Optimization,validation and application of an assay for the activity of HMG-CoA reductase in vitro by LC–MS/MS☆
2015-12-21JingWngJiYeSunChunJieShYuFengShoYnHongLiuYouXinLiZhenWenDunWnHuiLiu
Jing Wng,Ji-Ye Sun,Chun-Jie Sh,Yu-Feng Sho,Yn-Hong Liu,You-Xin Li, Zhen-Wen Dun,Wn-Hui Liu,,*
aSchool of Pharmacy,Yantai University,Yantai 264005,China
bState Key Laboratory of Long-acting and Targeting Drug Delivery System,Shandong Luye Pharmaceutical Co.,Ltd.,Yantai 264003,China
cPeking University WBL Biotech Co.,Ltd.,Beijing 100080,China
Original Article
Optimization,validation and application of an assay for the activity of HMG-CoA reductase in vitro by LC–MS/MS☆
Jing Wanga,1,Ji-Ye Sunb,1,Chun-Jie Shab,Yu-Feng Shaob,Yan-Hong Liua,You-Xin Lib, Zhen-Wen Duanc,Wan-Hui Liua,b,*
aSchool of Pharmacy,Yantai University,Yantai 264005,China
bState Key Laboratory of Long-acting and Targeting Drug Delivery System,Shandong Luye Pharmaceutical Co.,Ltd.,Yantai 264003,China
cPeking University WBL Biotech Co.,Ltd.,Beijing 100080,China
A R T I c L E I N F o
Article history:
12 June 2015
Accepted 29 June 2015
Available online 3 July 2015
Xuezhikang LC–MS/MS HMG-CoA reductase Mevalonolactone Quality control
A stable HMG-CoA reductase(HMGR)reaction in vitro was developed by a sensitive,selective and precise liquid chromatography–tandem mass spectrometry(LC–MS/MS)method.The optimized enzyme reaction condition contained 1.5μg of HMGR,20 nM of NADPH with 50 min of reaction time.The method was validated by several intra-and inter-day assays.The production transitions of m/z 147.0/59.1 and m/z 154.0/59.1 were used to detect and quantify mevalonolactone(MVAL)and MVAL-D7,respectively.The accuracy and precision of the method were evaluated over the concentration range of 0.005–1.000μg/mL for MVAL and 0.010–0.500μg/mL for lovastatin acid in three validation batch runs.The lower limit of quantitation was found to be 0.005μg/mL for MVAL and 0.010μg/mL for lovastatin acid. Intra-day and inter-day precision ranged from 0.95%to 2.39%and 2.26%to 3.38%for MVAL,1.46%to 3.89% and 0.57%to 5.10%for lovastatin acid,respectively.The results showed that the active ingredients in Xuezhikang capsules were 12.2 and 14.5 mg/g,respectively.This assay method could be successfully applied to the quality control study of Xuezhikang capsule for the fi rst time.
©2015 Xi'an Jiaotong University.Production and hosting by Elsevier B.V.Allrights reserved.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
1.Introduction
As a major membrane lipid,cholesterol has numerous biological functions.Many human diseases such as cardiovascular diseases are correlated with enzyme defects,which may lead to the abnormal regulation of cholesterol biosynthesis and the accumulation of its intermediate products[1–3].Mevalonate(MVA) pathway,a cholesterol biosynthetic pathway,is proceeded in the endoplasmic reticulum[4].HMGR is responsible for catalyzation of HMG-CoA to CoA and MVA by four-electron reductive deacylation [5].MVA equilibrates with MVAL under natural pH conditions. HMGR plays a key role in the regulation of sterol biosynthesis and cholesterol production,which makes it an attractive target in the in-depth development of hypolipidemic drugs.
Analytical methods for assay of HMGR activity,including radioisotope(RI)technique,high performance liquid chromatography(HPLC),and chromatography coupled mass spectrometry, have been reported.The primary RI method measures the radioactivity in[14C]mevalonic acid(MVA)or 5-[32P]phospho-MVA produced from labeled HMG-CoA,which is simple but requires the handling of radiolabeled materials[6–9].Although HPLC method[10]and gas chromatography mass spectrometry(GC–MS) [11–13]are used to overcome disadvantages of RI method,these methods also have cumbersome natures such as low sensitivity for HPLC,complicated sample derivatization steps and a long analytical process to eliminate interfering peaks for GC–MS[14–16]. Liquid chromatography–tandem mass spectrometry(LC–MS/MS) has been used more readily than GC–MS to analyze relatively polar compounds due to its high speci fi city,accuracy and multiplexing options[17–20],even for small molecular weight compounds like MVA or MVAL,scanning in multiple reaction monitoring(MRM) for speci fi c mass transitions[21–23].
