Interpreting genotype×environment interactions for grain yield of rainfed durum wheat in Iran
2015-12-21RezMohmmdiEztollhFrshdfrAhmedAmri
Rez Mohmmdi*,Eztollh Frshdfr,Ahmed Amri
aCampus of Agriculture and Natural Resources,Razi University,Kermanshah,Iran
bDryland Agricultural Research Institute(DARI),AREEO,Sararood branch,P.O.Box 67145-1164,Kermanshah,Iran
cInternational Center for Agricultural Research in the Dry Areas(ICARDA),Rabat,Morocco
Interpreting genotype×environment interactions for grain yield of rainfed durum wheat in Iran
Reza Mohammadia,b,*,Ezatollah Farshadfara,Ahmed Amric
aCampus of Agriculture and Natural Resources,Razi University,Kermanshah,Iran
bDryland Agricultural Research Institute(DARI),AREEO,Sararood branch,P.O.Box 67145-1164,Kermanshah,Iran
cInternational Center for Agricultural Research in the Dry Areas(ICARDA),Rabat,Morocco
A R T I C L E I N F O
Article history:
24 August 2015
Accepted 30 September 2015
Available online 14 October 2015
Durum wheat Classification Ordination analysis Environmentalcovariables
Clustering genotype×environment(GE)interactions and understanding the causes of GE interactions are among the most important tasks in crop breeding programs.Pattern analysis(cluster and ordination techniques)was applied to analyze GE interactions for grain yield of 24 durum wheat(Triticum turgidum L.var.durum)genotypes(breeding lines and old and new cultivars)along with a popular bread wheat(Triticum aestivum)cultivar grown in 21 different rainfed environments during the 2010-2013 cropping seasons.To investigate the causes of GE interaction,several genotypic and environmental covariables were used.In a combined ANOVA,environment was the predominant source of variation, accounting for 81.2%ofthe totalsum ofsquares(TSS),and the remaining TSS due to the GE interaction effect was almost seven times that of the genetic effect.Cluster analysis separated the environments into four groups with similar discriminating ability among genotypes,and genotypes into five groups with similar patterns in yield performance. Pattern analysis confirmed two major environmental clusters(cold and warm),and allowed the discrimination and characterization ofgenotype adaptation.Withinthe cold-environment cluster,severalsubclusters were identified.The breeding lines were mostadapted to moderate and warm environments,whereas the old varieties were adapted to cold environments.The results indicated that winter rainfall and plant height were among the environmental and genotypic covariables,respectively,that contributed most to GE interaction for grain yield in rainfed durum wheat.
©2015 Crop Science Society of China and Institute of Crop Science,CAAS.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
1.Introduction
Durum wheat accounts for a small part of the world wheat industry,representing approximately 5%of acreage and 10% of total wheat production.World durum wheat production in recent years has been approximately 30 million tons,with the European Union,Canada,and the United States accounting for nearly 60%of totalproduction.Durum wheat is one of the most important crops in the Mediterranean areas,mainly in the Central and West Asia and North Africa(CWANA)region[1].This region produces about 13 million tons annually,with Turkey,Syria,Morocco,Tunisia,Algeria,and Iran accounting for 84%of that production[1].Rainfall and temperature in Mediterranean dryland areas show large and unpredictable fluctuations within and among cropping seasons.Durum wheat in Iran is cultivated across diverse environments, ranging from warm lowlands to cold highlands.The improvement of a crop's productivity under stress conditions requires genotypes with stress tolerance and yield stability[2].
To ensure valid genotype recommendation,a common practice among breeders is to repeat yield trials over years to validate recommendations.With this approach,crossover-type genotype×environment(GE)interactions are frequently observed[3],limiting the adaptation of new varieties to only certain environments.In durum wheat,as in many other crops, insufficient yield stability has been recognized as one of the main factors responsible for the gap between yield potential and actual yield,particularly in drought-prone environments [4,5].
The GE interaction,defined as the variation in relative performance of genotypes in different environments[6],is challenging to plant breeders because it complicates the selection of superior genotypes,thereby reducing genetic progress[7].If GE interactions are present,breeders need to identify stable genotypes with relatively consistent performance across a range of environments.
Several statistical methods have been proposed to investigate GE interactions.These range from univariate parametric models to multivariate models.Joint regression is the most popular of the univariate methods because of its simplicity of calculation and application[8].Among the multivariate methods, pattern analysis has been successfully applied to analyzing GE interactions in multi-environment trials(MET)[9-12].Pattern analysis consists of the complementary procedures of classification(clustering)and ordination[13,14].GE interaction data obtained from regional yield trials can be investigated by pattern analysis[14-16]to identify genotypes with similar responses across environments and to identify environments that produce similar discriminations among the genotypes growing in them.Cluster analysis summarizes the complexity ofthe data while retaining most ofits information by permitting the description of responses with relatively few genotype clusters,environment clusters,or both[17].Biplot analysis summarizes the data by representing the patterns ofthe data in a smallnumber of dimensions.
Numerous methods have been used in the search for understanding of the causes of GE interaction[18].These methods can be categorized into two major strategies.The first involves factorial regression analysis of the GE matrix upon environmentalfactors,genotypic traits,or combinations of both[19].The second involves correlation or regression analysis in which the environmental and/or genotypic interaction principal component(PC)scores of the GE matrix are related to environmental and/or genotypic covariables[20,21]. By relating the PC1 and PC2 scores to environmentalconditions and genotypic traits,an understanding of the environmental and genotypic basis of the non-crossover and crossover GE interactions can be achieved[22].
The main objectives of this study were to(i)classify the GE interactions for grain yield of 25 wheat genotypes grown in 21 different rainfed environments and(ii)investigate the causes of GE interactions in durum wheat yield trials in Iran.
