硝酸改性活性炭上氧/氮官能团对脱汞性能的促进作用
2015-12-29徐文青刘瑞辉朱廷钰
佟 莉 徐文青 亓 昊 周 璇 刘瑞辉 朱廷钰,*
(1中国科学院过程工程研究所,湿法冶金清洁生产技术国家工程实验室,北京市过程污染控制技术研究中心,北京100190;2中国科学院大学,北京100049)
硝酸改性活性炭上氧/氮官能团对脱汞性能的促进作用
佟 莉1,2徐文青1亓 昊1,2周 璇1刘瑞辉1朱廷钰1,*
(1中国科学院过程工程研究所,湿法冶金清洁生产技术国家工程实验室,北京市过程污染控制技术研究中心,北京100190;2中国科学院大学,北京100049)
采用硝酸氧化手段对活性炭进行了表面处理,并在固定床反应器上测试了其脱除单质汞的性能.研究表明,在模拟烟气中硝酸改性活性炭能有效脱除单质汞.采用元素分析、Brunauer-Emmett-Teller(BET)比表面积、扫描电子显微镜(SEM)、拉曼(Raman)光谱、Boehm滴定、程序升温脱附(TPD)和X射线光电子能谱(XPS)等手段研究了活性炭表面官能团对其脱汞性能的影响.结果表明:硝酸氧化处理能同时增加活性炭表面含氧官能团和含氮官能团的含量.与改性活性炭的物理性质相比,其化学性质对脱汞性能的影响更大,单质汞主要被改性活性炭氧化为HgO而去除.在脱汞反应中,羰基、酯基和酸酐等含氧官能团可能是活性吸附位点,反应后这些官能团被还原为羟基或者醚基;而吡咯等含氮官能团可能是活性催化位点.此外,基于上述表征结果提出了硝酸改性活性炭表面官能团的脱汞机制.
单质汞;活性炭;硝酸;氧官能团;氮官能团
©Editorial office ofActa Physico-Chimica Sinica
1 Introduction
Mercury,which is very harmful to human beings and ecosystems because of its high volatility,persistence,and bioaccumulation,has attracted increasing attention in recent years.1,2It has been reported that coal combustion is one of the primary anthropogenic mercury emission sources in the world.3Mercury emitted from coal combustion is primarily in the forms of elemental mercury(Hg0),oxidized mercury(Hg2+),and particlebound mercury(HgP).4,5Hg2+and HgPcan be removed using existing pollution control units,such as baghouses/electrostatic precipitator (ESPs)or wet scrubbers.6,7However,Hg0is the most difficult to remove because of its relatively low water solubility(6×10-5g·L-1at 25°C)and high vapor pressure(0.246 Pa at 25°C).4Therefore, it is imperative to do more studies on Hg0removal.
Activated carbon(AC)injection is regarded as one of the most effective techniques for Hg0removal due to its large surface area and various active functional groups located on the edges of the graphene layers.8,9However,the high cost limits the development of AC injection techniques.10Therefore,many researchers have focused on chemical modification methods recently,which could introduce extra active atoms,such as Cl,Br,and I,ontoAC surfaces to enhance Hg0removal efficiencies,in order to reduce the required amount of AC.11-13Although AC modified by halides showed high performance for Hg0removal,the changes in operating conditions may result in the secondary emissions of hydrogen halides,which severely corrodes pipes.Therefore,other chemical modification methods should be considered.
Previous studies have found that some specific oxygen functional groups on AC have high affinities for metals,14whereas nitrogen surface functionalities are able to increase the catalytic activity of the carbon in the oxidation reactions for certain pollutant,such as SO2.15,16Some researchers have investigated the effects of oxygen functional groups onAC on mercury adsorption as well.For example,Li et al.17studied the effect of AC surface moisture on mercury adsorption and concluded that surface oxygen complexes were closely associated with Hg0adsorption. Meanwhile,Skodras et al.18studied the role of AC surface chemistry in mercury adsorption and concluded that lactones were possible active sites that favored Hg0adsorption.However,there are lack of literature about the effect of nitrogen functional groups on Hg0removal.Based on the above studies,we used nitric acid (HNO3)to modify theAC,expecting to obtain the environmentally friendly cost-effective activated carbon by the idea of introducing functional groups.
In the present study,the removal efficiency for Hg0on HNO3-modified AC was investigated.Elemental analysis,BET specific surface area,Raman spectra,Boehm titrations,temperature programmed desorption(TPD),and X-ray photoelectron spectroscopy(XPS)were used to characterize the samples.The possible reaction mechanisms were discussed based on the experimental and characterization results.
2 Materials and methods
2.1 Sample preparation
The activated carbon used in this experiment was purchased from the Chengde Jibei Yanshan activated carbon plant in Hebei province.The nitric acid(AR)was supplied by Beijing Chemical Works.Prior to use,pristine activated carbon(AC-P)was crushed and sieved to 40-80 mesh(0.45-0.20 mm).It was then washed with distilled water to remove dust and dried for 24 h at 120°C. The modified activated carbon was prepared using the incipientwetness impregnation method with 6 mol·L-1HNO3.The mixture was placed in an ultrasonic cleaner operating at 25°C,150 W,and 40 kHz for 1 h.After treatment,the sample was washed with distilled water until the pH value of the washed water was approximately 7.Finally,the HNO3treatment sample was dried in an oven at 120°C for 12 h and cooled down to room temperature in the desiccators for use.The HNO3-modified activated carbons were denoted asAC-N.