Xuezhikang,extracted from red yeast rice,is a Chinese traditional medicine which is widely used in the treatment of cardiovascular diseases.Previous clinical studies show that it also has anti-in fl ammatory effects[24],and can improve endothelial function[25].The hypolipidemic mechanism of Xuezhikang is effi cient inhibition of HMGR by natural lovastatin which is the major component of it.Besides,Xuezhikang also contains the homologs of lovastatin,unsaturated fatty acids,fl avonoids,plant sterols and other bioactive substances[26].Although active ingredients of Xuezhikang are very important for clinical studies,currently,thereis no method of active assay that can be used in quality control of Xuezhikang production.Quanti fi cation of Xuezhikang requires a speci fi c and sensitive method which is suitable for the routine analysis of biological samples.Some quanti fi ed components of Xuezhikang rather than the quanti fi cation methods applied have been reported in the literature.The methods used include HPLC for Xuezhikang[27,28],as well as micellar electrokinetic capillary chromatography[29],nuclear magnetic resonance(NMR)evaluation[30],and chromatography coupled mass spectrometry technology to detect several components such as monacolins,pigments and citrinin in red yeast rice[31–34].However,no method was reported for quanti fi cation of total active ingredients of Xuezhikang by inhibition of HMG-CoA reductase in vitro with HPLC-tandem mass spectrometry(MS/MS).
With MVAL-D7as the internal standard,we studied a highthroughput and sensitive enzyme method to quantify the active ingredients of Xuezhikang.The validated method and enzyme reaction system in vitro were used for the fi rst time to analyze the active ingredients of Xuezhikang,and can be developed for the quality control of Xuezhikang to ensure its clinical ef fi cacy.
2.Materials and methods
2.1.Chemicals and reagents
HMG-CoA,HMGR,NADPH,MVAL,DL-dithiothreitol,formic acid (purity>96%),ammonium formate and ammonium hydroxide(28% NH3in H2O,purity>99.99%)were purchased from Sigma-Aldrich (St.Louis,MO,USA).The internalstandard used in the assay method was DL-mevalonolactone-4,4,5,5,6,6,6,-D7(purity>99%)from CDN Isotopes(Pointe-Claire,Quebec,Canada).Lovastatin acid(96%)was purchased from Toronto Research Chemicals(North York,Canada). Xuezhikang capsules were obtained from Peking University WBL Biotech Co.,Ltd.(Beijing,China).HPLC-grade methanol and acetonitrile were purchased from MERCK(Darmstadt,Germany).ISOLUTE ENV+(3 mL/100 mg)was purchased from Biotage,Inc.(Uppsala, Sweden).De-ionized water was further puri fi ed by a Milli-Q water purifying system(Millipore Corporation,Bedford,MA,USA).
2.2.Instrumentations and conditions
The system consists of an API 4000 mass spectrometer(Applied Biosystems Sciex,Ontario,Canada)with an electrospray ionization (ESI)probe and an Agilent RRLC series HPLC system(Agilent Technologies,Palo Alto,CA,USA).Chromatography was performed on a ZORBAX SB-C18(150 mm×4.6 mm,5μm)at the room temperature using an isocratic elution with ammonium formate (10 mM,pH 8.0)–acetonitrile(70:30,v/v).The sample injection volume was 30μL and the cycle time was 5 min per injection at a fl ow rate of 0.8 mL/min with split.The retention time of the analyte and the internal standard was 1.46 min.
Multiple reaction monitoring(MRM)at unit resolution was employed to monitor the transitions of the protonated forms of MVAL at m/z 147.0→59.1 and MVAL-D7at m/z 154.0→59.1 in the negative ion mode.Optimized MS conditions were described as follows:curtain gas,gas 1 and gas 2(all nitrogen)with 20,45 and 45 units,respectively;dwell time with 100 ms;ion spray voltage with-4500 V;source temperature with 500°C;declustering potentials with-40 eV;collision energies with-18 eV.
2.3.Preparation of stock and working solutions,standards and quality control samples
The stock solutions of MVAL and lovastatin acid were prepared according to dissolving reference standards.These stock solutions were then used to prepare the working solutions of MVAL and lovastatin acid in acetonitrile:a mixture of 10 mM of ammonium formate and HPLC grade acetonitrile(70:30,v/v)by appropriate dilution.Calibration curve standards consisting of eight concentration levels were prepared in a concentration range of 0.005–1.000μg/mL for MVAL and 0.010–0.500μg/mL for lovastatin acid.Similarly, quality control(QC)standards were prepared for the lower limit of quanti fi cation(LLOQ QC),low quality control(LQC),medium quality control(MQC)and high quality control(HQC)for the analytes within the calibration curve range.The calibration curve standards and QC samples were stored at 2–8°C for method validation.All the stock solutions were stored at-35°C for further use.
2.4.Preparation of Xuezhikang samples
Six capsules of Xuezhikang were taken from two batches,respectively,and the capsule contents of each batch were mixed and milled.The content(0.3 g)was accurately weighed,and dissolved with 75%ethanol for ultrasound treatment about 10 min.The supernatant was obtained by centrifuging at 2000 r/min for 5 min. The transformed Xuezhikang solutions were diluted to 25.0 and 75.0 ng/mL after adding 0.5 M of potassium hydroxide solution, which transformed lovastatin into lovastatin acid in Xuezhikang as sample solutions.
2.5.HMG-CoA reductase reaction in vitro
The preparation of enzyme activity assay was conducted according to the study[7]with some modi fi cations using HMG-CoA reductase(073M4047V 4.70 U/mL)prepared by diluting 10μL of puri fi ed human HMG-CoA reductase to a fi nal volume of 1.6 mL with enzyme reaction buffer.The HMG-CoA(0.025μM)was diluted with enzyme reaction buffer which contains potassium phosphate buffer(100 mM,pH 7.4),EDTA(0.1 mM),DTT(5 mM), and NADPH(0.016μM),achieving a fi nal reaction volume of 140μL.Samples were incubated for 50 min at 37°C,whereupon the reaction was terminated by the addition of HCl(20μL,5 M)for 15 min at 37°C.A sample(2.14 mL)containing water(1 mL),HCl (0.9 mL,0.1 M),MVAL-D7(100μL)and reaction solutions(140μL) was added to each tube in order.The solutions were vortexed for 20 s and stored at room temperature for at least 10 min to ensure the lactonization of biosynthetic MVA.
