Antimicrobial and antiproliferative prospective of kosinostatin–a secondary metabolite isolated from Streptomyces sp.☆
2015-12-21VinygmRmuSurmniynSuSuurmniynVijykumr
Vinygm Rmu,Surmniyn Su,Suurmniyn Vijykumr,*
aP.G.and Research Department of Botany and Microbiology,A.V.V.M.Sri Pushpam College(Autonomous),Poondi,Thanjavur,Tamil Nadu,IndiabK.K Biotec Lab Service,Chennai,Tamil Nadu,India
Original Article
Antimicrobial and antiproliferative prospective of kosinostatin–a secondary metabolite isolated from Streptomyces sp.☆
Vinayagam Rambabua,Subramaniyan Subab,Suburamaniyan Vijayakumara,*
aP.G.and Research Department of Botany and Microbiology,A.V.V.M.Sri Pushpam College(Autonomous),Poondi,Thanjavur,Tamil Nadu,IndiabK.K Biotec Lab Service,Chennai,Tamil Nadu,India
A R T I c L E I N F o
Article history:
17 November 2014
Accepted 20 November 2014
Available online 29 November 2014
Kosinostatin Secondary metabolite Streptomyces sp. Cytotoxic activity MCF-7
Cancer is a communal health hazard worldwide.The present investigation attempts to evaluate antimicrobial and anticancer potential of kosinostatin on mammary carcinoma cell line(MCF-7).The anticancer and antiproliferative activities of kosinostatin were analyzed on MCF cell line by MTT assay and cytotoxicity assays like lactate dehydrogenase(LDH)and glutathione(GSH).The secondary metabolite kosinostatin exhibited its apoptotic nature by expressing p53 protein.Collectively,the results acquired from this study promise that kosinostatin shows the potent anticancer activity.
©2015 Xi'an Jiaotong University.Production and hosting by Elsevier B.V.All rights reserved.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/3.0/).
1.Introduction
Naturalproducts are inspiration for a signi fi cant quantity of the new small-molecule chemical entities established as drugs.Microbial natural products are an important source of both existing and new drugs[1].Investigation into prokaryotic and eukaryotic organisms produced natural products with pharmacological and medicinal possessions shows the way for clinical consequence.
Antibiotics and analogous natural products,being secondary metabolites,have been regarded as imperative resources that could produce potential chemotherapeutic agents[2].The convenient signi fi cance of antibiotics and other secondary metabolites is remarkable in that they are widely used in the human therapies including antibacterial,antifungal,antiprotozoal,antinematode,anticancer,antiviral and anti-in fl ammatory activities[3].A fi lamentous,non-motile Gram-positive bacterium,Actinomycetes,is known to produce secondary metabolite which has valuable clinical traits[4,5].The species belonging to the genus Streptomyces are known to produce a greater number of discovered bioactive secondary metabolites acting as antibiotics,antiviral drugs,herbicides, insecticides,antitumor agents and immunomodulators agents[6,7].
Cancer is a class of disease in which a group of cells display the traits of uncontrolled proliferation of cells.Nearly all cancers are caused by abnormalities in the genetic materialof the transformed cells[8].Breast cancer is the primary cause of cancer-related death in women,and one in 10 of all new cancers is diagnosed worldwide each year[9].The progress of breast cancer is coupled with modi fi cation of the fragile equilibrium between cell propagation and apoptotic cell death,cellular redox status,deregulation of cellular differentiation and endocrine derangement[10,11].
Natural products are still important sources to discover new anticancer agents,mainly secondary metabolites,produced by organisms in response to externalstimuli such as nutritionalchanges, infection and competition.Natural products produced by plants, fungi,bacteria,insects and animals have been isolated as biologically active pharmacophores.Kosinostatin,a quinocycline antibiotic (Fig.1),was isolated from the culture fi ltrate of Streptomyces violaceusniger strain HAL64.Kosinostatin inhibits the growth of Grampositive bacteria strongly and Gram-negative bacteria and yeasts moderately.It shows cytotoxicity against various cancer cell lines and inhibits human DNA topoisomerase Ila[12].The present investigation signi fi es antimicrobial and anticancer potential of kosinostatin against mammary carcinoma cell line(MCF-7).