2.2 Sample characterization
Elemental analysis of the samples was performed using an elemental analyzer(Vario Macro Cube,Elementar).
The physical morphology was obtained using a scanning electron microscopy(SEM)(JSM 6700F,JEOF)with a field emission gun.
The porous structure characteristics were determined by nitrogen adsorption at-196°C using an automatic porosity analyzer (AutosorbiQ,Quantachrome).The surface area was calculated from the nitrogen adsorption isotherms using the BET equation. The total pore volume was obtained by converting the amount of nitrogen gas adsorbed at a relative pressure(p/p0)of 0.99 to the volume of the liquid adsorbate.The micropore volume was calculated using the Horvath-Kawazoe(HK)method.The Raman spectra were collected with a Raman spectrometer(LabRAM HR800,Horiba Jobin Yvon)using a 514.5 nm Ar+laser beam as the excitation source.
The surface oxygen functional groups of the samples were quantitatively measured using the Boehm method.19Approximately 0.50 g of theAC sample was added to 50 mLbase(NaOH, Na2CO3,and NaHCO3)solution,respectively.The vials were sealed and shaken for 24 h at 25°C and then filtered.Atotal of 10 mL of the filtrate was back titrated with HCl.The oxygen groups were evaluated based on the hypotheses that the NaHCO3only reacted with the carboxylic groups,Na2CO3reacted with the carboxylic and lactonic groups,and NaOH reacted with the carboxylic,lactonic,and phenolic groups.
The chemical states and elemental speciation of the samples were analyzed using X-ray photoelectron spectroscopy(ESCALAB 250,Thermo)with a monochromated Al KαX-ray source(hν= 1486.6 eV).The spectrometer operated in a vacuum better than 10-7Pa and at a power of 150 W.The binding energy was calibrated using the contaminated carbon(284.6 eV).
2.3 Experimental apparatus and removal capacity measurement
A schematic flow chart of Hg0removal is shown in Fig.1.The test rig was composed of an Hg0vapor-generating unit,a simu-lated flue gas feed system,a fixed-bed reactor,and a continuous online mercury analyzer.The Hg0vapor was generated from an Hg0permeation tube(Mercury S56-HE-SR,VICI Metronics), which was placed in a sealed U-shaped glass tube immersed in a water bath maintained at 60°C.Abranch of 200 mL·min-1N2was used as a carrier to introduce the Hg0vapor into the gas mixing chamber.An initial Hg0concentration of(136.0±0.3)μg·m-3was used in each experiment.All pipelines that Hg0passed through were heated to approximately 90°C to prevent mercury deposition to its internal surface.The other feed gases were supplied by gas cylinders when necessary and the balance of N2was adjusted by mass flow controllers with a total flow rate of 600 mL·min-1.
After the gases were fully mixed in the gas mixing chamber, they were pressurized to a fixed-bed quartz reactor.The reactor was 4 mm in inner diameter quartz with a thermocouple outside the wall of the reactor to control the temperature of the furnace. In each test,an approximately 50 mg sample was used to evaluate the adsorption efficiency of Hg0on theAC sample.The Hg0vapor concentrations were continuously monitored using an online mercury analyzer(RA-915M,Lumex)with the data recorded automatically on a computer(one measurement every 5 s).The tail gases were purified by activated carbon before they were discharged into the atmosphere.Before each test,a blank test was performed to examine the amount of Hg0adsorption on the pipeline.The results showed that in all cases,the Hg0adsorption concentrations on the pipeline were below 0.4 μg·m-3.The samples were purged with N2atmosphere for 30 min before each Hg0removal test.The Hg0removal capacity of breakthrough curves was defined by the following equation:

where Coutand Cinare the Hg0concentrations at the inlet and outlet of the fixed-bed quartz reactor,respectively.
The TPD experiment was employed to analyze the Hg product on theAC.30 mg of theAC samples were placed in a quartz reactor.After the samples were kept in Hg0vapor for 2 h at 120°C, it was purged with N2at the flow rate of 600 mL·min-1until the outlet Hg0concentration was constant for at least 30 min.Then, the samples were heated from 120 to 800°C with a ramp rate of 10°C·min-1in N2atmosphere.The effluent gas Hg0concentration was continuously measured using an online mercury analyzer.

Fig.1 Schematic flow chart of Hg0removal
3 Results and discussion
3.1 Mercury removal capacity test
The Hg0adsorption capabilities onAC were evaluated at 120°C. To test the properties of the AC-N sample for Hg0removal and shorten the experiment time,an initial mercury concentration of (136.0±0.3)μg·m-3,which is much higher than that of coal combustion flue gas(0.3-35.0 μg·m-3),20was used.As shown in Fig.2,it can be seen that significant differences on the Hg0removal between AC-P and AC-N samples were observed.The breakthrough curve showed that the HNO3treatment greatly enhanced the Hg0removal in the N2atmosphere.This demonstrated that HNO3treatment could enhance the removal capability for Hg0onAC samples.