2.6.Extraction procedure
For the extraction of MVAL and IS(MVAL-D7),we employed the solid-phase extraction(SPE),in which solid-phase extraction cartridges(3 mL/100 mg)were conditioned with 1 mL of water,2 mL of methanol–water(50:50,v/v)and 3 mL of methanol.Methanol was washed by 1 mL of 0.1 M HCl,and the samples(see sample preparation)were loaded onto the solid phase carriers under low vacuum condition.Then cartridges were washed by 1 mL of 0.1 M HCl and 1 mL of water,and dried naturally.Cartridges were eluted with 0.5 mL of methanol for 5 times.The combined elutes were evaporated to dryness at 40°C in nitrogen evaporator,and the dry residues were reconstituted in 200μL of 0.2%ammonium hydroxide,followed by a mixture of 10 mM of ammonium formate and HPLC grade acetonitrile(70:30,v/v)in a vortex mixer for 20 s.The reconstituted samples were transferred into auto-sampler vials,and 30μL of sample was injected into the LC–MS/MS system for analysis.
2.7.Method validation for MVAL and reaction system
The full method validation was performed according to the guideline for the validation of bio-analytical methods set by the Food and Drug Administration(FDA)[35].The validation wasperformed to evaluate the method in terms of speci fi city,LLOQ, linearity,precision,accuracy,matrix effect,extraction recovery, and stability.
Speci fi city was evaluated by the analysis of enzyme reaction system in six different HMG-CoA reductase sources.Three precision and accuracy batches of calibration curve standard at eight different concentration levels ranging from 0.005 to 1.000μg/mL for spiked MVAL and 0.010 to 0.500μg/mL for lovastatin acid were prepared and analyzed.Weighted(w=1/x2)linear regression was used to construct the calibration curves of spiked MVAL and lovastatin acid.The accuracy and precision of intra-and inter-batch assay was evaluated by analyzing six replicates of QC samples at four concentration levels(0.005,0.010,0.080,and 0.800μg/mL for MVAL,and 0.010,0.025,0.050,and 0.100μg/mL for lovastatin acid) through three different analytical batches.Recovery of MVAL was evaluated by comparing the peak areas response of the extracted samples with that of the unextracted stock solutions at the three QC concentration levels of 0.010,0.080 and 0.800μg/mL of MVAL. Matrix effect of MVAL was analyzed by six replicates of QC samples.The matrix factor(MF)of each analyte or IS was obtained by calculating peak area ratio of post-extracted spiked samples with peak area of neat solutions.Extraction recovery was determined by comparing peak areas of extracted QC samples with those of the post-extracted spiked samples at corresponding concentrations.The stability of MVAL was performed at three QC levels,each with six replications.The stability results were evaluated with the freshly spiked calibration curve(CC)and QC samples,and the percentage concentration deviation was calculated.
2.8.Method application
20μL of each concentration of Xuezhikang samples and lovastatin acid working solutions were respectively added into the optimized HMG-CoA reductase reaction system to inhibit HMGR, which led to the reduction of MVAL compared with the amount of that when no inhibitor was added into the HMGR reaction(negative control).The inhibition rate to HMGR was calculated as follows:

The amount of lovastatin acid and other HMGR inhibitors in Xuezhikang samples was calculated as lovastatin acid equivalents obtained from a calibration curve constructed from the plot of inhibition rate of HMGR versus the concentration of lovastatin acid.Concentrations were calculated using logistic dose response in pharmacology/chemistry in software Origin 8.0:

where y is the inhibition rate to HMGR,X is the concentration of lovastatin acid(ng/mL),A1,A2,X0and P are parameters originated from software.Concentration of HMGR inhibitors in Xuezhikang was calculated by inhibition rate of Xuezhikang in the formula.
3.Results and discussion
3.1.Optimization of enzyme reaction
Enzyme reaction was observed under some test conditions.The results revealed that 1.5μg of HMGR,20 nM of NADPH and 50 min of reaction time were the optimized enzyme reaction conditions.
Enzyme reaction was quickly performed on ice to avoid HMGR protein degradation.Factors such as the amount of enzyme and NADPH,pre-incubation time and incubation time could affect the enzyme reaction,which was expressed as the transformation effi ciency of substrate in spiked samples against a calibration curve. Therefore,we studied the enzyme reaction under different conditions in order to obtain the optimized reaction conditions.