2.Materials and methods
2.1.Drug and materials
Dimethyl sulfoxide(DMSO)was purchased from Med Koo Biosciences,Inc.,Chapel Hill,North Carolina,USA.RPMI-1640,DMEM and sodium pyruvate were purchased from Biochrom,Berlin,Germany.Kosinostatin and ethidium bromide were purchased from Sigma-Aldrich,Bangalore,India.Human mammary carcinoma cell line(MCF-7)was obtained from National Center for Cell Sciences(NCCS),Pune,India.Penicillin–streptomycin and fetal bovine serum were purchased from Gibco,Germany.Trypsin–Ethylene diamine tetra acetic acid(EDTA)was obtained from Hi-media Laboratories,Mumbai,India.Cell culture plates and dishes were purchased from TPP,Trasadingen,Canton,Switzerland.Rabbit antimouse immunoglobulin(IgG)was purchased from Genei,Bangalore,India.Nitrocellulose membrane and polyvinylidene di fl uoride (PVDF)membrane were purchased from Millipore Co.,MA,USA. Primary antibodies(Ab's)p53 were purchased from Abcam,Cambridge,USA.All other chemicals including solvents were of highest purity and of analytical grade marketed by Glaxo Laboratories and SISCO Research Laboratories(SRL),Mumbai,India.

Fig.1.The chemical structure of kosinostatin(Quinocycline B).
2.2.Instrumentation
The MTT assay was done using ELx800 Absorbance Microplate Reader,BioTek Instruments,India.Lactate dehydrogenase(LDH) assay was determined using Biochemical Analysis System Mindray BS-420,Shanghai,China.Estimation of GSH was done using a TOC analyzer(TOC-VCPN 5000A,Shimadzu,Japan).
2.3.Drug preparation
Kosinostatin was dissolved in DMSO(the fi nal concentration of DMSO was not exceeded 0.1%(v/v)and did not affect the cell survival),prepared in serum-free Roswell Park Memorial Institute (RPMI)medium,fi ltered by a 0.045 mm syringe fi lter and stored at 4°C.
2.4.Agar well diffusion and determination of minimum inhibitory concentration(MIC)
Bacterial broth culture was prepared to a density of 108cells/mL and the aliquot was spread evenly by L rod onto Muller Hinton agar.Then,the plated medium was allowed to dry at room temperature for 30 min.Kosinostatin(5μM and 10μM) was aseptically introduced into an agar well and the plates were incubated at 37°C for 24 h.The formation of clear inhibition zone on the Muller Hinton agar was measured.The experiment was performed in triplicate.
2.5.Human MCF-7 maintenance and treatment
Human MCF-7 cells were routinely grown as monolayer cultures at 37°C in a humidi fi ed atmosphere of 5%CO2and 95%O2in Dulbecco Modi fi ed Eagles Medium(DMEM)containing 10%(v/v) fetal bovine serum(FBS),penicillin(50 IU)and streptomycin (50μg/mL).Kosinostatin was dissolved in DMSO,prepared in DMEM,fi ltered by a 0.045 mm syringe fi lter and stored at 4°C for the cell culture protocols.
2.6.MTT cell viability assay
MCF-7 cells were seeded in 96 well microplate(1×104cells/ well in 180μL medium)and routinely cultured in a humidi fi ed incubator(37°C in 95%CO2)for 24 h.Kosinostatin(1,2,5 and 10μM)was added in a serial concentration and re-incubated for 24 h.MTT(3-(4,5 dimethyl thiozal-2-yl)-2,5-diphenyl tetrazolium bromide)assay was performed as described[13].The viability of the cells was assessed by MTT assay,which was based on the reduction of MTT by the mitochondrial dehydrogenase of intact cells to a purple formazan product.Then the medium was discarded and 30μL of tetrazolium dye(5 mg/mL in PBS)was added to every well and re-incubated for 4 h.After removing the un-transformed MTT reagent,100μL of DMSO was added to dissolve the formazan crystals formed.The amount of formazan was determined by measuring the absorbance at 570 nm using an ELISA reader.
The percentage of cell survival was calculated by the following formula:
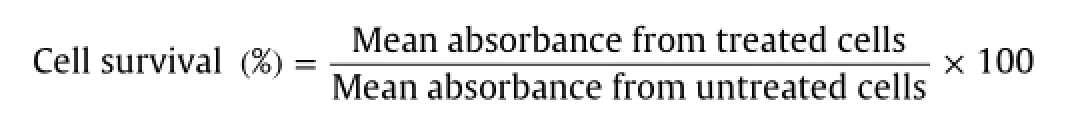
The cells were incubated at 37°C in a controlled humidi fi ed atmosphere of 5%CO2and 95%air for 48 h and the following experiments were carried out.