2.Materials and methods
2.1.Plant material and climatic data
Twenty four durum wheat genotypes(Table 1)including 21 breeding lines(G1-G21),one new(G22)and two old cultivars (G23,G24),along with one popular old bread wheat cultivar (G25)were tested in seven rainfed research stations representative of the major rainfed durum wheat-growing areas in Iran, during three cropping seasons(2010-2013),resulting in 21 environments(combinations of location and year).The seven stations were Maragheh(M11,M12,and M13 representing the 2011,2012,and 2013 seasons),Qamloo(Q11,Q12,Q13),Shirvan (S11,S12,S13),Uromieh(U11,U12,U13),Ardebil(A11,A12,A13) (cold locations);Kermanshah(K11,K12,K13)(moderately cold location);and Ilam(I11,I12,I13)(warm location)(Table 2).In each environment,the experimental layout was a randomized complete block design with three replications.Plot size was 7.2 m2(6 rows,6 mlong,with 20-cmrow spacing).Management practices recommended for each location were followed in all yield trials.The grain yields were measured on a plot basis and converted to kg ha-1for the statistical analyses.
In addition to grain yield,drought adaptive traits including days to heading(DTH),days to maturity(DTM),plant height (PLH),and 1000-kernel weight(TKW)were measured for the genotypes in each environment.These traits were used as genotypic covariables.Climatic variables including monthly rainfall,minimum and maximum temperature,average temperature,freezing days,relative humidity,and evaporation were obtained from climatological stations established at the research stations and used as environmental covariables.
2.2.Data analysis
Combined analysis of variance(ANOVA)for grain yield data was performed to determine the effects of genotype(G), environment(E),and GE interaction effects.Pattern analysis was performed using the IRRISTAT statistical program on the basis of standardized mean data for each environment, following Fox and Rosielle[23].Hierarchical agglomerative clustering[9]was applied to the GE interaction data matrix with incremental sums of squares[24]as the fusion criterion. In other words,in any part of the dendrogram,members or groups were joined to minimize the new within-group sums of squares.Dendrograms were constructed on the basis of fusion levels using the Ward method[24]to examine similarities in pattern of traits of interest among genotypes (in response to environments)and environments(in discriminating among genotypes).Classification efficacy was determined by the sum of squares retained in the reduced GE interaction data matrix.Ordination was performed on the environment standardized mean yield data using the singular-value decomposition(SVD)algorithm with results represented by a biplot[25,26].The data were modeled in two dimensions,and the efficacy of the model was determined by the proportion of the sum of squares accounted for by eachcomponent.In biplots,the genotype groups and environment groups allowed characterization of genotypes with similar performance within specific environments.By this method,it is possible to reduce the dimensionality ofa data matrix,so that the variation may be better described.
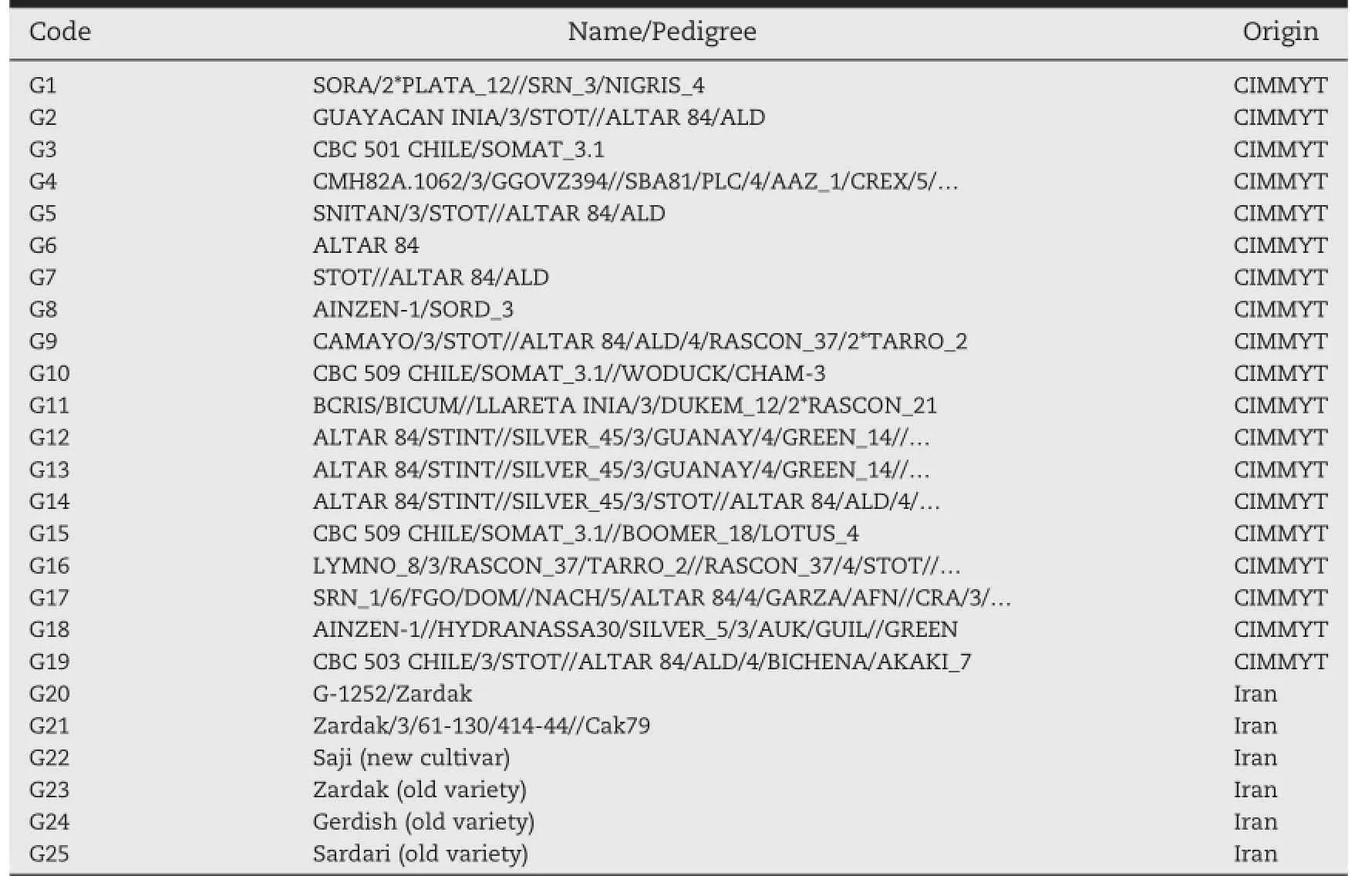
Table 1–Code,name,and origin of the durum wheat genotypes tested in 2011–2013 under rainfed conditions in Iran.