There might be the following two reasons responsible for the increase of mercury removal capacity of the AC-N samples:(1) the physical characteristics,such as the BET surface area,the total pore volume,and the micropore volume;18,21(2)the chemical characteristics,such as the active functional groups and elements.11,22
3.2 Effects of physical characteristics on mercury removal
To clarify the nature of the increase in the Hg0adsorption capacity,characterizations of the AC were performed.The physical morphologies of the AC-P and AC-N carbon were characterized using SEM technique,as shown in Fig.3.The basic morphology property of the AC-N sample was similar to that of the AC-P sample.However,some etching marks were formed on the walls of theAC-N sample.
As shown in Table 1,the BET surface area,total pore volume, and micropore volume of theAC-N decreased compared toAC-P, which were probably caused by collapses and blockage by deposition of functional groups14,23during HNO3oxidation.However, combined with the results of the breakthrough curves for Hg0removal shown in Fig.2,the slight difference of physical characteristics was unlikely to result in such significant changes of Hg0removal capacity betweenAC-P andAC-N samples.Therefore,it was inferred that the removal efficiencies were not closely correlated with the physical characteristics of the activated carbons. The changes in physical characteristics of theAC-P carbon agreed well with the results of SEM images.
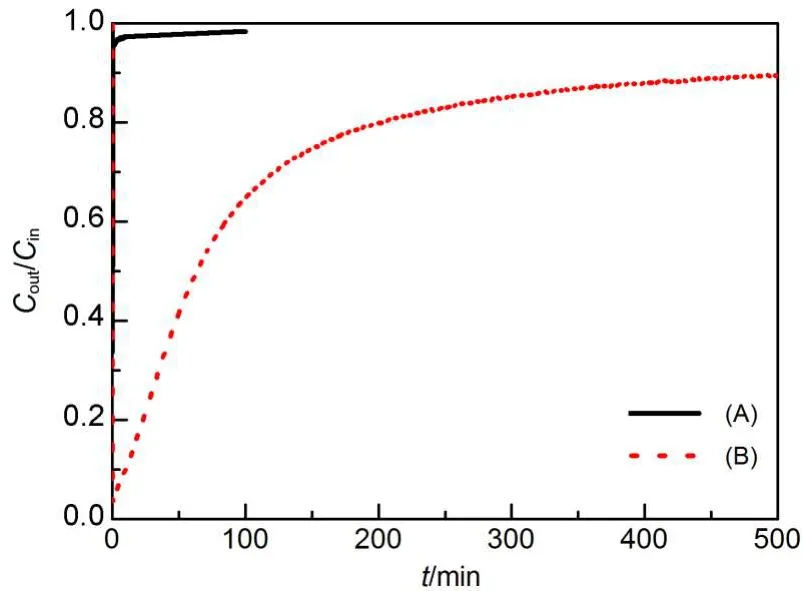
Fig.2 Breakthrough curves of Hg0on(A)AC-Pand(B)AC-N samples in N2atmosphere

Fig.3 SEM images of(a)theAC-Pand(b)AC-N samples
3.3 Effects of chemical functional groups on mercury removal
3.3.1 Raman spectra
As discussed above,the Hg0removal capacity was not exclusively determined by the physical characteristics,so the chemical characteristics must be the main reason for the increase in the Hg0removal capacity.To further clarify the interactions between Hg0andAC samples,Raman spectra were collected to characterize the fine structures of the AC-P and AC-N samples,and their correspondingAC-P-Hg andAC-N-Hg samples after mercury removal test.As shown in Fig.4,two prominent peaks appeared at approximately 1340 cm-1(D band)and 1600 cm-1(G band),respectively.The band intensity ratio(ID/IG)was commonly used as an indicator to characterize the extent of disorder of carbon materials.24FromTable 2,it can be seen that the ID/IGvalue of theACN sample was larger than that of the AC-P sample,which meant that HNO3slightly ruined the structure of graphene layer.The ID/ IGvalue was negatively proportional to the size of graphite crystallite surface layer,25while the size of graphite crystallite surface layer was positively proportional to the catalytic oxidation performance for AC.26Thus,it was inferred that the AC derived from the same matrix with larger ID/IGvalue might facilitate Hg0removal.Furthermore,compared toAC-P andAC-N samples,the ID/IGvalues of AC-P-Hg and AC-N-Hg samples increased,indicating that the adsorbed Hg0slightly changed the order graphite layer structures ofAC-PandAC-N samples.Therefore,the Raman results might predict that a chemical reaction most likely occurred in the process of the Hg0removal.