2.7.LDH leakage assay
LDH assay was performed by the method reported by Grivell et al.[14].LDH activity was expressed as moles of NADH used per minute per well.Based on the above studies,kosinostatin at the concentrations of 5 and 10μM was selected for further experiments.
2.8.Estimation of glutathione(GSH)
Total reduced glutathione was determined by the method reported by Moron et al.[15].1 mL of 5%TCA was added to human hepatoma cell line(1×106cells).The precipitate was removed by centrifugation.To an aliquot of the supernatant,2 mL of 5,5′-dithiobis-(2-nitrobenzoic acid)(DTNB)reagent was added to make a fi nalvolume of 3 mL.The absorbance was read at 412 nm against a blank containing TCA instead of sample.Aliquots of the standard solution were treated similarly.The amount of reduced glutathione was expressed asμg of GSH/mg protein.
2.9.Quanti fi cation of protein concentration
The total protein concentration in MCF-7 cells(controland allyl isothiocyanate(AITC)treated cells)was quanti fi ed by the method reported by Lowry et al.[16]using bovine serum albumin(BSA)as a standard.The protein concentration in cell lysate was expressed as mg protein.
2.10.Expression of p53 proteins by Western blotting analysis
MCF-7 cells(1×106/mL)were treated with the AITC at the concentrations of 2,5 and 10μMfor 48 h at 37°C.Cells were lysed with 10μL of lysis buffer.Cell proteins were separated in sodium dodecyl sulfate polyacrylamide gel elecrophorosis(SDS-PAGE).4% stacking gel and 10%resolving gel were used to separate the proteins.After electrophoresis,gelwas placed over a nitrocellulosemembrane,and separate blotting was done for each protein.The gel and the PVDF membrane were packed by three cut-pieces of Whatman fi lter paper(No.3).The arrangement was covered on both sides with absorbers(provided with the system)and clipped. The arrangement was immersed in a tank containing blotting buffer.A current of 25 mA was passed through overnight.
Then,the membranes were removed from the system and immersed in methanolfor a minute.The membranes were blocked by treating with the blocking buffer for 1 h at 37°C.After washing, the membranes were incubated with anti-mouse p53(1:100)and anti-mouse caspase-3(1:1000)for 6 h at 37°C.After three washes in phosphate buffered solution(PBS)/0.1%Tween 20,the membranes were incubated with Horse Raddish Peroxidase(HRP) conjugated anti-mouse IgG antibody for 1 h at 37°C.The protein bands appeared were then visualized and photographed.The band intensity for p53 was normalized with that of the internal control βactin.
3.Results
In the present study,the evaluation of antibacterial activity of kosinostatin was studied using agar well diffusion method.The results revealed variability in the inhibitory concentration of each concentration against pathogenic bacteria.Kosinostatin showed signi fi cant antimicrobial activity against Candida albicans,Micrococcus luteus and Saccharomyces cervicea,but it showed minimum zone of inhibition against Staphylococcus aureus and Bacillus subtilis when compared with the previously mentioned genus.Kosinostatin at a concentration of 10μM showed signi fi cant effect compared with that at 5μM.Thus,it revealed dose-dependent inhibitory pattern against the above mentioned microorganisms (Fig.2).
3.1.MTT cell viability assay
The result shown in Fig.3 is the cell viability of control and kosinostatin treated(1,2,3,5 and l0μM)MCF-7 cells.Kosinostatin noticeably inhibited the mammary cells after 24 h of treatment. The results showed that the treatment with kosinostatin markedly reduced the viability of MCF-7 cells in a dose-dependent manner.
3.2.Lactate dehydrogenase(LDH)leakage
The levels of LDH released into the medium of control and kosinostatin treated(5,10μM)MCF-7 cells are presented in Fig.4. From the result,it was observed that LDH activities signi fi cantly elevated(p<0.05)in the medium containing 10μM of kosinostatin when compared to the control.

Fig.2.Determination of minimum inhibitory concentration(MIC).