Table 2–Description of the durum wheat testing environments under rainfed conditions in Iran.
A stepwise-regression procedure for selecting the most significant environmental covariables was used.The environmentalcovariables entered in the modelwere used to interpret GE interaction.A correlation analysis between genotypic/ environmental PC1 and PC2 scores from pattern analysis andgenotypic/environmental covariables was applied to identify most sources of GE interaction in rainfed durum wheat MET data.
3.Results
3.1.Climatic data,variance components,and mean yield performance
3.2.Genotype and environment classification
Environments were classified into four groups(Fig.1)and genotypes into five groups(Fig.2).In the classification of environments,five environments(S11,Q11,Q12,A11,andA12),corresponding to cold environments with medium yield potential were the first to be separated as a single group(the LGp-35 group).The low-yielding environments S12,S13,U11, K13,and U13 were included in the LGp-36 group.At the next split,the five low-to-high-yielding environments A13,U12, M12,M13,and Q13 were separated as the LGp-37 group.The major split formed the last cluster,the LGp-38 group.This cluster represented the high-yielding environments K11,K12, M11,I11,I12,and I13 as LGp-38 group that was different from the other environmentgroups(LGp-35,LGp-36 and LGp-37)with low to medium yield performance,indicating the presence of two mega-environment groups(cold and warm)in rainfed durum wheat regional yield trials in Iran(Fig.1).
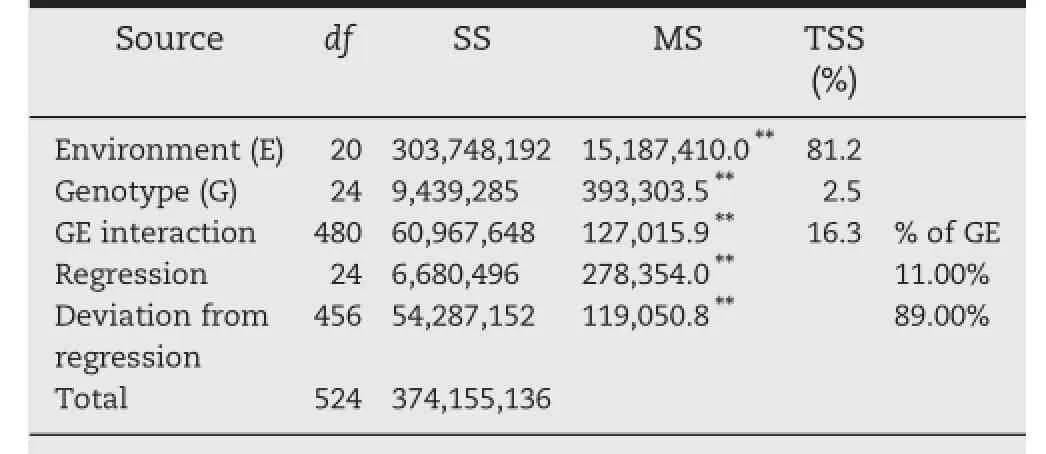
Table 3–Analysis of variance for grain yield of 25 durum wheat genotypes grown in 21 different rainfed environments.
The genotype dendrogram clearly indicated the presence of two major groups(GGp-25 versus the other groups)in the final cluster of maximum dissimilarity(Fig.2).Group GGp-25, with the highest yield potential(1969 kg ha-1),contained the old wheat cultivar(Sardari)with high adaptation to cold rainfed highland regions of Iran.The other four clusters contained the durum wheat genotypes.Genotype group GGp-40,the first to be separated on the dendrogram,consisted of four durum breeding lines(G12,G16,G18,and G19)with the lowest yield performance.This group had mean performance below the grand mean in all environment groups.At the next split,group GGp-43 consisted of durum breeding lines G3,G7, G8,G10,G14,and G15 with relatively high yield potential (1592 kg ha-1).The yield of this group in moderately cold and some cold environments was higher than those of other breeding line groups(GGp-40 and GGp-44).Group GGp-44 contained two durum breeding lines(G20 and G21)and two old durum varieties(G23 and G24)with low yield performance. The last group(GGp-45),with average yield potential,consisted of a new durum cultivar(G22)and durumbreeding lines G1,G2, G4,G5,G6,G11,G13,and G17.