Various amount of HMG-CoA reductase(0.25,0.50,0.75,1.00, 1.50,1.75,and 2.00μg)were respectively added into the inactivated reaction mixture composed of sodium phosphate buffer(200 mM, pH 7.4)and DL-dithiothreitol(DTT)(10 mM).NADPH with a relatively higher concentration may reduce the test sensitivity,as the nicotinamide adenine dinucleotide in NADPH exerts a great effect on ion suppression[36].Therefore,NADPH with different concentrations(2.5,5.0,7.5,10.0,12.5,15.0,17.5,and 20.0 nM)was tested in the enzyme assay so as to choose an appropriate amount which has no impact on enzyme activity measurement but meets the need of the substrate.As the pre-incubation time determines the binding capacity between inhibitor and enzyme,HMGR was pre-incubated for several intervals of5,10,15,20,25,and 30 min at 37°C.According to the enzyme activity,2.5 nM of HMG-CoA was added in the reaction system to trigger the reaction.The previous studies show that the amount of reaction product is closely related to the amount of enzyme[37].The complete reaction mixture(with a fi nal volume of 140μL)was incubated at 37°C and stopped at fi xed intervals(15,30, 45,60,75 and 90 min)by 20μL of 5 MHCl.With the lactonization of the reaction with HCl,0.1 M HCl was added to promote the production of MVAL.
Fig.1 shows,according to the signi fi cant variation of transformation ef fi ciency,the amount of HMGR and NADPH,and the reaction time exert greater impacts on HMG-CoA reductase reaction. However,pre-incubation time has little effect on enzyme reaction.
3.2.Method validation
3.2.1.Selectivity and linearity
Interfering peaks were not observed at the retention time of MVAL and MVAL-D7.The blank sample(without IS and MVAL), MVAL standard solution(0.010μg/mL)and sample generated from the enzyme reaction were injected under the established LC–MS/ MS conditions,respectively.Their representative chromatograms are shown in Fig.2.
The calibration curve of MVAL and the inhibition rate curve of Lovastatin acid were both reliably reproducible over the standard concentrations.Good correlation was obtained within a range of 0.005–1.000μg/mL for MVAL(r=0.9968)and 0.010–0.500μg/mL for lovastatin acid(r=0.9980).
3.2.2.Precision and accuracy
The results of precision and accuracy are shown in Table 1.The intra-day precision(RSD)was less than 2.39%and 3.89%for MVAL and lovastatin acid at each QC level,respectively,and the inter-day precision(RSD)was less than 3.38%and 5.10%.The accuracy(RE) was within 101.63%of the target values.The above values were within the acceptable range and indicated that the developed method and reaction system were accurate and precise.
3.2.3.Matrix effect
The matrix effect was minimal based on peak areas ratios of MVAL and MVAL-D7in post-extraction samples(low,medium and high QC)with peak area of the corresponding neat solutions.Table 2 shows that these ratios at three QC concentration levels were 83.83%,87.83%and 93.68%with RSD of 1.92%,5.65%and 1.88%, respectively;and the mean ratio of MVAL-D7was 87.02%with a RSD of 4.02%.The percentage coef fi cient of variance of both the analytes and internal standard was within the acceptance.
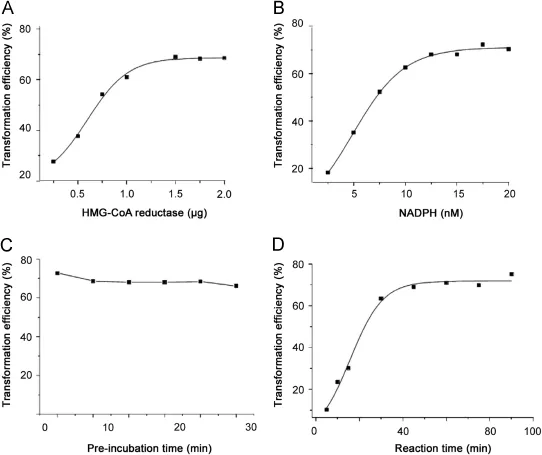
Fig.1.Relation of HMG CoA reductase activity(substrate transformation)and the amountof HMG-CoA reductase(A),NADPH(B),pre-incubation time(C)and reaction time(D).

Fig.2.Representative MRM chromatograms of MVAL in enzyme reaction matrix.(A)blank matrix;(B)blank matrix spiked with 0.010μg/mL of MVAL(left)and MVAL-D7(right);(C)realmatrix sample generated by enzyme reaction(left)and MVAL-D7(right).
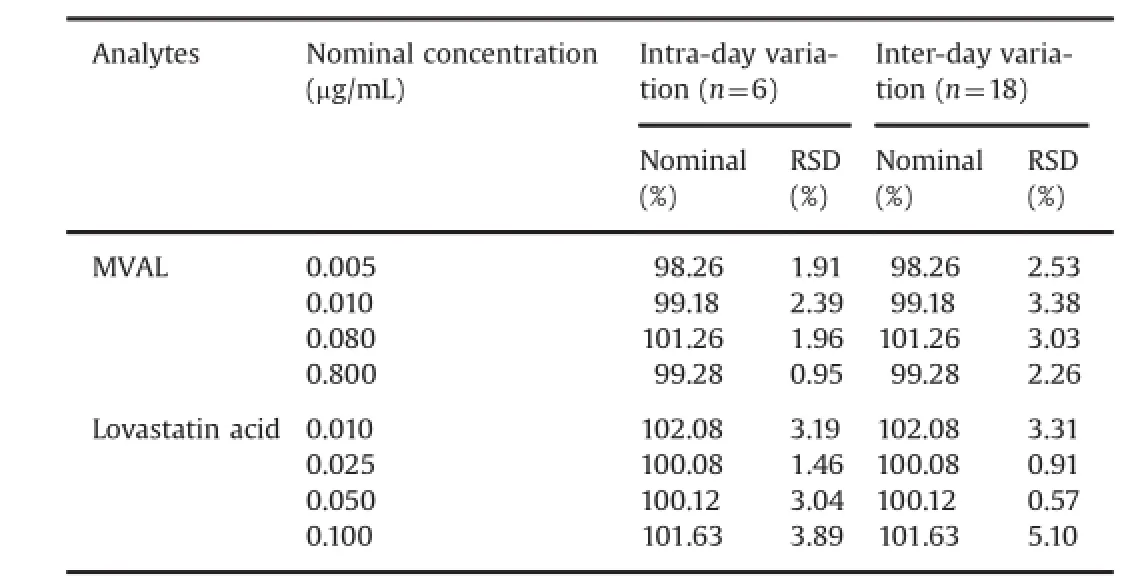
Table 1 Intra-day and inter-day accuracy and precision of the method for MVAL and lovastatin acid.
