黑萨福克和杜泊绵羊超数排卵效率比较及分析
2015-12-23易金云,李继成,任超等
黑萨福克和杜泊绵羊超数排卵效率比较及分析
易金云1,2,李继成3,任 超3,董德权3,刘国世1*,吕文发2*
(1.吉林农业大学动物科技学院,吉林 长春 130118;
2.中国农业大学动物科技学院,北京 100193;
3.北京奥鑫牧业有限公司,北京 101309)
本试验的目的是比较黑萨福克和杜泊绵羊超数排卵效率的差异,并进一步分析差异产生的原因。20只黑萨福克绵羊和10只杜泊绵羊用FSH递减法超数排卵,获得的胚胎用于移植103只受体。结果表明,黑萨福克绵羊平均回收胚胎11.0±7.1枚,杜泊绵羊14.3±6.7枚,差异不显著(P>0.05);移植2枚可用胚的黑萨福克组和杜泊组产羔受体绵羊,可用胚发育效率无差异(75%和80%,P>0.05);整体水平上,黑萨福克绵羊获得后代的几率显著低于杜泊绵羊(44.7%和88.9%,P<0.05)。说明两品种绵羊回收的胚胎数量没有差异,但质量有差异;黑萨福克超数排卵效率低于杜泊绵羊,但与母羊繁殖力无关,可能因公羊精液品质或者输精方式所致。
黑萨福克;杜泊;超数排卵;胚胎移植;人工输精
修回日期:2015-03-02
自然生理环境下,绵羊卵巢一个情期只有一到两个卵泡成功排卵。外源FSH可以解除优势卵泡对其它卵泡发育的抑制,在合适时间注射FSH,可获得更多胚胎[1]。孕酮栓除使发情同步,由于孕酮水平上升,卵泡募集得更多,发育得更大[2]。
绵羊超数排卵效果不稳定,连续超数排卵得到的胚胎往往是第一次或第二次最好[3-5]。孕酮栓埋植天数不同,绵羊发情排卵也不同[6]。不同个体的卵巢对外源激素反应有差异,诱导分泌的雌激素水平高低不同,卵泡群动态变化多种多样[7,8,9]。同一品种绵羊排卵数在较大范围内波动,而不是固定的一或两枚。
不同品种绵羊超数排卵是否有差异存在不同观点[10-12]。评价超数排卵多是比较黄体数和冲胚数等,没有纳入胚胎移植结果,但结论可能因胚胎鉴定时肉眼误判而存在缺陷。因此,结合胚胎移植分析超数排卵是否因品种而不同,并找出原因,对品种挑选和方案改进有重要意义。
1 材料与方法
试验分两批进行,每一批10只黑萨福克和5只杜泊绵羊。试验流程见图1。
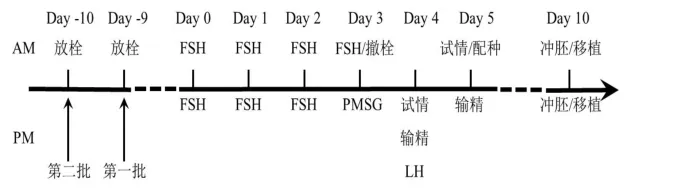
图1 试验流程
供体绵羊发情周期任意某天,阴道放入CIDR栓(新西兰),第二批放栓晚第一批1 d。第一批放栓第10 d,第二批第11 d,设为第0 d。第0 d上午到第3 d上午,以剂量递减方式每12 h注射一次FSH(宁波,下同),最后一针的同时撤栓。撤栓12 h,注射PMSG;31~33 h,试情;34 h,发情母羊输精;36 h,注射LH;50 h,再次试情,公羊交配;57 h,再次输精。第10 d,供体绵羊腹腔内窥镜法回收胚胎,挑选桑葚胚和囊胚,移入与供体绵羊同步发情处理的受体绵羊子宫角。
试验在2012年4月下旬至5月上旬完成,受体绵羊9月下旬至10月中旬产羔。
数据用SPSS18.0独立样本T检验、非参数检验或者卡方检验分析。平均数以“均值±标准差”表示,P>0.05为不相关或差异不显著,P<0.05为相关或差异显著。
2 结果与分析
2.1 超数排卵效果(见表1)

表1 黑萨福克和杜泊绵羊超数排卵效果比较

图2 黑萨福克和杜泊绵羊冲胚数分布