3.3.Mean-performance plots
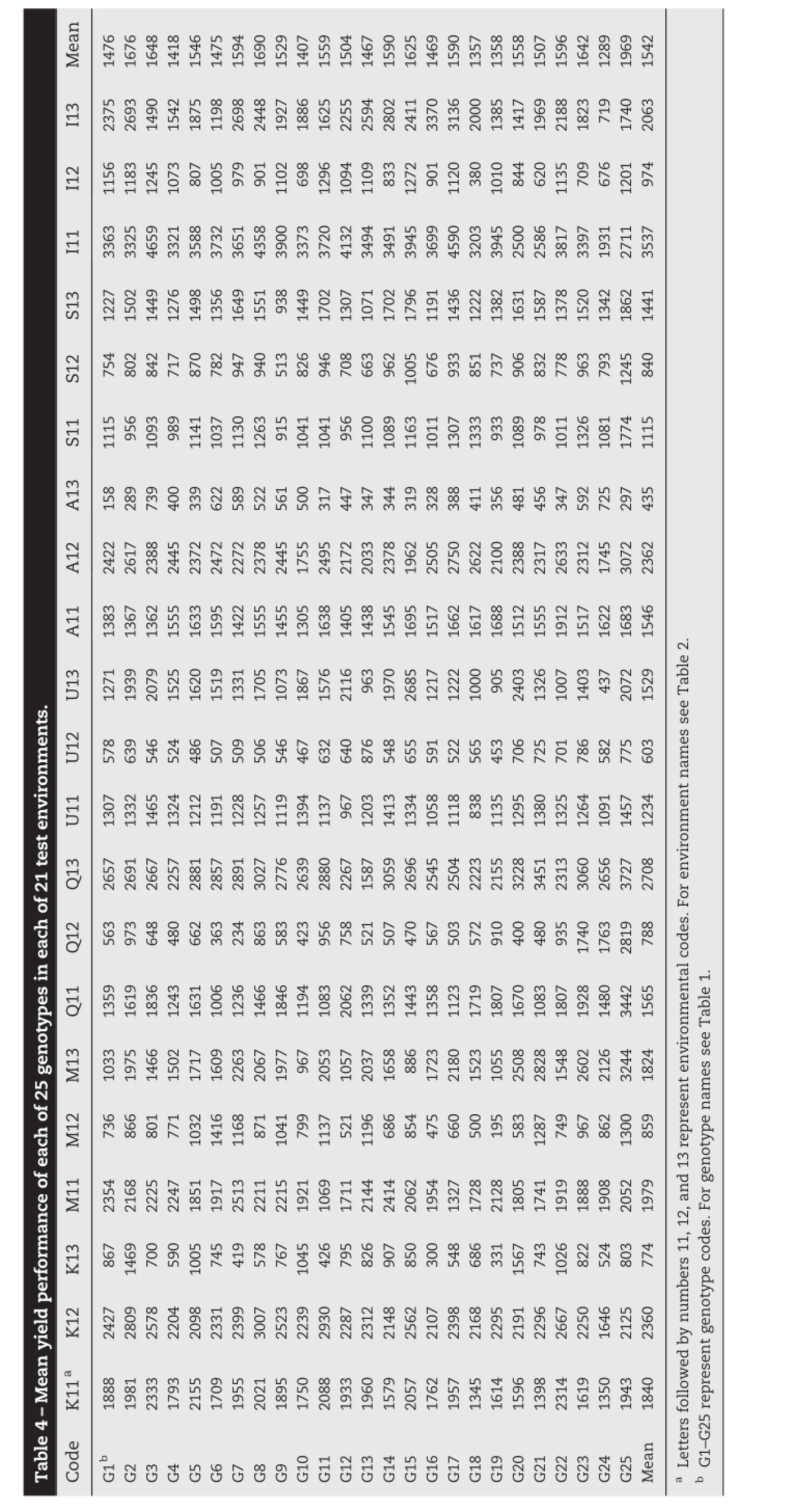
The results of classification analysis based on grain yield data for environments and genotypes,as well as their group numbers and memberships,are presented in Table 5.The mean performance of each genotype group in each environment group based on GE interaction effects is presented in Fig.3.The response plot of the yield of the five genotype groups showed various patterns of adaptation to different environment groups.The genotype group GGp-25,followed by GGp-44,showed the highest interaction with environmental groups,and thus displayed specific adaptation to only certain locations and may have unstable yield across all environmental groups(Fig.3).Genotype group GGp-40 showed negative interaction with all environment groups and thus is the poorest group and can be discarded.Genotype groups GGp-43 and GGp-45 showed the lowest interaction with environmental groups and can be regarded as stable groups. The results indicated that genotypic groups consisting of durum breeding lines were poorly adapted to LGp-35(the cold-environment group)and,in contrast,GGp-25 consisting of mainly the bread wheat cultivar was poorly adapted to LGp-38(the warm-environment group),indicating the poor adaptation of durum breeding lines to cold environments in comparison to the old bread wheat cultivar(Sardari).The
Among the environments,precipitation(October-June)varied from 177 to 399 mm,average minimum temperatures ranged from-2.5 to 8.5°C,and average maximum temperatures varied from 8.4 to 24.5°C.The low rainfall and low temperature at most of the stations resulted in both drought and cold stresses,which are both limiting factors for durum wheat production in cold rainfed highland areas of Iran.
The results of combined analysis of variance for grain yield of 25 genotypes in 21 environments are given in Table 3. Highly significant differences were observed between genotypes and between environments and their interactions (P<0.01).Partitioning of TSS indicated that the environments accounted for 81.2%of the TSS and the remaining sum of squares(SS)due to GE interaction effect was almost seven times that of the genetic effect(Table 3).A linear regression analysis may be used to evaluate the stability of genotypes across environments.Linear regression accounted for 11%of the GE interaction,indicating that a linear approach cannot be expected to account for the integrated effect of different limiting factors(cold,drought,temperature,etc.)on yield performance of the genotypes investigated.
Mean yields varied from 435 to 3537 kg ha-1across the 21 diversified rainfed environments,indicating large variation in yield potential of genotypes(Table 4).The mean yield for an individual genotype in an individual environment ranged from 158 kg ha-1for genotype G1 in environment A13 to 4659 kg ha-1for G3 in environment I11(Table 4).environment groups LGp-36 and LGp-37 were those that best discriminated genotype groups,given that the genotypic groups were better separated in these environmental groups (Fig.3).
?