Table1 Elemental content and physical characteristics of theAC
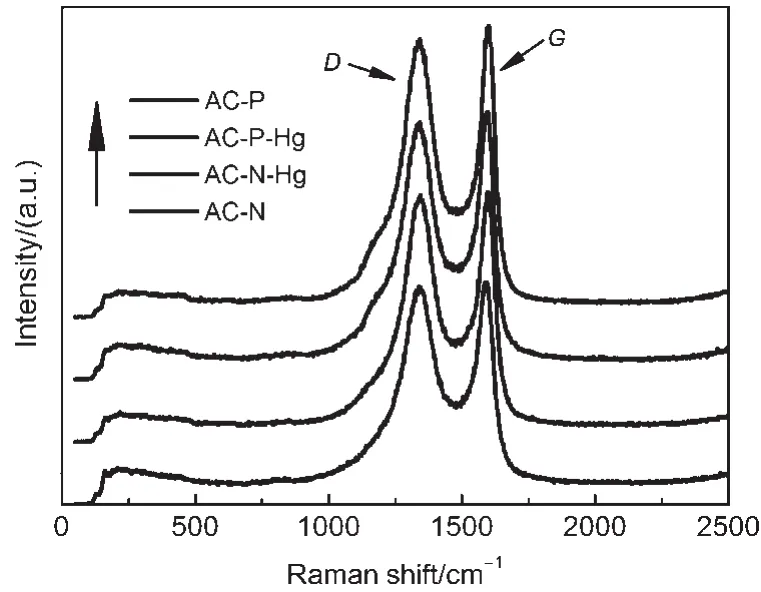
Fig.4 Raman spectra of theAC-P,AC-N,AC-P-Hg, andAC-N-Hg samples
3.3.2 Elemental content of theAC samples
To find out the active elements responsible for Hg0removal on AC,elemental analysis was carried out to determine the amount of oxygen and nitrogen elements introduced onto the activated carbon surface.As shown in Table 1,both AC-P and AC-N samples are primarily composed of N,O,and C.The N and O contents of the AC-P sample were 0.817%and 4.607%,and they increased to 1.076%and 8.002%for AC-N,respectively.The results indicated that HNO3oxidation increased the content of the oxygen and nitrogen elements onAC as expected.
3.3.3 Boehm titrations
To investigate the effect of oxygen surface functional groups on Hg0adsorption,Boehm titrations were used to quantitatively characterize theAC-PandAC-N samples.As observed from Table 2,the phenolic,carboxylic,and lactonic oxygen functional group contents ofAC-P sample were 41,8,and 86 mmol·kg-1,respectively,whereas those onAC-N sample increased to 169,143,and 214 mmol·kg-1.Therefore,it was confirmed that HNO3oxidation much favored the formation of C=O and less favored the C—O oxygen groups on AC-N.The trend for Boehm titrations results agreed with the elemental analysis results.Furthermore,thecomparison analysis between the Hg0breakthrough curve and the quantities of surface oxygen functional groups of the AC-P and AC-N samples might suggest that the amount of the adsorbed Hg0was correlated to certain oxygen functional groups on the carbon surfaces,especially the C=O oxygen-containing groups whose content increased the largest.
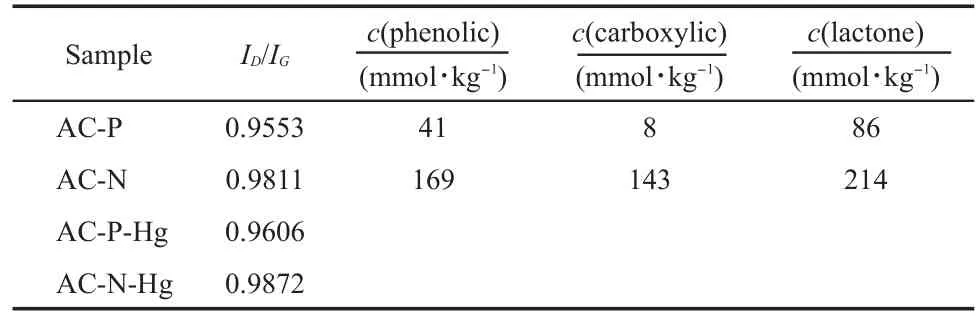
Table2 ID/IGvalues of theAC-P,AC-N,AC-P-Hg,andAC-N-Hg samples and Boehm titrations results of the oxygen functional group contents onAC-PandAC-N samples
3.4 Identification of the mercury removal mechanism
3.4.1 Hg production analysis
The Hg0product adsorbed on the AC is very important for establishing the Hg0removal pathways on HNO3-modifiedAC.To understand the mechanism of the Hg0removal,the Hg 4f spectra were collected.Fig.5 shows the Hg 4f XPS spectra of theAC-NHg sample,which was obtained by separating from the interference signals of Si 2p spectra.The two peaks at 100.7 and 104.9 eV were confirmed as the Hg 4f7/2and Hg 4f5/2doublet for Hg2+due to its higher binding energy than the reference point for the Hg0at 99.7 eV.27Thus,it was inferred that an oxidation-reduction mechanism must be involved in the Hg0removal on theAC-N-Hg sample,transforming Hg0to Hg2+.