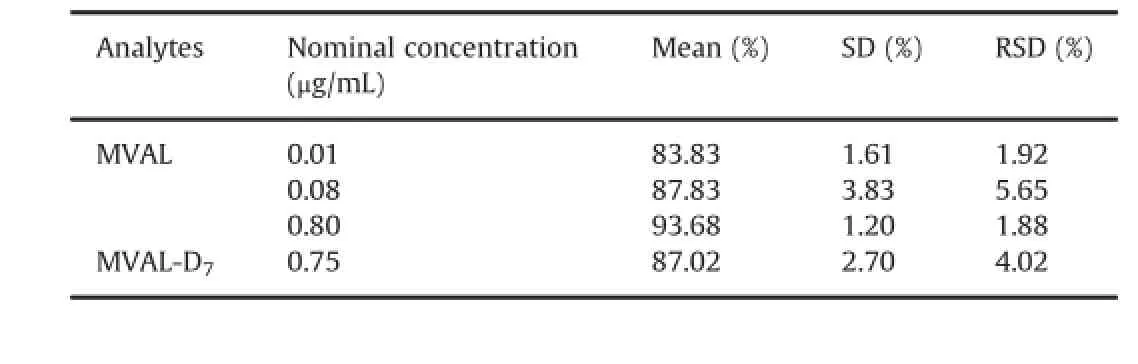
Table 2 Matrix effect evaluation for MVAL and MVAL-D7.
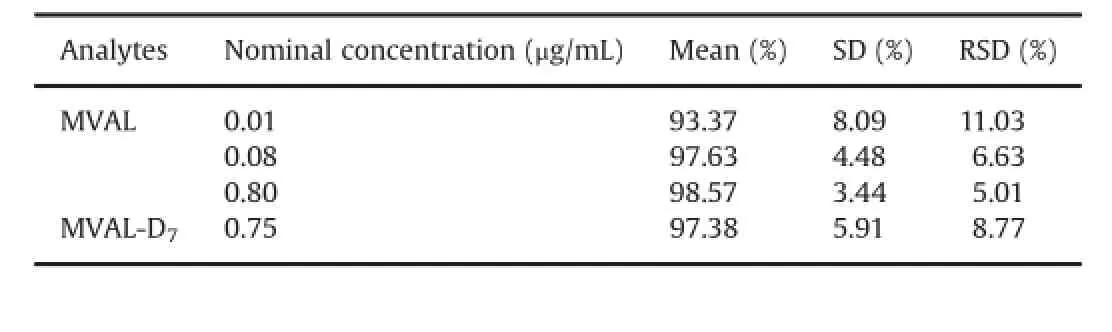
Table 3 Extraction recovery evaluation for MVAL and MVAL-D7.
3.2.4.Extraction recovery
The extraction recovery of MVAL from the low,medium and high QC samples ranged from 93.37%to 98.57%with a maximum RSD of 11.03%.The extraction recovery of internal standards was 97.38%with a RSD of 8.77%.The results in Table 3 revealed that the SPE approach employed in the present work provided reproducible recoveries for MVAL and internal standard.
3.2.5.Stability
The stability studies of MVAL were performed at three QC concentration levels(low,medium and high)in six replicates (n=6).The stability of MVAL was evaluated in storage at room temperature for 4 h,as well as after three freeze/thaw cycles and processed samples stored at the autosampler temperature(25°C) for 24 h.The long-term stability was also evaluated after the storage of QC samples at-35°C for 122 days.The results of all stabilities are given in Table 4.
笔者针对事故车辆类型、事故发生时间段、肇事人年龄段、性别、是否酒驾、是否持驾照、是否本人车辆、肇事时间和肇事次数等指标对事故中的车和人进行详细分析,从事故发生根源入手,分析事故发生原因,从而为预防事故的措施出台提供可靠理论基础。
3.3.Method application
The developed and validated method was used for the determination of total lovastatin acid obtained from Xuezhikang capsules.The prepared Xuezhikang sample solutions were added into the HMG-CoA reductase reaction system in vitro.Lovastatinacid standard curve was established to evaluate the active ingredient.Table 5 shows that the contents of active ingredients in Xuezhikang capsules which were back-calculated by inhibition curves of lovastatin acid were tested to be 12.2 and 14.5 mg/g, overtopping the sum of lovastatin and lovastatin acid in the same batch of Xuezhikang detected directly by HPLC(12.1 and 12.5 mg/ g).The results indicated that there may be other ingredients which also play the role in inhibiting HMG-CoA reductase in Xuezhikang.