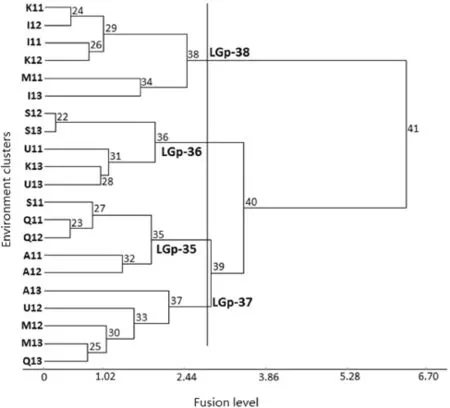
Fig.1–Environment dendrogram showing hierarchical classification of 21 different rainfed environments using standardized grain yield data for 25 genotypes.LGp stands for location group.
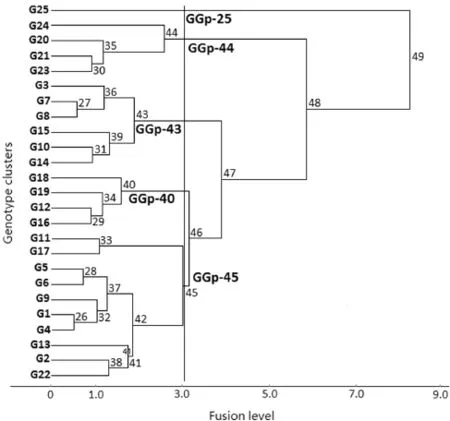
Fig.2–Genotype dendrogram showing hierarchicalclassification of 25 genotypes using environment standardized grain yield data across 21 diverse rainfed environments.GGp stands for genotype group.
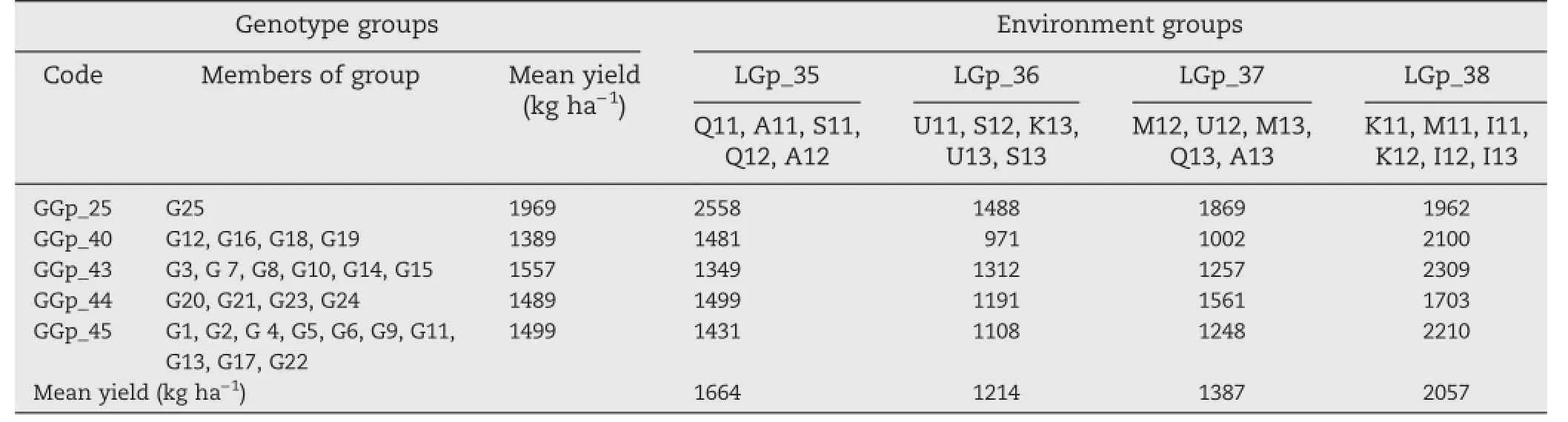
Table 5–GE interaction groups and membership based on grain yields of 25 genotypes grown in 21 rainfed environments. Mean yields for genotypic and environmental groups are original values.
3.4.Ordination analysis
The results of ordination analysis are presented in the biplot (Fig.4)as suggested by Gabriel[25]and Kempton[26].The first two PCs in the biplotexplained 41.6%(PC1=24.5%,PC2=17.1%) of the total sum of squares for GE interaction.Consequently only the first two PCs with moderate cumulative variance percentage of components were plotted for ordination analysis, and this approach could notfully depictthe scatter ofgenotypes and environments in the space.The position and perpendicular projection of genotype points relative to environment points could be used to determine whether a genotype was specifically adapted to a given environment.
The genotype and environment groups derived from the cluster analysis discussed above are indicated by the closed loops in Fig.4.Genotypes close to the origin of the biplot are average in performance or are not wellmodeled by the analysis. Genotypes that are close together are similar in performance, while adjacent environments cause similar discrimination among genotypes.Generally,genotypes and environments within clusters were in close proximity,whereas some clusters were more diffuse.Some genotypes had similar responses over environments;generally,genotype performance differed considerably.Genotype groups that were positioned in high hand side of biplot with an environment group tended to show higher grain yield,reflecting better adaptation to that environment group[26].Genotype group GGp-25 showed the highest adaptation to cold environments(LGp-35 and LGp-36). Genotypes in group GGp-43 were positively associated with the environment groups LGp-36(cold environment)and LGp-38 (mild coldenvironments),while genotypic group GGp-44 showed the highestadaptation to LGp-37(cold environments).Genotypic group GGp-45 consisted of genotypes that were close to the origin of the biplot,indicating average performance.
3.5.Causes of GE interaction represented by PC1 and PC2
Both genotypic and environmental PC1 and PC2 scores had both positive and negative values.Thus,both PC1 and PC2 summarize the most important part of the crossover GE interaction in rainfed durum wheat MET.Any genotypic traits and environmental factors that are highly correlated with the PC1 and/or PC2 scores can thus be interpreted as possible genotypic and environmental causes of crossover GE interaction[22].