Adsorption and desorption processes on the adsorbent surface depend on the adsorption energies,and chemical bonds between the adsorbed molecules and the adsorption sites are often quite strong,which could be differentiated from the physisorptions.28Consequently,to further confirm the Hg0production adsorbed on the AC-N-Hg,TPD technique was used.As shown in Fig.6,the primary Hg0desorption peak of the AC-N-Hg sample was observed at 403°C.The chemisorbed mercury evolved at temperature high to 400-500°C.28Thus,it was inferred that chemisorption of Hg2+was the main product.Lopez-Anton et al.29reported that the desorption peaks of HgO appeared at approximately 430°C.Rumayor et al.30reported that the desorption peaks of Hg(NO3)2appeared at approximately 280°C.Consequently,on the basis of previous studies,it was supposed that the peak at 403°C is associated with the decomposition of HgO,which is in agreement with the Hg 4f XPS result.

Fig.5 (A)Hg 4f7/2,(B)Si 2p3/2,(B)Si 2p1/2,and(D)Hg 4f5/2XPS spectra of theAC-N-Hg sample

Fig.6 TPD results ofAC-N-Hg sample after mercury removal at 120°C for 2 h in N2atmosphere
3.4.2 O 1s XPS spectral analysis
To further understand the mechanism of Hg0removal on the HNO3-modified activated carbon,the active species present on the AC surface were discussed.Therefore,the carbon samples were further analyzed using XPS to identify the chemical state and changes of the relative content of oxygen and nitrogen functional groups on the AC.The optimum curve fitting of the O 1s region illustrated in Fig.7,was achieved by deconvoluting into five peaks:31,32peak A(531.0-531.9 eV)corresponds to carbonyl oxygen atoms;peak B(532.3-532.8 eV)corresponds to carbonyl oxygen atoms in esters,anhydrides as well as oxygen atoms in hydroxyls or ethers;peak C(533.1-533.8 eV)corresponds to oxygen atoms in esters and anhydrides;peak D(534.3-535.4 eV) corresponds to oxygen atoms in carboxyl groups;and peak E (536.0-536.5 eV)corresponds to adsorbed water and/or oxygen. Peak(B-C)represents hydroxyl or ether groups and was calculated by subtracting peak C from peak B.The area percentages of the peaks shown in Table 3 reflected the relative molar content of each group.
The spectral area percentages of the carbonyl,ester,and anhydride groups for theAC-N sample were 24.9%and 29.0%,and those percentages decreased to 21.6%and 23.4%for the AC-NHg sample.This result might be because carbonyl,ester,and anhydride groups were consumed as reactants during the reaction between Hg0and the sample.Thus,it implied that the carbonyl, ester or anhydride groups were beneficial for Hg0adsorption. However,the spectral area percentage of hydroxyl or ether groups was 9.0%for the AC-N sample,and this percentage increased to 25.5%for the AC-N-Hg sample.This result might be because hydroxyl or ether groups were generated during the reaction between Hg0and the sample.Thus,it was likely that hydroxyl or ether groups hindered Hg0adsorption because these groups influenced the equilibrium of the reaction between Hg0and the active oxygen groups on the sample.The spectral area percentage of the carboxyl groups had no obvious variations;both were 6.6% before and after the reaction between Hg0and the sample,which might indicate that carboxyl groups were not involved in the reaction between Hg0and the sample.The results for O 1s were similar to the theoretical study results obtained by Liu et al.33andverified the Boehm titrations results.Based on the above analysis results,the pathways for Hg0oxidation on theAC-N sample could be as follows:the carbonyl and ester groups were strong oxidizers, which could accept two electrons from the Hg0outer shell and convert it to Hg2+,and subsequently being reduced to hydroxyl or ether groups.
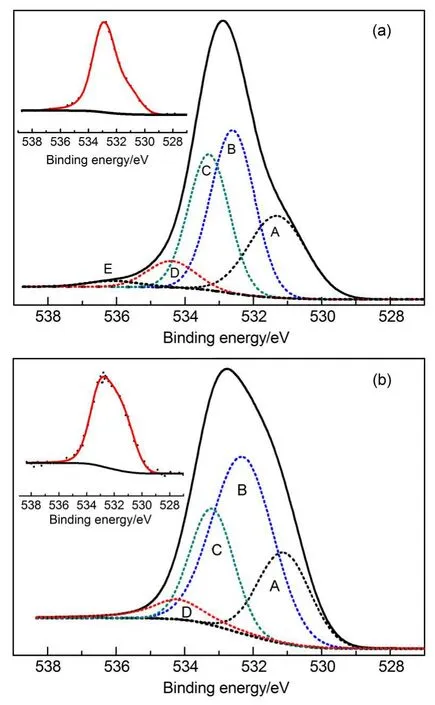
Fig.7 Deconvolution of the O 1s XPS spectra of the (a)AC-N and(b)AC-N-Hg samples

Table3 Relative content of the different oxygen groups onAC samples
3.4.3 N 1s XPS spectral analysis
To further understand the mechanism of the Hg0removal,we also examined the N 1s XPS spectra of the AC-N and AC-N-Hg samples,and the results are shown in Fig.8.Both of the N 1s spectra of the AC-N and AC-N-Hg samples showed two obvious peaks:34peak A(400.1-400.4 eV)corresponds to pyrrolic groups and peak B(404.9-405.6 eV)corresponds to chemisorbed nitrogen oxides.