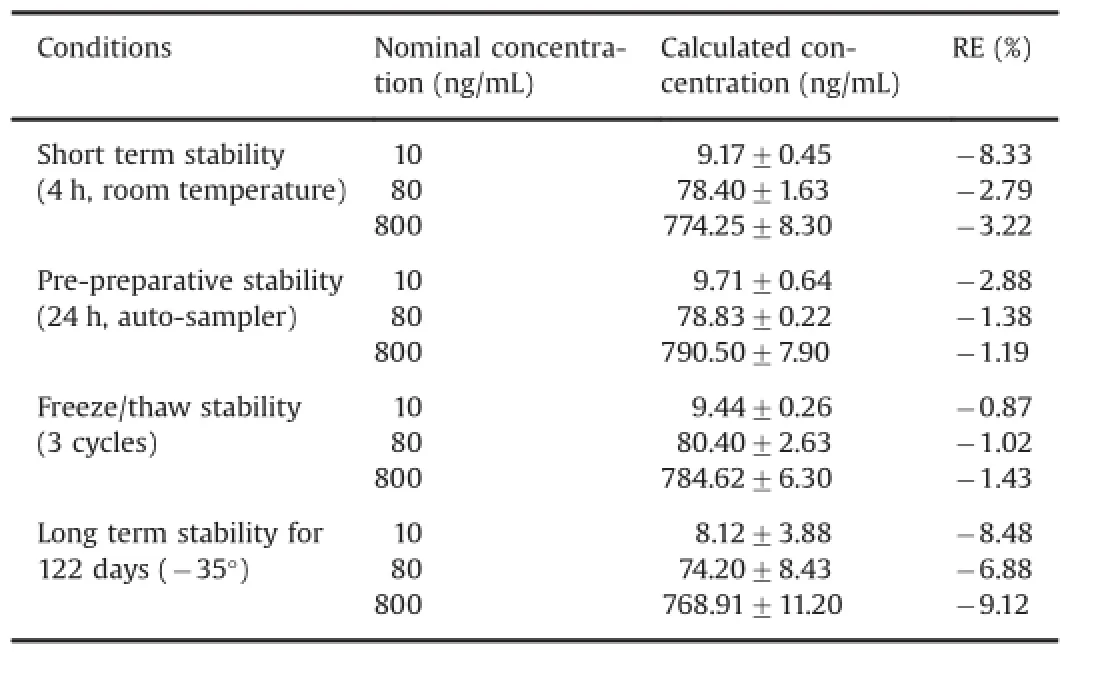
Table 4 Stability data of MVAL in processed QC samples for different stability activities in different conditions(n=6).
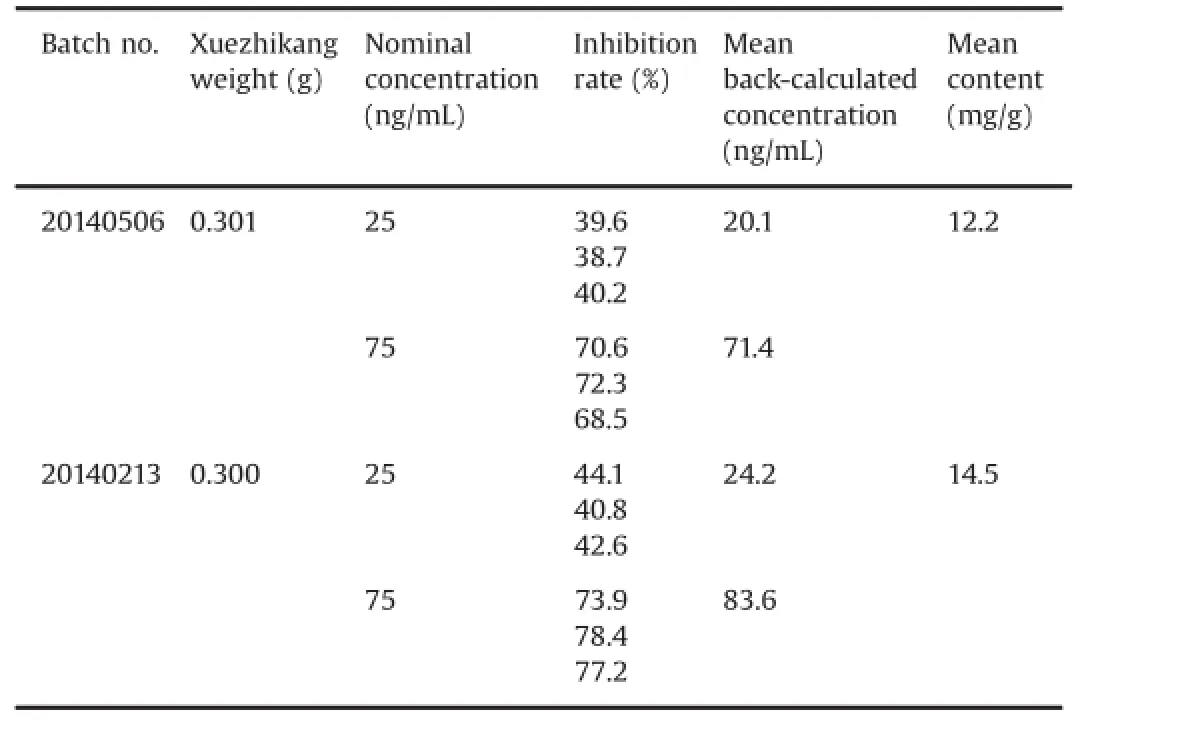
Table 5 The content of active ingredients in Xuezhikang calculated from lovastatin acid standard curve.