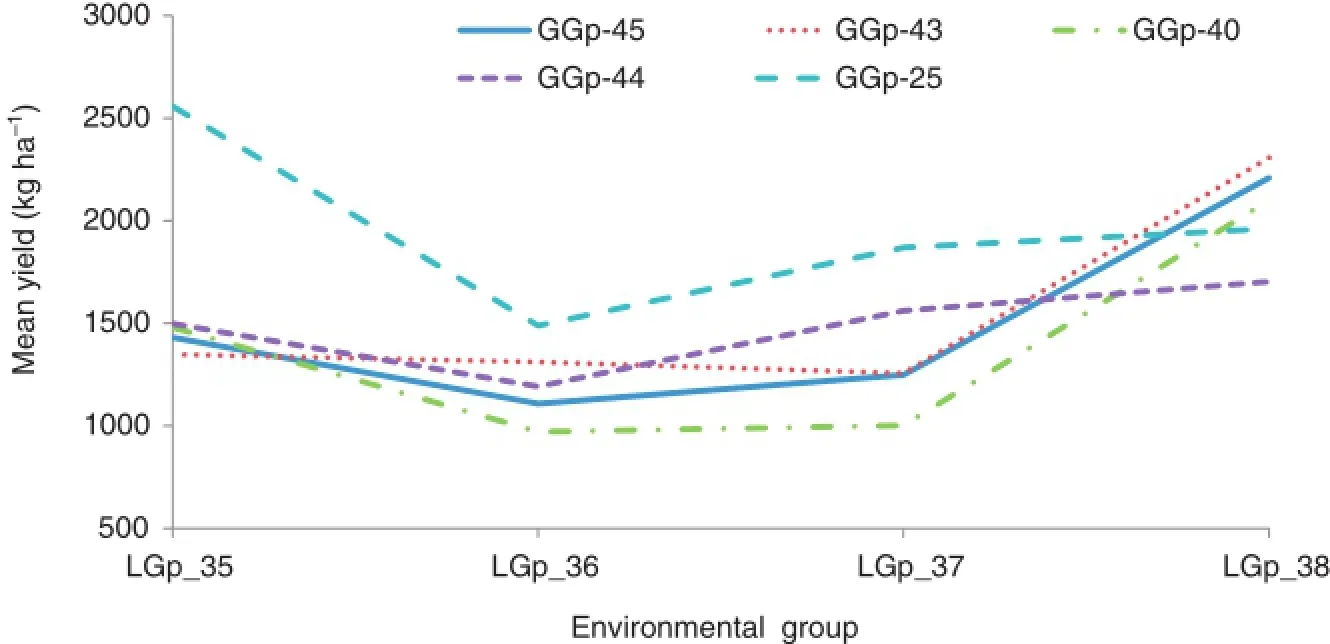
Fig.3–Mean performance plots of five genotype groups over four environment groups based on yield data.GGp stands for genotype group and LGp for location group.
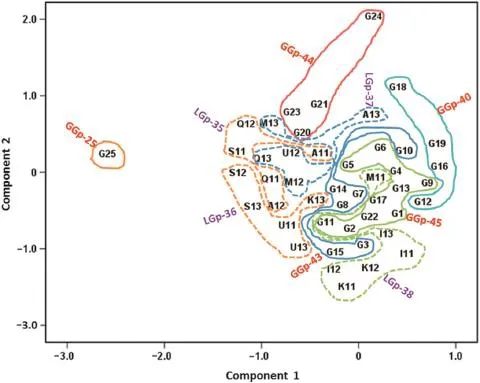
Fig.4–Ordination biplot based on the first two components(PC1 and PC2)obtained from grain yield of 25 genotypes grown in 21 rainfed environments.The loops enclose genotype(GGp-25,GGp-40,GGp-43,GGp-44,and GGp-45)and environment (LGp-35,LGp-36,LGp-37,and LGp-38)groups.
PC1 scores were positively correlated with environmental variables such as winter rainfall(P<0.01)and winter average and maximum temperatures(P<0.05)(Table 6).This finding suggests that environments with greater rainfall and higherwinter temperature tended to have greater PC1 scores.PC1 showed negative correlations with 1000-kernelweight(P<0.01) and plant height(P<0.05)indicating that lower height and lower grain weight tended to contribute to GE interaction.

Table 6–Correlation coefficients between the first two PCs from pattern analysis and various environmental/ genotypic co-variables.
4.Discussion
In this study most of the TSS was explained by the environment(82.1%of TSS),reflecting a much wider range of environment main effects than genotype main effects.For the majority of METs,environment accounts for the maximum variation[11,12].The observed pattern of GE interaction for grain yield in durum wheat yield trials supports a hypothesis of the presence in Iran of differentially adapted durum wheat genotypes[27,28].
Pattern analysis separated environments on the basis of temperature.There were extreme differences in the discrimination effects of cold-and warm-stressed environments on performance of different genotypes.For a specificenvironment,successful breeding should result in progression toward a positive association with that environment.The independent environment groups differing in temperature indicate that breeding lines are better adapted to warm and moderately cold environments.In this case,the most cold adapted genotypes would be located on the left side of the biplot(Fig.4)and the most warm-and moderately cold-adapted genotypes would be located on the opposite side.Genotypic group GGp-25,the bread wheat cultivar,was adapted mostly to cold rainfed environments,while the breeding lines in groups GGp-40 and GGp-45 were well adapted to warm and moderately cold rainfed environments.
In the present study,pattern analysis,which has previously been shown to be very effective for analyzing GE interactions [12,13,16,29],allowed a sensible and usefulsummary ofa GE data set,and assisted in examining the natural relationships and variations in genotype performance among various environmental groups.It has also assisted with refining the breeding strategy for durum wheat in Iran with the identification of two contrasting warm and cold mega-environments[28].