2.2 可用胚质量
两组受体绵羊可用胚移植和产羔情况见表2。
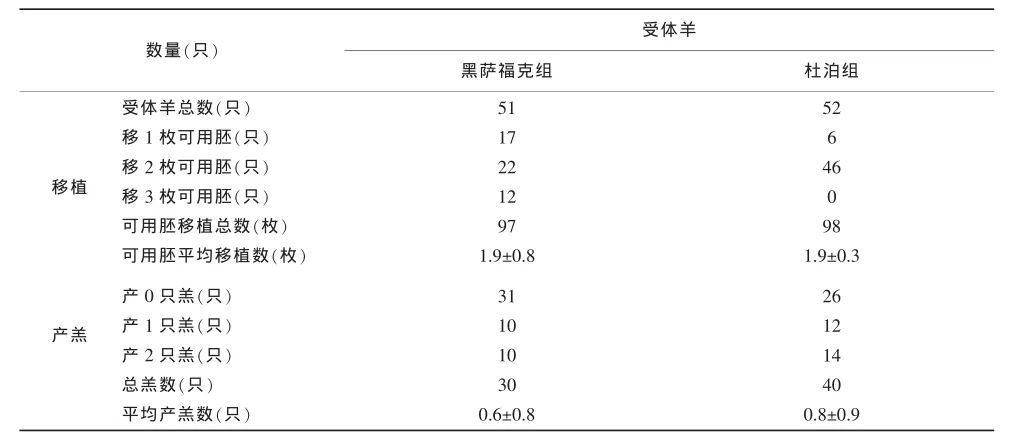
表2 两组受体绵羊可用胚移植和产羔情况
2.2.1供体品种与所有受体绵羊产羔的关系
由表2可知,两组可用胚平均移植数均为1.9枚。黑萨福克组39.2%即20只受体绵羊产羔;杜泊组50.0%即26只受体绵羊产羔。产羔受体绵羊比例相差10.8个百分点。

表3 供体品种与所有受体绵羊产羔的关系
为研究可用胚质量是否有差异,先将每一组受体绵羊按是否产羔分为两组(表3),分析供体品种是否影响受体绵羊产羔。
经卡方检验,受体绵羊是否产羔与供体品种不相关(P>0.05),即供体品种不影响受体绵羊产羔。
2.2.2 供体品种与产羔受体绵羊移植可用胚发育效率的关系
由表2可知,两组都是移植2枚可用胚的受体绵羊最多,黑萨福克组22只,占43.1%;杜泊组46只,占88.5%。黑萨福克组总产羔30只,杜泊组40只,相差10只。为此,选出移植2枚可用胚且产羔的受体绵羊(表4),进一步分析供体品种是否影响产羔受体绵羊移植可用胚的发育。
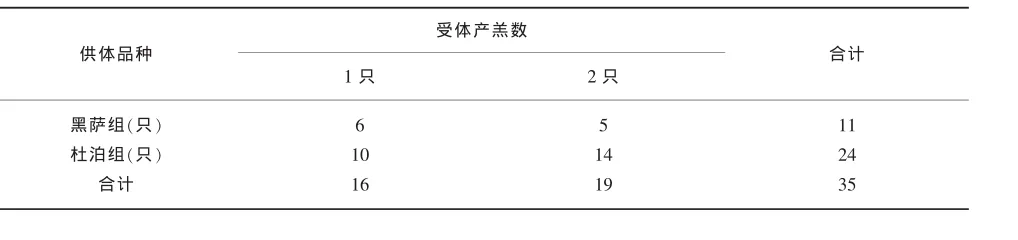
表4 移植2枚可用胚的产羔受体绵羊,产羔数与供体品种的关系
表4经卡方检验,移植2枚可用胚的产羔受体绵羊,产羔数与供体品种无关(P>0.05)。其中,黑萨福克组受体绵羊平均产羔1.5只,即75%(1.5/2)的胚胎发育产羔;杜泊组平均产羔1.6只,80%(1.6/2)的胚胎发育产羔。供体品种不影响产羔数,产羔受体绵羊移植的可用胚发育效率无差异。
供体品种既不影响受体绵羊产羔,又不影响产羔受体绵羊移植的可用胚发育效率,说明两品种绵羊可用胚质量无差异。
2.3 所有胚胎(可用胚和不可用胚)质量
两品种绵羊后代数/冲胚数见表5。

表5 黑萨福克和杜泊绵羊后代数/冲胚数
黑萨福克绵羊回收胚胎219枚,受体绵羊产羔30只,平均13.7%(30/219)的胚胎发育到羔羊;杜泊绵羊回收胚胎129枚,受体绵羊产羔40只,平均31.0%(40/129)的胚胎发育到羔羊。
黑萨福克绵羊19只冲出胚胎,9只有后代,占47.4%;杜泊绵羊9只冲出胚胎,8只有后代,占88.9%。黑萨福克绵羊后代获得率比杜泊低41.5个百分点。
2.3.1供体品种与后代获得的关系
为研究两品种绵羊胚胎(包括不可用胚)的发育效率是否有差异,先将冲出胚胎的绵羊按是否有后代分为两类(后代数/冲胚数=或>0),分析供体品种是否影响后代的获得。
表5中后代数/冲胚数的分布经卡方检验,供体品种与后代数/冲胚数是否大于0显著相关(P<0.05),即是否有后代与品种有关。黑萨福克绵羊冲出胚胎但没有后代的几率比杜泊高。
2.3.2 两品种有后代的绵羊胚胎质量比较
进一步比较表5中有后代的两品种绵羊后代数/冲胚数,分析有后代的供体绵羊冲出的胚胎,质量是否存在品种间差异。
经T检验,有后代的黑萨福克和杜泊绵羊后代数/冲胚数无差异(P>0.05),且分别有24.6%和31.9%的胚胎发育到羔羊。即如果有后代,两品种绵羊胚胎质量无差异。
供体品种影响后代的获得,但不影响有后代的供体绵羊胚胎发育,说明黑萨福克和杜泊绵羊的胚胎整体发育效率(13.7%和31.0%)有差异,即超数排卵效率有差异。
3 讨论