4.Conclusion
We developed and validated a method for the quantitation of Xuezhikang with enzyme reaction in vitro,and acquired some innovative points.Firstly,we optimized the HMG-CoA reductase reaction which converts HMG-CoA to CoA and MVA using an LC–MS/MS method for detection;secondly,we investigated the stability of the HMG-CoA reductase reaction system which was reproducible;thirdly,the observed method was successfully used in quantitation of the active ingredients of Xuezhikang capsules for the fi rst time.The method is particularly valuable for the investigation of active ingredients,and can be applied to the quality process control of Xuezhikang.
Acknowledgments
This work was supported by the National Basic Research Program of China(973 Program)(No.2012CB724003).We also would like to thank Taishan Scholar Projects for their kindly supports in the method development.
References
[1]R.Zetterström,The 1964 Nobel Prize for the discovery of the biosynthesis of cholesterol,Acta Paediatr.98(2009)1223–1227.
[3]F.D.Porter,G.E.Herman,Malformation syndromes caused by disorders of cholesterol synthesis,J.Lipid Res.52(2011)6–34.
[4]E.S.Istvan,M.Palnitkar,S.K.Buchanan,et al.,Crystalstructure of the catalytic portion ofhuman HMG-CoA reductase:insights into regulation of activity and catalysis,EMBO J.19(2000)819–830.
[5]E.S.Istvan,J.Deisenhofer,Structural mechanism for statin inhibition of HMGCoA reductase,Science 292(2001)1160–1164.
[6]S.Goldfarb,H.C.Pitot,Improved assay of 3-hydroxy-3-methylglutaryl coenzyme A reductase,J.Lipid Res.12(1971)512–515.
[7]S.Shefer,S.Hauser,V.Lapar,et al.,HMG CoA reductase of intestinal mucosa and liver of the rat,J.Lipid Res.13(1972)402–412.
[8]G.Nicolau,S.Shefer,G.Salen,et al.,Determination of hepatic 3-hydroxy-3-methylglutaryl CoA reductase activity in man,J.Lipid Res.15(1974)94–98.
[9]G.Popjak,G.Boehm,T.S.Parker,et al.,Determination of mevalonate in blood plasma in man and rat,J.Lipid Res.20(1979)716–728.
[10]M.Mozzicafreddo,M.Cuccioloni,A.M.Eleuteri,et al.,Rapid reverse phase-HPLC assay of HMG-CoA reductase activity,J.Lipid Res.51(2010)2460–2463.
[11]A.Honda,J.Shoda,N.Tanaka,et al.,Simultaneous assay of the activities of two key enzymes in cholesterol metabolism by gas chromatography-mass spectrometry,J.Chromatogr.565(1991)53–66.
[12]M.Axelson,O.Larsson,J.Zhang,et al.,Structuralspeci fi city in the suppression of HMG-CoA reductase in human fi broblasts by intermediates in bile acid biosynthesis,J.Lipid Res.36(1995)290–298.
[13]B.H.Woollen,P.C.Holme,W.J.Northway,et al.,Determination of mevalonic acid in human urine as mevalonic acid lactone by gas chromatography-mass spectrometry,J.Chromatogr.B 760(2001)179–184.
[14]L.Li,J.Sun,Y.X.Sun,et al.,LC–ESI–MS determination of lovastatin in human plasma,Chromatographia 67(2008)621–625.
[15]M.J.Morris,J.D.Gilbert,Y.K.Hsieh,Determination ofthe HMG–CoA reductase inhibitors simvastatin,lovastatin,and pravastatin in plasma by gas chromatography/chemical ionization mass spectrometry,Biol.Mass Spectrom.22 (1993)1–8.
[16]T.Takano,S.Abe,S.Hata,A selected ion monitoring method for quantifying simvastatin and its acid form in human plasma,using the ferroceneboronate derivative,Biol.Mass Spectrom.19(1990)577–581.
[17]M.Y.Ndong-Akoume,D.Mignault,S.Perwaiz,et al.,Simultaneous evaluation of HMG-CoA reductase and cholesterol 7a-hydroxylase activities by electrospray tandem MS,Lipids 37(2002)1101–1107.
[18]M.Abrar,P.D.Martin,Validation and application of an assay for the determination of mevalonic acid in human plasma by liquid chromatography tandem mass spectrometry,J.Chromatogr.B 773(2002)103–111.
[19]M.Jemal,A.Schuster,D.B.Whigan,Liquid chromatography/tandem mass spectrometry methods for quantitation of mevalonic acid in human plasma and urine:method validation,demonstration ofusing a surrogate analyte,and demonstration ofunacceptable matrix effect in spite of use of a stable isotope analog internal standard,Rapid Commun.Mass Spectrom.17(2003) 1723–1734.
[20]G.S.Saini,T.A.Wani,A.Gautam,et al.,Validation of LC–MS/MS method for quanti fi cation of mevalonic acid in human plasma and an approach to differentiate recovery and matrix effect,J.Lipid Res.47(2006)2340–2345.