Year-to-year variation in weather has a strong effect on the degree of stress experienced by crops,prompting the use of testing environments to represent target stress environments. van Oosterom et al.[21]found that for two clusters(low-yield and high-yield)identified in their environments,the same locations fellinto different groups in different years,meaning that the locations were not repeatable.In our experiments,we have not been able to ensure repeatability of the cold and drought stresses under rainfed conditions,and thus repeatability was not shown by the pattern analysis(except for the Ilam location,corresponding to the warm environments I11, I12,and I13).
Analytical approaches to GE interaction analysis are important for enhancing the value of METs and gaining an understanding of causes of GE interactions[22,30].The techniques used to interpret GE interactions involve the characterization of trialsites according to environmentalfactors,using either direct measurements,calculated indices,or variables derived from crop growth models.These covariates can then be analyzed in combination with modern multivariate techniques such as pattern analysis,AMMI(additive main effect and multiplicative interaction)or GGE(G+GE)biplots to identify patterns of GE interactions and identify criticalfactors driving the interactions [22,31].These methods have been demonstrated successfully in a range of other crops[18,19,29,32,33].
Our results indicated that both PC1 and PC2 scores had both positive and negative values,resulting in crossover GE interaction and leading to inconsistent yield performance of genotypes across environments.Most of the environmental and genotypic covariables were more highly correlated with PC2 than with PC1 scores,indicating that the contribution of most covariables can be defined in relation to PC2 scores. However,when PC1 and PC2 were considered together,winter rainfalland plant height contributed most to GE interaction in rainfed durum wheat yield trials in Iran.The results confirmed that plant height is an important trait responsible for observed GE interaction and suggest that GE interaction could be reduced by optimizing plant height(by selecting plants with medium height).In this strategy,extremely tallgenotypes can be discarded with confidence even at early stages of breeding. Among the environmental covariables,winter rainfall was the main contributor to GE interaction and may be the most effective in identifying superior genotypes for different environments.However,genotype evaluation in the presence of unpredictable GE interaction is a constant problem in crop breeding[34].
In conclusion,we tested genetic materials under two limiting factors(cold and drought)of durum yield production in highland rainfed conditions of Iran.Because oflower mean yield in colder environments,there was clear discrimination between cold-tolerant(old varieties)and cold-susceptible (breeding lines)genotypes.Clear discrimination was not possible for drought because all trials were conducted under rainfed conditions but with different rainfall distribution patterns.However,in our case,GE interaction was influenced mainly by the temperature factor.Pattern analysis allowed the discrimination and characterization of adaptation of genotypes to cold environments,although several sub-environment clusters were identified within the cold-environment cluster that may be associated with levels of cold and drought stresses.An understanding of the causes of GE interaction can help identify covariables that contribute to better cultivar performance and environments that facilitate genotype evaluation.The results indicated that winter rainfall and plant height contributed most to complex interactions in rainfed durum wheat regional yield trials in Iran.
Acknowledgments
This research was a part of the regionaldurum wheat research project of the Dryland Agricultural Research Institute(DARI)of Iran and was supported by the Agricultural Research,Education and Extension Organization(AREEO)(0-15-15-89102).We thank all members of the durum project who contributed to the implementation of the field work.
利用微生物或其组分抑制植物病害的生物防治可代替化学杀真菌剂,也是一种生态的、有效的农业病原菌防治方法。几个研究小组报道了壳聚糖酶的体外抗真菌活性,它们可用于提高植物对不同植物病原真菌的抗性[28-30]。Kouzai等人报道了壳聚糖酶活性在植物抗病性中的分子机制。植物病原真菌在感染过程中改变细胞壁成分,避免宿主裂解酶降解,细胞壁几丁质向壳聚糖的转化可能是病原体的感染原因之一。
R E F E R E N C E S
[1]J.P.Brennan,A.Aw-Hassan,K.J.Quade,T.L.Nordblom, Impact of ICARDA Research on Australian Agriculture, Economic Research Report No.11.,NSWAgriculture,Wagga Wagga,2002.
[2]R.Mohammadi,A.Amri,Genotype×environment interaction and genetic improvement for yield and yield stability of rainfed durum wheat in Iran,Euphytica 192 (2013)227-249.
[3]R.C.Yang,Mixed-modelanalysis of crossover genotype-environment interactions,Crop Sci.47(2007) 1051-1062.
[4]M.Tollenaar,E.A.Lee,Yield potential,yield stability and stress tolerance in maize,Field Crop Res.75(2002)161-169.
[5]L.Cattivelli,F.Rizza,F.W.Badeck,E.Mazzucotelli,A.M. Mastrangelo,E.Francia,C.Marè,A.Tondelli,A.M.Stanca, Drought tolerance improvement in crop plants:an integrated view from breeding to genomic,Field Crops Res.15(2008) 1-14.
[6]M.Cooper,D.E.Byth,Understanding plant adaptation to achieve systematic applied crop improvement-a fundamental challenge,in:M.Cooper,G.L.Hammer(Eds.),Plant Adaptation and Crop Improvement,CABIPublishing, Wallingford 1996,pp.5-23.
[7]I.Romagosa,P.N.Fox,Genotype×environment interaction and adaptation,in:M.D.Hayward,N.O.Bosemark,I. Romagosa(Eds.),Plant Breeding:Principles and Prospects, Chapman&Hall,London 1993,pp.373-390.
[8]H.C.Becker,J.Leon,Stability analysis in plant breeding,Plant Breed.101(1988)1-23.
[9]W.T.Williams,Pattern Analysis in Agricultural Science, Elsevier Scientific Publishing Company,Amsterdam,the Netherlands,1976.
[10]S.C.Chapman,J.Crossa,G.O.Edmeades,Genotype by environment effects and selection for drought tolerance in tropical maize:I.Two mode pattern analysis of yield, Euphytica 95(1997)1-9.
[11]B.I.G.Haussmann,D.E.Hess,B.V.S.Reddy,S.Z.Mukuru,M. Kayentao,H.G.Welz,H.H.Geiger,Pattern analysis of genotype environment interaction for striga resistance and grain yield in African sorghum trials,Euphytica 122(2001) 297-308.