Fig.3.MTT cell viability assay on control and kosinostatin treated MCF-7 cell line.
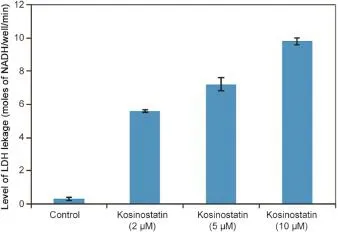
Fig.4.Level of LDH leakage in control and kosinostatin treated MCF-7 cell lines.
3.3.GSH level
It is well known that the toxicity of anti-proliferating drugs may basically depend on the intracellular level of reduced GSH [17].GSH has an important activity in protecting cells and cellular components against oxidative damage as well as in detoxi fi cation. It is often found that GSH levels are increased in the drug resistant cancer cells when compared to the drug sensitive cells.Inhibition of GSH synthesis or modulation of GSH storages in tumors to reduce anticancer drugs resistance might contain a novel anticancer strategy[18].The levels of GSH content in controland kosinostatin treated MCF-7 cells were estimated.The result revealed signi fi cant (p<0.05)depletion of GSH in kosinostatin treated mammary cells at the concentrations of 5 and 10μM when compared to the control cells(Fig.5).
3.4.Expression of p53 proteins
The p53 gene was involved as a regulator in the process of apoptosis.After DNA damage in cell types,p53 can trigger the genetically altered cells to be eliminated by inducing apoptosis [19].In addition to its DNA damage response,p53 is also involved in the response by abnormal or stress conditions such as hypoxia and oxidative stress.The expression of p53 in control and kosinostatin treated(2,5 and 10μM)MCF-7 cells by Western blotting is presented in Fig.6.Treatment of kosinostatin on MCF-7 cells showed an increased band intensity of 53 kDa protein compared to the control.The accumulation of p53 protein indicated the expression of tumor suppressor protein induced apoptosis by kosinostatin on MCF-7 cells.This result strongly suggested that kosinostatin treatment stimulated the apoptosis.

Fig.5.Level of GSH in control and kosinostatin treated MCF-7 cells.
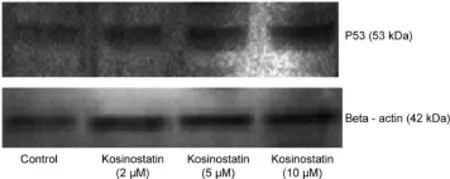
Fig.6.Western blotting analysis of controland kosinostatin treated MCF-7 cells.
4.Discussion
The result of MIC revealed that kosinostatin(5 and l0μM)was active against C.albicans,S.cervicea,M.luteus,S.aureus,and B. subtilis.
The reason for different sensitivities between fungus and bacteria could be ascribed to the morphological differences between these microorganisms,Gram-positive bacteria having only an outer peptidoglycan layer which is not an effective permeability barrier[20].The reason for different sensitivities between bacteria could be attributed to the morphological differences between these microorganisms[21].Kosinostatin might inhibit the fungi through interfering with germination and fi lamentous formation [22].From our result,it could be concluded that kosinostatin has a higher ability to inhibit fungi than inhibit bacteria in a dose-dependent manner.
The progress in drug resistance phenotype is accompanied by the changes in different biological features of malignant cells,including morphological ones.In vitro screening models provide preliminary data in selecting the drugs for clinical trials[23].The cytotoxic cells were grown under controlled conditions,outside of their natural environment.In this assay,cell death and cell viability were estimated[24].The antiproliferative activity and cytotoxicity of kosinostatin against MCF-7 cell lines were determined by MTT assay[25].This analysis revealed the anticancer potential of kosinostatin in MCF-7 cells.Kosinostatin at a concentration of 5μM shows a good evidence that it is cytotoxic to cancer cells.The kosinostatin treated group showed a decrease in viability of MCF-7.Streptomyces sp.secondary metabolites reduced the cell viability in a dose-dependent manner.The secondary metabolite reduced the cellviability up to 50%at the concentrations of 5 and 10μMon MCF-7 cells and the inhibition was in a dose-dependent manner, which signi fi es that kosinostatin has better antiproliferative activity towards mammary carcinoma cell line at minimum concentration.The various compounds such as vitamins,carotenoids, terpenoids,fl avonoids,polyphenols,alkaloids,tannins,saponins, enzymes and minerals might be responsible for the antiproliferative activity[26].