[21]T.A.Wani,A.Samad,M.Tandon,et al.,The effects of rosuvastatin on the serum cortisol,serum lipid,and serum mevalonic acid levels in the healthy Indian male population,AAPS PharmSciTech 11(2010)425–432.
[22]E.Kindt,G.Szekely-Klepser,S.T.Fountain,The validation of a simple LC–MS/ MS method for determining the levelof mevalonic acid in human plasma, Biomed.Chromatogr.25(2011)323–329.
[23]J.Waldron,C.Webster,Liquid chromatography-tandem mass spectrometry method for the measurement of serum mevalonic acid:a novel marker of hydroxymethylglutaryl coenzyme A reductase inhibition by statins,Ann.Clin. Biochem.48(2011)223–232.
[24]J.J.Li,S.S.Hu,C.H.Fang,et al.,Effects of xuezhikang,an extract of cholestin,on lipid pro fi le and C-reactive protein:a short-term time course study in patients with stable angina,Clin.Chim.Acta 352(2005)217–224.
[25]Z.L.Lu,W.R.Kou,B.M.Du,et al.,Effect of Xuezhikang,an extract from red yeast Chinese rice,on coronary events in a Chinese population with previous myocardial infarction,Am.J.Cardiol.101(2008)1689–1693.
[26]J.Y.Ma,Y.G.Li,Q.Ye,et al.,Constituents ofred yeast rice,a traditional Chinese food and medicine,J.Agric.Food Chem.48(2000)5220–5225.
[27]Z.B.Huang,Y.Xu,H.Zhang,et al.,Simultaneous determination of two Monascus metabolites in red yeastrice by HPLC using fl uorescence detection,Food Chem.127(2011)1837–1841.
[28]B.Nigović,M.Završki,M.Sertić,et al.,Simple and fast voltammetric method for assaying monacolin K in red yeast rice formulated products,Food Anal. Methods 8(2015)180–188.
[29]B.Nigović,M.Sertić,A.Mornar,Simultaneous determination oflovastatin and citrinin in red yeast rice supplements by micellar electrokinetic capillary chromatography,Food Chem.138(2013)531–538.
[30]D.W.Lachenmeier,Y.B.Monakhova,T.Kuballa,et al.,NMR evaluation of total statin content and HMG-CoA reductase inhibition in red yeast rice(Monascus spp.)food supplements,Chin.Med.7(2012)8–13.
[31]M.Sertić,A.Mornar,B.Nigović,A rapid pro fi ling of hypolipidemic agents in dietary supplements by direct injection tandem mass spectrometry,J.Food Compos.Anal.34(2014)68–74.
[32]B.Avula,P.A.Cohen,Y.H.Wang,et al.,Chemical pro fi ling and quanti fi cation of monacolins and citrinin in red yeast rice commercialraw materials and dietary supplements using liquid chromatography-accurate QToF mass spectrometry:Chemometrics application,J.Pharm.Biomed.Anal.100(2014) 243–253.
[33]A.Mornar,M.Sertić,B.Nigović,Development of a rapid LC/DAD/FLD/MSnmethod for the simultaneous determination ofmonacolins and citrinin in red fermented rice products,J.Agric.Food Chem.61(2013)1072–1080.
[34]Y.G.Li,F.Z.,Z.T.Wang,et al.,Identi fi cation and chemical pro fi ling of monacolins in red yeast rice using high-performance liquid chromatography with photodiode array detector and mass spectrometry,J.Pharm.Biomed.Anal.35 (2004)1101–1112.
[35]Guidance for Industry,Bioanalytical Method Validation U.S.Department of Health,Human Services,Food and Drug Administration,Center for Drug Evaluation and Research(CDER),Center for Veterinary Medicine(CVM),BP, 2001.〈http://www.fda.gov/cder/guidance/4252fnl.htm〉.
[36]K.Patricia,K.Joachim,F.Isabel,et al.,Measurement of HMG-CoA reductase activity in different human cell lines by ultra-performance liquid chromatography tandem mass spectrometry,Biochem.Biophys.Res.Commun.443 (2014)641–645.
[37]B.Michele,P.Raffaella,G.Chiara,et al.,Novel and simple high-performance liquid chromatographic method for determination of 3-hydroxy-3-methylglutarylcoenzyme A reductase activity,J.Chromatogr.B 819(2005)307–313.
27 February 2015
in revised form
☆Peer review under responsibility of Xi'an Jiaotong University.
at:Schoolof Pharmacy,Yantai University,Yantai264005, China.
E-mail address:wanhuiliu@ytu.edu.cn(W.-H.Liu).1Authors contributed equally to this work.
猜你喜欢
杂志排行
Journal of Pharmaceutical Analysis的其它文章
- Application of analytical instruments in pharmaceutical analysis
- Comparative study of adsorptive role of carbonaceous materials in removal of UV-active impurities of paclitaxel extracts☆
- In vitro–in vivo studies of the quantitative effect of calcium, multivitamins and milk on single dose cipro fl oxacin bioavailability☆
- Antimicrobial and antiproliferative prospective of kosinostatin–a secondary metabolite isolated from Streptomyces sp.☆
- Quanti fi cation of tolvaptan in rabbit plasma by LC–MS/MS:Application to a pharmacokinetic study☆
- Identi fi cation,synthesis and characterization of process related des fl uoro impurity of ezetimibe and HPLC method validations☆