此次试验,两品种绵羊超数排卵效率的差异主要体现在胚胎质量上。有后代的黑萨福克和杜泊绵羊,可用胚分别有75%和80%发育到羔羊;而所有胚胎(可用胚和不可用胚)分别有24.6%和31.9%发育到羔羊。而对于所有(有后代和无后代)绵羊,黑萨福克和杜泊绵羊回收的胚胎分别有13.7%和31.0%发育产羔,相差16.3个百分点,导致黑萨福克绵羊后代获得率显著低于杜泊绵羊。由胚胎质量在整体而非有后代个体上的差异,可知黑萨福克绵羊不可用胚胎(包括发育失败的移植胚)太多,平均可用胚(5.1枚)显著少于杜泊绵羊(11.3枚)可佐证这一点。
此外,超数排卵效率还体现在胚胎数量上,即排卵数或者冲胚数。
Lassoued等(2003)发现,同一种绵羊繁殖力较高品系的中型卵泡和排卵数(黄体数)多于繁殖力较低品系[8]。说明排卵数与母羊自身繁殖力有关。杜泊绵羊平均胎产仔数在1.45~1.60只之间[13,14],也有认为是1.28只[15];萨福克绵羊在1.46~1.92只之间[16,17]。总体上萨福克绵羊胎产仔数比杜泊高,繁殖力更高,募集卵泡数也应越多。
试验中,黑萨福克绵羊平均冲胚数与杜泊无差异。Guzik等(1995)同时对四种绵羊超数排卵,黄体数与冲胚数均无差异[10]。可能是绵羊繁殖力相差并不大,或者卵巢承受能力达到极限,无法体现高繁殖力绵羊的优势。
综上可知,两品种绵羊超数排卵效率出现差异,与母羊繁殖力无关,而可能因卵子受精状态影响胚胎质量所致。LH注射时间或公羊精液都可能影响卵子受精。
绵羊繁殖期内源性LH峰在撤栓38.2 h或36.5 h出现[18,19];繁殖低谷期LH峰较高峰时延迟3 h[20]。而外源性高峰在注射LH 5 min后即可出现[21]。此次试验超数排卵在4-5月进行,LH峰应在撤栓36~41 h左右,因此撤栓36 h注射LH,外源与内源高峰出现时间相近,卵泡可充分发育、排卵并受精。事实上,LH峰即使相差4 h也不影响卵子质量[11]。
萨福克公羊睾丸在春夏季重新发育,为秋季繁殖高峰期做准备[22,23];春季配种能力最弱,精液品质可能不稳定[24]。在非繁殖季节,胚胎受精失败的几率更高[20]。此次试验,杜泊绵羊受精率100%,而两批黑萨福克绵羊分别为100%和50%。精液品质波动可能是黑萨福克绵羊胚胎质量低于杜泊的原因之一。
Sayre等(1997)比较了腹腔镜与子宫颈输精的效率,前者胚胎受精率和母羊妊娠率均高于后者[25]。Ghalsasi等(1996)也有类似发现[26]。Fair等(2005)用腹腔镜法给两品种绵羊输精,胚胎受精率无差异,而用子宫颈法则差异显著[12]。可见,选用合适的输精方式,品种并不影响胚胎受精率。此次试验采用子宫颈法(自然交配为辅),可能母羊生殖道内分泌影响到了精子的运行及授精,从而降低了黑萨福克绵羊胚胎的质量[27]。
综上所述,黑萨福克绵羊回收胚胎数与杜泊无差异,但发育效率低于杜泊绵羊。两品种超数排卵效率有差异,但与母羊繁殖力无关,可能因黑萨福克公羊精液品质不稳定,或输精方式的缺陷所致。
[1]Soboleva TK, Peterson AJ, Pleasants AB, et al. A model of follicular development and ovulation in sheep and cattle[J]. Animal Reproduction Science, 2000,(58): 45-57.