LDH activity was determined by the colorimetric method[27], and LDH leakage analysis was performed to con fi rm cytotoxicity of the drug[28].Results of LDH cytotoxicity assay revealed that the treatment of cancer cells with kosinostatin caused the increase of cytotoxicity.The LDH assay also revealed that the cytotoxicity was increased in the kosinostatin secondary metabolite treated group of both MCF-7 cells.Kosinostatin treated cells prove the cytotoxic nature of secondary metabolites by leakage of LDH.As the concentration of kosinostatin was elevated,the release of LDH also increased in a concentration-dependent manner[29].Since lactate release of tumor cells is thought to be related to the glycolytic shift of cells contributing to the tumor development,lactate release would be an indicator of glycolytic activity in cells[30].
GSH concentration was changed during the cell cycle[31]. Therefore,GSH-dependent enzymes were also subject to such regulation,particularly as they could contribute to the differences between the cell lines and also be a factor in their sensitivity to cytotoxic drugs.High intracellular GSH levels were associated with apoptotic resistance,and GSH depletion itself was found to trigger cell death cascades[32].The study showed that treatment with kosinostatin involved in the progression of cell death.It is likely that generation of intracellular reactive oxygen species(ROS)and depletion of GSH are related to the induction of mitochondriadependent cell death in breast cancer cells.Thus,these fi ndings supported the hypothesis that oxidative stress plays a role as a common mediator of cell death by treatment with Streptomyces secondary metabolite kosinostatin.
Even though p53 is expendable for customary growth,it plays an essential role in the cellular response to DNA damage from both endogenous and exogenous sources providing a defensive effect against tumorigenesis[33].p53 is activated in response to DNA damage and many factors interact to signal and modulate this response[34],and p53 serves as a key relay for signals elicited by cellular stresses arising from diverse environmentalor therapeutic insults.This relay then activates a cell cycle arrest or cell death program,depending on the stimulus and cell type.The absence of p53 function disables the cell death or arrest programmes,thereby allowing the emergence of variants with various types of genomic alterations[35].In human,the p53 tumor suppressor gene is located on the short arm of chromosome 17 and its protein product is a negative regulator of the cell cycle in the G1 phase.Mutations in the p53 gene are the most frequent alteration in many types of human malignancy,including lung,colon and breast cancers[36].
Apoptosis is a highly regulated process of programmed celldeath, and disruption of this process reveals a major contributing factor in the pathology of cancer[37].Apoptosis is mediated by the action of caspases,a group of cysteine proteases which can be activated through two pathways,extrinsic and intrinsic pathways[38].Another major player in apoptosis process is p53,which is activated when mammalian cells are subjected to stress conditions such as hypoxia,radiation,DNA damage or chemotherapeutic drugs[39].It was reported that the expression of mutant p53 protein in breast cancer seems to be related to poor prognosis associated with a high histological grade,epidermal growth factor receptor(EGFR)positivity,and Bcl-2 and estrogen receptor(ER)negativity[40].In this study, the p53 expression in MCF-7 cells treated with kosinostatin was investigated.Kosinostatin administration on mammary cancer cell line illustrates increased band intensity of 53 kDa protein.The accretion of p53 protein indicates the expression of tumor suppressor protein induced apoptosis by kosinostatin on MCF-7 cells,which displays that kosinostatin management stimulates the apoptosis.
5.Conclusion
In conclusion,outcome of the present investigation suggests that the bioactive secondary metabolite from Streptomyces sp.,kosinostatin,probably gives a stop to proliferation of MCF-7 cells by induction of apoptosis.It also holds an excellent antimicrobial activity against various microbial populations.It is concluded that kosinostatin has antimicrobial property and antiproliferative prospective against MCF-7 cells as a novel source for new anticancer drugs.
Acknowledgments
The authors are thankful to the management of A.V.V.M Sri Pushpam College(Autonomus),Poondi,for providing necessary facilities and support to carry out this work.
References
[1]R.Solanki,M.Khanna,R.Lal,Bioactive compounds from marine actinomycetes,Indian J.Microbiol.48(2008)410–431.
[2]J.Berdy,New trends in the research of bioactive microbialmetabolites in chemistry and biotechnology of biologically active natural products,in:Proceedings of the 4th International Conference,Budapest,1987,pp.269–291.