[12]Y.Zhang,Z.He,A.Zhang,M.van Ginkel,G.Ye,Pattern analysis on grain yield performance of Chinese and CIMMYT spring wheat cultivars sown in China and CIMMYT, Euphytica 147(2006)409-420.
[13]D.E.Byth,R.L.Eisemann,I.H.Delacy,Two-way pattern analysis of a large data set to evaluate genotypic adaptation, Heredity 37(1976)215-230.
[14]M.Cooper,I.H.DeLacy,Relationships among analytic methods used to study genotypic variation and genotype-byenvironment interaction in plant breeding multienvironment trials,Theor.Appl.Genet.88(1994)561-572.
[15]G.Alagarswamy,S.Chandra,Pattern analysis of international sorghum multi-environment trials for grain-yield adaptation,Theor.Appl.Genet.96(1998)397-405.
[16]I.H.DeLacy,R.J.Redden,D.G.Butler,T.Usher,Analysis of line×environment interactions for yield in navy beans:3. Pattern analysis of environments over years,Aust.J.Agric. Res.51(2000)619-628.
[17]R.Shorter,D.E.Byth,V.E.Mungomery, Genotype×environment interactions and environmental adaptation:II.Assessment of environmentalcontributions, Aust.J.Agric.Res.28(1977)223-235.
[18]F.A.van Eeuwijk,J.B.Denis,M.S.Kang,Incorporating additional information on genotypes and environments in models for two-way genotype by environment tables,in:M.S. Kang,H.G.Gauch(Eds.),Genotype-by-Environment Interaction,CRC Press,Boca Raton,FL 1996,pp.15-49.
[19]C.P.Baril,J.B.Denis,R.Wustrnan,F.A.van Eeuwijk,Analyzing genotype by environment interaction in Dutch potato variety trials using factorial regression,Euphytica 82(1995)149-155.
[20]R.W.Zobel,M.G.Wright,H.G.Gauch,Statisticalanalysis of yield trial,Agron.J.80(1988)388-393.
[21]E.J.van Oosterom,D.Kleijn,S.Ceccarelli,M.M.Nachit, Genotype-by-environment interactions of barley in the Mediterranean region,Crop Sci.33(1993)669-674.
[22]W.Yan,L.A.Hunt,Interpretation of genotype×environment interaction for winter wheat in Ontario,Crop Sci.41(2001) 19-25.
[23]P.N.Fox,A.A.Rosielle,Reducing the influence of environmental main-effects on pattern analysis of plant-breeding environments,Euphytica 31(1982)645-656.
[24]J.H.Ward,Hierarchicalgrouping to optimize an objective function,J.Am.Stat.Assoc.58(1963)236-244.
[25]K.R.Gabriel,The biplot graphic display of matrices with application to principal component analysis,Biometrika 58 (1971)453-467.
[26]R.A.Kempton,The use of biplots in interpreting variety by environment interactions,J.Agric.Sci.103(1984)123-125.
[27]R.Mohammadi,A.Amri,R.Haghparast,D.Sadeghzadeh, M.M.Armion,M.Ahmadi,Pattern analysis of genotype-by-environment interaction for grain yield in durum wheat,J.Agric.Sci.147(2009)537-545.
[28]R.Mohammadi,R.Haghparast,A.Amri,S.Ceccarelli,Yield stability of rainfed durum wheat and GGE biplot analysis of multi-environment trials,Crop Pasture Sci.61(2010)92-101.
[29]O.S.Abdalla,J.J.Crossa,E.Autrique,I.H.DeLacy,Relationships among internationaltesting sites ofspring durum wheat,Crop Sci.36(1996)33-40.
[30]J.Voltas,H.Lopez-Corcoles,G.Borras,Use of biplot analysis and factorial regression for the investigation of superior genotypes in multi-environment trials,Eur.J.Agron.22(2005) 309-324.
[31]M.Vargas,J.Crossa,F.A.van Eeuwijk,E.Ramirez,K.Sayre, Using partial least squares regression,factorialregression, and AMMImodels for interpreting genotype×environment interaction,Crop Sci.39(1999)955-967.
[32]E.J.van Oosterom,F.R.Bidinger,V.Mahalakshmi,K.P.Rao, Effect of water availability pattern on yield of pearlmillet in semi-arid tropicalenvironments,Euphytica 89(1996) 165-173.
[33]W.Yan,N.A.Tinker,An integrated biplot analysis stem for displaying,interpreting,and exploring genotype×environment interaction,Crop Sci.45(2005) 1004-1016.
[34]P.J.Bramel-Cox,Breeding for reliability of performance across unpredictable environments,in:M.S.Kang,H.H.Gauch Jr. (Eds.),Genotype-by-Environment Interaction,CRCPress,Bota Raton,Florida 1996,pp.309-339.
1 April 2015
in revised form
at:Dryland Agricultural Research Institute(DARI),AREEO,Sararood branch,P.O.Box 67145-1164,Kermanshah, Iran.
E-mail address:r.mohammadi@areo.ir(R.Mohammadi).
Peer review under responsibility of Crop Science Society of China and Institute of Crop Science,CAAS.
猜你喜欢
杂志排行
The Crop Journal的其它文章
- Herbivore defense responses and associated herbivore defense mechanism as revealed by comparing a resistant wild soybean with a susceptible cultivar
- Effectofelevated[CO2]and nutrientmanagement on wetand dry season rice production in subtropicalIndia
- A nucleotide substitution at the 5′splice site of intron 1 of rice HEADING DATE 1(HD1)gene homolog in foxtail millet,broadly found in landraces from Europe and Asia
- Resistance to powdery mildew in the pea cultivar Xucai 1 is conferred by the gene er1
- Responses in gas exchange and water status between drought-tolerant and-susceptible soybean genotypes with ABA application
- Genetic gains in wheat in Turkey:Winter wheat for irrigated conditions
