythecombustionprocessintheconditionofnitrogenatmosphere.Thetestingtemperaturerangeis20~900 ℃andtheheatingrateis20 ℃/min.Thetestresultsarepresentedinfig. 8.
Fromfig.8,wecanseetheweightlossofthedrygelisdividedintothreeparts,accompaningwiththreechangesinthequantityofheat.From20 ℃to120 ℃,thefirstweightlossofdrygelisabout14%ofthetotalmass.Thedeviationmaybecausedbytheoverlapreactionofcombustion.DSCcurveshowsthisprocessisendothermic,resultingfromtheevaporationofthefreewaterfromthedrygel.Afterthen,thedrygelshouldcontainLiNO3,(NH4)2HPO4,FeSO4and citric acid.
From120 ℃to290 ℃,theTGcurveshowsthesecondweightlossisabout54%oftheoverallweightandtheDSCcurvedisplaysanexothermicreactioninthisstep.Theorganiccitricacidabsorbsheattoreachignitiontemperatureatfirstandthencombusts,releasingalargenumberofpost-combustionheat.Afterweightloss,thereshouldbeLiNO3,(NH4)2HPO4and FeSO4.
From290 ℃to800 ℃,theTGcurveshowsthethirdweightlossisabout9%oftheoverallweight.ItmaybeinferredthattheformationofLFPisderivedfromthedecompositionofnitrateandsulfate.TheweightlosscanbetheliberationofNH3,SO2and NO2(the wt.% of released gases is coincident with the third weight loss 9%).The reaction equation is as follow:
2LiNO3+2(NH4)2HPO4+2FeSO4+2C6H8O7+9O2→
2LiFePO4+2NH3↑+2SO2↑+2NO2↑+12CO2↑+14H2O
3.2.3ComplexationMechanismofCitricAcidCitrateisastrongcomplexingagentwhichcanformstablecomplexwithvariousmetalionsespeciallyinacidiccondition.Inthisexperiment,thecomplexationmechanismappliestotheformingprocessofthesol-gel.Metalions(Fe2+,Li+) and citric acid complex by half-and-half mole ratio to form sol[2-14]:
C6H8O7+Fe2+=C6H6O7Fe+2H+,C6H8O7+Li+= C6H7O7Li+H+.
Aftercomplexation,twokindsofcitratecomplexmoleculesareassociatedwithhydrogenbondingtoformgel.Buthydrogenbondingisnotstableanddisconnectedinheatingormoistatmosphere.Theobtaineddrygeliseasytoabsorbwater,whichwillleadtodeliquescence,sothedrygelshouldbeplacedinthefurnaceof600 ℃immediately.Otherwise,itwillhavethehardaggregateafterbeingcalcinedathightemperature.
3.2.4RedoxReactionTheredoxreactionmechanismappliestothecombustionprocessofdrygel.Citricacidrootisakindofstrongreductantion[11-12,15].Withtheincreasingofthecontentofcitricacid(n(citricacid)∶n(Fe2++Li+)≥1),the mixture decomposition changes from multistep decomposition to one-step decomposition with a sharp strong exothermic peak.It is a self-propagating combustion synthesis including two kinds of oxidation reduction reaction in the process:
2C6H8O7+9O2=12CO2↑+8H2O,C6H8O7=3C+3CO+4H2O.
Withtheconsumptionofoxygenincombustionreaction,somecitricacidcan’tcompletelycombust.TheycrackandformacarbonnetscoveringtheparticlewhichpreventstheoxidationofFe2+.Therefore,thefirstincompletecombustionredoxreactionandthesecondcarbonizationreactionistoprovidereducingatmospheretopreventthegeneratedLFPfrombeingoxidized.
4Conclusions
Ferrousnitrate,diammoniumhydrogenphosphateandlithiumnitrateareusedasmainrawmaterialstoprepareLFPbyauto-combustionmethod.TheresultsshowthatLFPissuccessfullypreparedbycombustionmethodwiththeadvantagesofshortreactiontime(6min),highpurity,nooxidationandnoneedtopassintotheinertgas.ThebestamountofcitricacidtosynthesizeLFPmaterialsisφ=n(CA)/n(LFP)=2.Citricacidactsasreductantandcomplexingagentinthewholereaction.AndthecrackingofcitricacidalsobringsincarbonpreventingtheoxidationofFe2+.
References:
XIAnjing.LithiumIronPhosphateElectrochemicalImpedanceSpectroscopyExperimentalStudy.Beijing:TsinghuaUniversity,2012.
HANCong,SHENXiangqian,ZHOUJianxin.PreparationofLiFePO4/Ni Composite Microspheres.J.Chin.Ceram.Soc.,2008,36(4):559-564.
TANGZhiyuan,QIURuiling,TENGGuopeng,etal.ResearchProgressofLiFePO4Cathode Material for Lithium Ion Batteries.Chem.Ind.Eng.Prog.,2008,27(7):995-1 000.
CAOYanbing,HUGuorong,DUKe,etal.SynthesisofLiFePO4Cathode Materials by Polyol Reduction Processing.ChineseJournalInorganicChemistry,2010,26(6):1 061-1 065.
SANGJunli,WANGQiaojuan,GUOXifeng.SynthesisandCharacterizationTechniqueofLiFePO4Cathode Material.InorganicChemicalsIndustry,2008,40(2):13-16.
PADHIAK,GOODENOUGHJB,NANJUNDASWAMYKS.Phospho-OlivinesasPositive-ElectrodeMaterialsforRechargeableLithiumBatteries.JournaloftheElectrochemicalSociety,1997,144(4):1 188-1 194.
SCROCEF,EPIFANIOAD,HASSOUNJ,etal.ANovelConceptfortheSynthesisofAnim-ProvedLiFePO4Lithium Battery Cathode.ElectrochemSolid-StateLetter,2002,5(3):47-50.
WANGXiaojian,RENJunxia,LIYuzhan,etal.SynthesisofCathodeMaterial“Carbon-Included”LiFePO4by Microwave Heating.ChineseJ.Inorg.Chem.,2005,21(2):249-252.
PROSINIPP,CAREWSKAM,SCACCIAS,etal.ANewSynthesisRouteforPreparingLiFePO4with Enhanced Electrochemical Performance.JournaloftheElectrochemicalSociety,2002,149(7):886-890.
[10]ZHANGPeixin,WENYanxuan,LIJianhong,etal.PerformanceandStructureofMg2+DopedLithiumIronPhosphatePreparedbyChemicalPrecipitationMethode.Chin.J.Funct.Mater.,2006,37(12):1 942-1 945.
[11]XIEHui,ZHOUZhentao.SynthesisandElectrochemicalPerformancesofLiFePO4/C Composites by Solid State Reduction Method.JournalofInorganicMaterials,2007,22(4):631-636.
[12]LIWenxia,YINSheng.LowTemperatureCombustionSynthesisofUltrafineCeramicPowder.J.Chin.Ceram.Soc.,1999,27(1):71-78.
[13]LIWenxia,YINSheng.OrganicMaterialsinCombustionSynthesis.MaterialsReview,2000,14(5):45-48.
[14]WANGXinyu,PIANYingchao,LIShipu,etal.ExplorationofMechanismforNanosizedHydroxyapatitePowdersPreparedbyAuto-CombustionMethodandInfluencingFactors.JournaloftheChineseCeramicSociety,2002,30(5):564-568.
[15]YAMADAA,HOSOYAM,CHUNGSAI-CHEONG,eta1.Olivine-TypeCathodes:AchievementsandProblems.J.PowerSources.,2003,119-121:232-238.