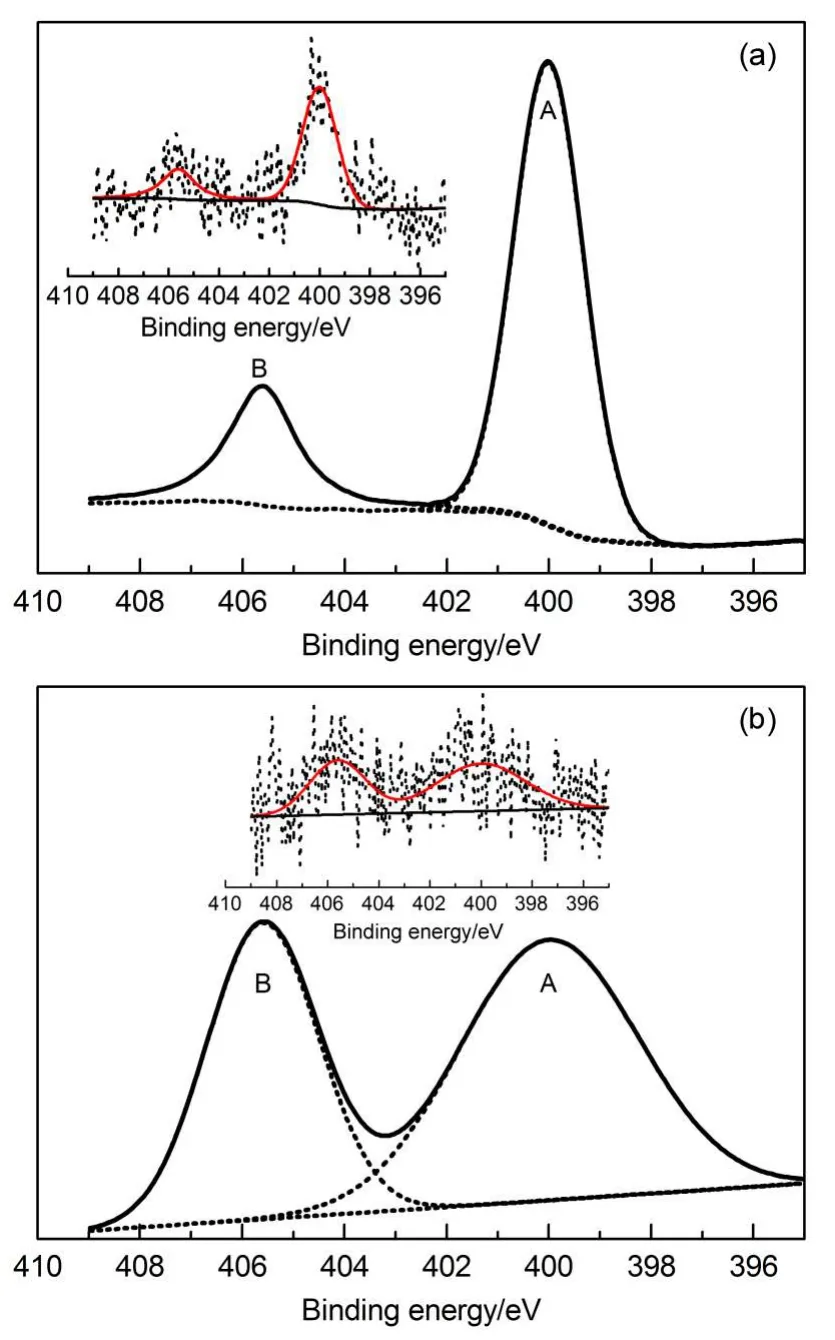
Fig.8 N 1s XPS spectra of the(a)AC-N and(b)AC-N-Hg samples
Result reported by the previous study35showed that nitrogen groups could promote Hg0removal.Because no other peaks were observed on AC-N-Hg sample after the Hg0removal test,which meant that no changes occurred to N atoms element on theAC-N sample,the catalytic oxidation reaction should partly contribute to the Hg0removal.Therefore,it was inferred that the pathways for Hg0oxidation on the AC-N sample might be also as follows: the N atoms in pyrrolic tautomers were of positive charges,resulting in loss of Hg0outer shell electrons to form certain intermediate species;then Hg2+bonded to nearby oxygen atoms to form HgO,and the nitrogen groups were converted back to their original states.The XPS results for Hg 4f,O 1s,and N 1s further supported our idea that chemical characteristics dominated the Hg0removal.
4 Conclusions
The HNO3-modifiedAC was prepared and studied for its ability to remove Hg0in simulated flue gas.It was effective for Hg0removal at 120°C.The characterization results indicated that Hg0removal was strongly affected by the chemical characteristics of the adsorbent.The removed Hg0was mainly oxidized to HgO on the HNO3-modified AC.The XPS results indicated that both theoxygen and nitrogen functional groups likely favored Hg0removal.The oxygen functional groups,such as carbonyl,ester,or anhydride groups,were likely the active adsorption sites.And the nitrogen functional groups,such as pyrrolic groups,were likely the active catalytic sites.Furthermore,the possible reaction pathways were revealed based on experimental results.