[3]Z.A.Bashir,A.Ahmad,S.M.Nor,etal.,Factors affecting bioactivity ofsecondary metabolites produced by Streptomyces sp.PT1 using plackett-burmandesign, Adv.Environ.Sci.Technol.6(2012)3043–3051.
[4]P.R.Anand,Isolation and characterization of oildegrading bacteria from oil contaminated soils of Vellore district Tamil Nadu India,J.Environ.Sci.Eng.52 (2010)113–116.
[5]P.Kampfer,The Family Streptomycetaceae,Part I:Taxonomy,the Prokaryotes: A Handbook on the Biology of Bacteria,Springer Berlin(2006),pp.538-604.
[6]J.Berdy,Bioactive microbial metabolites,J.Antibiot.58(2005)1–26.
[7]R.P.Maskey,E.Helmke,O.Kayser,et al.,Anti-cancer and antibacterial trioxacarcins with high anti-malaria activity from a marine Streptomycetes and their absolute stereochemistry,J.Antibiot.57(2004)771–779.
[8]K.Langeswaran,S.Perumal,S.Vijayaprakash,et al.,Therapeutic ef fi cacy of kaempferol against AFB1induced experimental hepatocarcinogenesis with reference to lipid peroxidation,antioxidants and biotransformation enzymes, Biomed.Prev.Nutr.2(2012)252–259.
[9]D.M.Parkin,L.M.Fernandez,Use of statistics to assess the global burden of breast cancer,Breast J.12(Suppl.1)(2006)S70–S80.
[10]M.R.Hussein,H.H.Ismael,Alterations of p53,Bcl-2,and hMSH2 protein expression in the normal breast,benign proliferative breast disease,in situ and in fi ltrating ductalbreast carcinomas in the Upper Egypt,Cancer Biol.Ther.3 (2004)983–988.
[11]R.Kumaraguruparan,D.Karunagaran,C.Balachandran,et al.,A comparative evaluation of heat shock-and apoptosis-associated proteins in mammary tumours,Clin.Chim.Acta 365(2006)168–176.
[12]M.Y.El-Naggar,Kosinostatin,a major secondary metabolite isolated from the culture fi ltrate of Streptomyces violaceusniger strain HAL64,J.Microbiol.45 (2007)262–267.
[13]V.Cardile,M.Renis,C.Seifo,et al.,Behavior of the new asbestos amphibole fl uorendenite in different lung cell systems,J.Biochem.Cell Biol.36(2004) 849–860.
[14]A.R.Grivell,M.N.Berry,The effects of phosphate and substrate-free incubation conditions on glycolysis in Ehrlich ascites tumor cells,Biochim.Biophys.Acta 1291(1994)83–88.
[15]M.S.Moron,J.W.Depierre,B.Mannervik,Levels of glutathione reductase and glutathione-S-transferase activities in rat lung and liver,Biochim.Biophys. Acta 582(1979)67–78.
[16]O.H.Lowry,N.J.Rosebrough,A.L.Farr,et al.,Protein measurement with the Folin-phenolreagent,J.Biol.Chem.193(1951)265–275.
[17]A.Troyano,C.Fernandez,P.Sancho,et al.,Effect of glutathione depletion on antitumor drug toxicity(apoptosis and necrosis)in U-937 human promonocytic cells,J.Biol.Chem.276(2001)47107–47115.
[18]C.M.Rudin,Z.Yang,L.M.Schumaker,et al.,Inhibition of glutathione synthesis reverses Bcl-2-mediated cisplatin resistance,Cancer Res.63(2003) 312–318.
[19]S.W.Lowe,E.M.Schmitt,S.W.Smith,et al.,P53is required for radiation induced apoptosis in mouse thymocytes,Nature 362(1993)847–849.
[20]R.Scherrer,P.Gerhardt,Molecular seiving by the Bacillium megaterium cell walland protoplast,J.Bacteriol.107(1971)718–735.
[21]E.B.Shirling,D.Gottlieb,Methods for characterization of Streptomyces species, Int.J.Syst.Bacteriol.16(1966)313–340.
[22]V.Manohar,C.Ingram,J.Gray,et al.,Antifungal activities of origanum oil against Candida albicans,Mol.Cell.Biochem.228(2001)111–117.