[2]Leyva V, Buckrell BC, Walton JS. Follicular activity and ovulation regulated by exogenous progestagen and PMSG in anestrous ewes[J]. Theriogenology, 1998,(50): 377-393.
[3]Bruno-Galarraga MM, Cueto M, Gibbons AE, et al. Repeatability of superovulatory response to successive FSH treatments in Merino sheep[J]. Small Ruminant Research, 2014,(120): 84-89.
[4]Forcada F, Abecia JA, Lozano JM, et al. Repeated superovulation of high-prolificacy Rasa Aragonesa ewes before culling as an inexpensive way to obtain high-quality embryos[J]. Livestock Production Science, 2000,(66): 263-269.
[5]Forcada F, Ait Amer-Meziane M, Abecia JA, et al. Repeated superovulation using a simplified FSH/eCG treatment for in vivo embryo production in sheep[J]. Theriogenology, 2011,(75): 769-776.
[6]Lehloenya KC, Greyling JPC. The ovarian response and embryo recovery rate in Boer goat does following different superovulation protocols, during the breeding season[J]. Small Ruminant Research, 2010,(88): 38-43.
[7]López Sebastián A, Gonzalez de Bulnes A, Santiago Moreno J, et al. Effects of follicular status at treatment on follicular development and ovulation in response to FSH in Spanish merino ewes[J].Theriogenology, 1999,(52): 505-514.
[8]Lassoued N, Rekik M, Gonzalez-Bulnes A, et al. Prolific strains of Barbarine sheep are characterized by increased ovulation rate due to extended period of ovulatory follicle recruitment and co-dominance effects[J]. Small Ruminant Research, 2013,(114): 134-139.
[9]Valasi I, Leontides L, Menegatos I, et al. Oestradiol concentration as a predictor of ovarian response in FSH stimulated ewe-lambs[J]. Animal Reproduction Science, 2007,(102): 145–151.
[10]Guzik A, Niemann H. Superovulation and recovery of zygotes suitable for microinjection in different breeds of sheep[J]. Animal Reproduction Science, 1995,(40): 215-227.
[11]Fair S, Hanrahan JP, Ward F, et al. The difference in embryo quality between Belclare and Suffolk ewes is not due to differences in oocyte quality[J]. Theriogenology, 2006,(66): 191-197.
[12]Fair S, Hanrahan JP, O’Meara CM, et al. Differences between Belclare and Suffolk ewes in fertilization rate, embryo quality and accessory sperm number after cervical or laparoscopic artificial insemination[J]. Theriogenology, 2005,(63): 1995-2005.
[13]Cloete SWP, Snyman MA, Herselman MJ. Productive performance of Dorper sheep[J]. Small Ruminant Research, 2000,(36): 119-135.
[14]Snyman MA, Olivier WJ. Productive performance of hair and wool type Dorper sheep under extensive conditions[J]. Small Ruminant Research, 2002,(45): 17–23.
[15]Schoeman SJ. A comparative assessment of Dorper sheep in different production environments and systems[J]. Small Ruminant Research, 2000,(36): 137-146.
[16]Schmidová J , Milerski M, Svitaková A, et al. Estimation of genetic parameters for litter size in Charollais, Romney, Merinolandschaf, Romanov, Suffolk, Sumava and Texel breeds of sheep[J]. Small Ruminant Research, 2014,(119): 33-38.