(1)Pavlish,J.H.;Sondreal,E.A.;Mann,M.D.;Olson,E.S.; Galbreath,K.C.;Laudal,D.L.;Benson,S.A.Fuel Process. Technol.2003,82,89.doi:10.1016/S0378-3820(03)00059-6
(2)Ko,K.B.;Byun,Y.;Cho,M.;Namkung,W.;Shin,D.N.;Koh, D.J.;Kim,K.T.Chemosphere2008,71,1674.doi:10.1016/j. chemosphere.2008.01.015
(3)Pirrone,N.;Cinnirella,S.;Feng,X.;Finkelman,R.B.;Friedli, H.R.;Leaner,J.;Mason,R.;Mukherjee,A.B.;Stracher,G.B.; Streets,D.G.;Telmer,K.Atmos.Chem.Phys.2010,10,5951. doi:10.5194/acp-10-5951-2010
(4)Galbreath,K.C.;Zygarlicke,C.J.Environ.Sci.Technol.1996,30,2421.doi:10.1021/es950935t
(5)Wang,P.Y.;Su,S.;Xiang,J.;You,H.W.;Cao,F.;Sun,L.S.; Hu,S.;Zhang,Y.Chemosphere2014,101,49.doi:10.1016/j. chemosphere.2013.11.034
(6)Yan,N.Q.;Liu,S.H.;Chang,S.G.Ind.Eng.Chem.Res.2005,44,5567.doi:10.1021/ie050377j
(7)Yan,R.;Liang,D.T.;Tsen,L.;Wong,Y.P.;Lee,Y.K.Fuel2004,83,2401.doi:10.1016/j.fuel.2004.06.031
(8)Xu,S.K.;Li,L.M.;Guo,N.N.;Su,Y.L.;Zhang,P.Acta Phys.-Chim.Sin.2012,28,177.[徐三魁,李利民,郭楠楠,苏运来,张 朋.物理化学学报,2012,28,177.]doi:10.3866/ PKU.WHXB201111181
(9)Yang,J.L.;Zhou,J.B.Acta Phys.-Chim.Sin.2013,29,377. [杨继亮,周建斌.物理化学学报,2013,29,377.]doi:10.3866/ PKU.WHXB201212101
(10)Granite,E.J.;Pennline,H.W.;Hargis,R.A.Ind.Eng.Chem. Res.2000,39,1020.doi:10.1021/ie990758v
(11)Hu,C.X.;Zhou,J.S.;He,S.;Luo,Z.Y.;Cen,K.F.Fuel Process.Technol.2009,90,812.doi:10.1016/j. fuproc.2009.03.020
(12)Niksa,S.;Naik,C.V.;Berry,M.S.;Monroe,L.Fuel Process. Technol.2009,90,1372.doi:10.1016/j.fuproc.2009.05.022
(13)Tan,Z.Q.;Xiang,J.;Su,S.;Zeng,H.C.;Zhou,C.S.;Sun,L. S.;Hu,S.;Qiu,J.R.J.Hazard.Mater.2012,239,160.
(14)Chingombe,P.;Saha,B.;Wakeman,R.J.Carbon2005,43, 3132.doi:10.1016/j.carbon.2005.06.021
(15)Kisamori,S.;Kuroda,K.;Kawano,S.;Mochida,I.;Matsumura, Y.J.;Yoshikawa,M.Energy&Fuels1994,8,1337.doi: 10.1021/ef00048a023
(16)Stohr,B.;Boehm,H.P.;Schlogl,R.Carbon1991,29,707.doi: 10.1016/0008-6223(91)90006-5
(17)Li,Y.H.;Lee,C.W.;Gullett,B.K.Carbon2002,40,65.doi: 10.1016/S0008-6223(01)00085-9
(18)Skodras,G.;Diamantopoulou,I.;Sakellaropoulos,G.P. Desalination2007,210,281.doi:10.1016/j.desal.2005.12.082
(19)Pradhan,B.K.;Sandle,N.K.Carbon1999,37,1323.doi: 10.1016/S0008-6223(98)00328-5
(20)Serre,S.D.;Silcox,G.D.Ind.Eng.Chem.Res.2000,39, 1723.doi:10.1021/ie990680i
(21)Tan,Z.;Qiu,J.;Zeng,H.;Liu,H.;Xiang,J.Fuel2011,90, 1471.doi:10.1016/j.fuel.2010.12.004
(22)Maroto-Valer,M.M.;Zhang,Y.;Granite,E.J.;Tang,Z.; Pennline,H.W.Fuel2005,84,105.doi:10.1016/j. fuel.2004.07.005
(23)Gadiou,R.;Didion,A.;Gearba,R.I.;Ivanov,D.A.;Czekaj,I.; Kötz,R.;Vix-Guterl,C.J.Phys.Chem.Solids2008,69, 1808.doi:10.1016/j.jpcs.2008.01.006
(24)Shimodaira,N.;Masui,A.J.Appl.Phys.2002,92,902.doi: 10.1063/1.1487434
(25)Melanitis,N.;Tetlow,P.L.;Galiotis,C.Mater.Sci.1996,31, 851.doi:10.1007/BF00352882
(26)Wang,X.Experimental Study onAdsorption of Mercury by Activated Carbon Fibres at Low Temperature.Ph.D. Dissertation,Huazhong University of Science and Technology, Wuhan,2006.