[23]P.Skehan,R.Storeng,D.Scudiero,et al.,New colorimetric cytotoxicity assay for anticancer drug screening,J.Natl.Cancer Inst.82(1990)1107–1112.
[24]M.Igarashi,T.Miyazawa,The growth inhibitory effect of conjugated linoleic acid on a hepatoma cell line Hep G2,is induced by a change in fatty acid in cells,Biochem.Biophys.Acta Mol.Cell Biol.Lipids 1530(2001) 162–171.
[25]K.Nawab,M.Yunus,A.A.Mahdi,et al.,Evaluation of anticancer properties of medicinalplants from the Indian sub-continent,Mol.Cell.Pharmacol.3(2011) 21–29.
[26]J.Gandhiappan,R.Rengasamy,Antiproliferative activity of Solanum anguivi against cancer cell lines,Der.Pharm.Lett.4(2012)875–880.
[27]J.King,Practical,Clinical Enzymology,D Van Nostrand Company Ltd.(1965), pp.83-93.
[28]T.Megha,F.Ferrari,A.Benvenuto,et al.,p53 mutation in breast cancer,correlation with cell kinetics and cell of origin,J.Clin.Pathol.55(2002)461–466.
[29]O.Craciunescu,D.Constantin,A.Gaspar,et al.,Evaluation of antioxidant and cytoprotective activities of Arnica montana L.and Artemisia absinthium L. ethanolic extracts,Chem.Cent.J.6(2012)97.
[30]J.Kao,K.Salari,M.Bocanegra,et al.,Molecular pro fi ling of breast cancer cell lines de fi nes relevant tumor models and provides a resource for cancer gene discovery,PLoS One 4(2009)e6146.
[31]O.Feron,Pyruvate into lactate and back:from the Warburg effect to symbiotic energy fuel exchange in cancer cells,Radiother.Oncol.92(2009)329–333.
[32]H.O'Hara,T.Terasima,Variations of cellular sulfhydrylcontent during the cell cycle of HeLa cells and its correlation to cyclic change of X-ray sensitivity,Exp. Cell.Res.58(1969)182–185.
[33]R.Franco,J.A.Cidlowski,Apoptosis and glutathione:beyond an antioxidant, Cell Death Differ.16(2009)1303–1314.
[34]M.Hollstein,D.Sidransky,B.Vogelstein,et al.,p53 mutations in human cancers,Science 253(1991)49–53.
[35]G.M.Wahl,S.P.Linke,T.G.Paulson,et al.,Maintaining genetic stability through TP53 mediated checkpoint control,Cancer Surv.29(1997)183–219.
[36]A.G.Knudson,Cancer genetics,Am.J.Med.Genet.111(2002)96–102.
[37]I.Hedenfalk,D.Duggan,Y.Chen,et al.,Gene expression pro fi les in hereditary breast cancer,N.Engl.J.Med.344(2001)539–548.
[38]R.W.Johnstone,A.A.Rue fl i,S.W.Lowe,Apoptosis:a link between cancer genetics and chemotherapy,Cell 108(2002)153–164.
[39]X.Saelens,N.Festjens,L.Vande Walle,et al.,Toxic proteins released from mitochondria in celldeath,Oncogene 23(2004)2861–2874.
[40]H.F.Horn,K.H.Vousden,Coping with stress:multiple ways to activate p53, Oncogene 26(2007)1306–1316.
15 August 2014
in revised form
☆Peer review under responsibility of Xi'an Jiaotong University.
.Tel.:+91 9003311921;fax:+91 4374239328.
E-mail address:svijaya_kumar2579@rediffmail.com(S.Vijayakumar).
杂志排行
Journal of Pharmaceutical Analysis的其它文章
- Application of analytical instruments in pharmaceutical analysis
- Comparative study of adsorptive role of carbonaceous materials in removal of UV-active impurities of paclitaxel extracts☆
- In vitro–in vivo studies of the quantitative effect of calcium, multivitamins and milk on single dose cipro fl oxacin bioavailability☆
- Optimization,validation and application of an assay for the activity of HMG-CoA reductase in vitro by LC–MS/MS☆
- Quanti fi cation of tolvaptan in rabbit plasma by LC–MS/MS:Application to a pharmacokinetic study☆
- Identi fi cation,synthesis and characterization of process related des fl uoro impurity of ezetimibe and HPLC method validations☆