[17]Notter DR. Effects of ewe age and season of lambing on prolificacy in US Targhee, Suffolk, and Polypay sheep[J]. Small Ruminant Research, 2000,(38): 1-7.
[18]Saifullizam AK, Routly JE, Smith RF, et al. Effect of insulin on the relationship of estrous behaviors to estradiol and LH surges in intact ewe[J]. Physiology & Behavior, 2010,(99): 555-561.
[19]Fergani C, Saifullizam AK, Routly JE, et al. Estrous behavior, luteinizing hormone and estradiol profiles of intact ewes treated with insulin or endotoxin[J]. Physiology & Behavior, 2012,(105): 757-765.
[20]Mitchell LM, Dingwall WS, Mylne MJA, et al. Season affects characteristics of the pre-ovulatory LH surge and embryo viability in superovulated ewes[J]. Animal Reproduction Science, 2002,(74): 163–174.
[21]Dobson H, Campbell BK, Scaramuzzi RJ. Use of a GnRH antagonist in conjunction amplitude, high frequency LH pulses to follicular growth without an LH surge and in ewes[J]. Animal Reproduction Science, 1997,(46): 213-222.
[22]Milczewski V, Chahad-Ehlers S, Spercoski KM, et al. Quantifying the effect of seasonality on testicular function of Suffolk ram in lower latitude[J]. Small Ruminant Research, 2015.
[23]Sanford LM, Dickson KA. Prolactin regulation of testicular development and sexual behavior in yearling Suffolk rams[J]. Small Ruminant Research, 2008,(77): 1-10.
[24]Mandiki SNM, Derycke G, Bister JL, et al. Influence of season and age on sexual maturation parameters of Texel, Suffolk and Ile-de-France rams 1. Testicular size, semen quality and reproductive capacity[J]. Small Ruminant Research, 1998,(28): 67-79.
[25]Sayre BL, Lewis GS. Fertility and ovm fertilization rate after laparoscopic or transcervical intrauterine artificial insemination of oxytocin-treated ewes[J]. Theriogenology, 1997,(46): 267-275.
[26]Ghalsasi PM, Nimbkar C. Evaluation of laparoscopic intrauterine insemination in ewes[J]. Small Ruminant Research, 1996,(23): 69-73.
[27]Fair S, Hanrahan JP, Donovan A, et al. Hormonal relationships during the periovulatory period among ewe breeds known to differ in fertility after cervical artificial insemination with frozen thawed semen[J]. Animal Reproduction Science, 2007,(97): 284-294.
Comparative Analysis of Superovulation Efficiency Between Black Suffolk and Dorper Ewes
YI Jin-yun1.2,LI Ji-cheng3,REN Chao3,DONG De-quan3,LIU Guo-shi1*,LV Wen-fa2*
(1. College of Animal Science and Technology, Jilin Agricultural University, Changchun 130118, China;
2. College of Animal Science and Technology, China Agricultural University, Beijing 100193, China;
3.Beijing Aoxin Animal Husbandry Co., Ltd,Beijiang 101309, China)
The purpose of the current study was to evaluate the differences and their causes of the efficiency of superovulation between Black Suffolk and Dorper ewes. Superovulation of Black Suffolk ewes(n=20)and Dorper ewes(n=10)was performed with dosages declined FSH, followed by embryo transplant procedure immediately. The mean number of ova/embryo did not vary(P>0.05)between Black Suffolk and Dorper ewes, with 11.0±7.1 and 14.3±6.7, respectively. There is no difference(P>0.05)between the receptors of Black Suffolk and Dorper, which received two valid embryos and lambed ultimately, in the development rate of valid embryo(75% and 80%, respectively). Overall, a significant lower birth rate was observed in Black Suffolk ewes compared to Dorper ewes(44.7% and 88.9%, respectively; P<0.05). In conclusion, the quality but not the quantity of ova/embryo recovered varied between breeds of ewes. Moreover, the Dorper was superior in superovulation efficiency to the Black Suffolk, which could be attributed to the quality of semen and mode of artificial insemination, irrespective of fecundity of ewes.
black suffolk;dorper;superovulation;embryo transplant;artificial insemination
S821.3+6
A
1003-6377(2015)03-0029-07
国家科技支撑项目(2011BAD19B02-4)和转基因重大专项(2014ZX08008-002B,2011ZX08008-005)
易金云(1990-),湖南衡阳,男,硕士。
刘国世,E-mail:gshliu@cau.edu.cn;吕文发,
E-mail:wenfa2004@163.com
2015-02-13,