(27)Sasmaz,E.;Kirchofer,A.;Jew,A.D.;Saha,A.;Abram,D.; Jaramillo,T.F.;Wilcox,J.Fuel2012,99,188.doi:10.1016/j. fuel.2012.04.036
(28)Tan,Z.;Su,S.;Qiu,J.;Kong,F.;Wang,Z.;Hao,F.;Xiang,J. Chem.Eng.J.2012,195-196,218.
(29)Lopez-Anton,M.A.;Perry,R.;Abad-Valle,P.;Díaz-Somoano, M.;Martínez-Tarazona,M.R.;Maroto-Valer,M.M.Fuel Process.Technol.2011,92,707.doi:10.1016/j. fuproc.2010.12.002
(30)Rumayor,M.;Diaz-Somoano,M.;Lopez-Anton,M.A.; Martinez-Tarazona,M.R.Talanta2013,114,318.
(31)Zhou,J.H.;Sui,Z.J.;Zhu,J.;Li,P.;Chen,D.;Dai,Y.C.;Yuan, W.K.Carbon2007,45,785.doi:10.1016/j.carbon.2006.11.019
(32)Zielke,U.;Hüttinger,K.J.;Hoffman,W.P.Carbon1996,34, 983.doi:10.1016/0008-6223(96)00032-2
(33)Liu,J.;Cheney,M.A.;Wu,F.;Li,M.J.Hazard.Mater.2011,186,108.doi:10.1016/j.jhazmat.2010.10.089
(34)Biniak,S.;Szymanski,G.;Siedlewski,J.;Swiatkowski,A. Carbon1997,35,1799.doi:10.1016/S0008-6223(97)00096-1
(35)Xu,L.S.Study onAdsorption and Oxidation Removal of SO2/ NOx/Hg at Low-Temperature by ModifiedActivated Carbon Fiber.Ph.D.Dissertation,Huazhong University of Science and Technology,Wuhan,2007.[许绿丝.改性处理活性炭纤维吸附氧化脱除SO2/NOx/Hg的研究[D].武汉:华中科技大学, 2007.]
Enhanced Effect of O/N Groups on the Hg0Removal Efficiency over the HNO3-Modified Activated Carbon
TONG Li1,2XU Wen-Qing1QI Hao1,2ZHOU Xuan1LIU Rui-Hui1ZHU Ting-Yu1,*
(1Beijing Engineering Research Center of Process Pollution Control,National Engineering Laboratory for Hydrometallurgical Cleaner Production Technology,Institute of Process Engineering,Chinese Academy of Sciences,Beijing 100190,P.R.China;2University of Chinese Academy of Sciences,Beijing 100049,P.R.China)
HNO3-modified activated carbon(AC)was prepared to determine its mercury removal ability on a fixed-bed reactor.In this study,the HNO3-modified AC was found to be effective for mercury removal in simulated flue gas.The original sample,the HNO3-modified sample and the production sample were characterized by elemental analysis,Brunauer-Emmett-Teller(BET)specific surface area measurements,scanning electron microscopy(SEM),Raman spectra,Boehm titrations,temperature programmed desorption(TPD)technique, and X-ray photoelectron spectroscopy(XPS).The results show that HNO3treatment increases the content of oxygen and nitrogen on the AC.Compared with the physical characteristics of HNO3-modified AC,the effects of its chemical characteristics on mercury removal are more significant.The Hg0is mainly oxidized to HgO by the HNO3-modifiedAC.The oxygen functional groups,possibly carbonyls,esters or anhydrides were found to be the adsorption sites for mercury removal,and these groups were reduced to hydroxyl groups or ether groups. The N-functional groups,possibly pyrrolic tautomers,were found to be the active catalytic sites.The mechanism for Hg0removal by HNO3-modified activated carbon is proposed based on the characterization results.
Elemental mercury;Activated carbon;HNO3;Oxygen functional group; Nitrogen functional group
O647
10.3866/PKU.WHXB201412251www.whxb.pku.edu.cn
Received:October 13,2014;Revised:December 25,2014;Published on Web:December 25,2014.
∗Corresponding author.Email:tyzhu@ipe.ac.cn;Tel/Fax:+86-10-82544821.
The project was supported by the National Key Basic Research Program of China(973)(2013CB430005)and National Hi-Tech Research and Development Program of China(863)(2013AA065501,2013AA065404).
国家重点基础研究发展规划项目(973)(2013CB430005)和国家高技术研究发展计划项目(863)(2013AA065501,2013AA065404)资助
