烃基取代茚基钌羰基化合物的合成及晶体结构
2015-12-15刘英春马志宏商成喜韩占刚郑学忠
刘英春 马志宏 商成喜 韩占刚 郑学忠 林 进*,
(1河北师范大学化学与材料科学学院,石家庄050024)
(2河北医科大学基础医学院,石家庄050017)
(3承德护理职业学院,承德067000)
烃基取代茚基钌羰基化合物的合成及晶体结构
刘英春1马志宏2商成喜3韩占刚1郑学忠1林 进*,1
(1河北师范大学化学与材料科学学院,石家庄050024)
(2河北医科大学基础医学院,石家庄050017)
(3承德护理职业学院,承德067000)
配体C9H7R(R=CH2CH2CH3(1),CH(CH3)2(2),C5H9(3),CH2C6H5(4),CH2CH=CH2(5))分别与Ru3(CO)12在二甲苯或庚烷中加热回流,得到了6个双核配合物[(η5-C9H6R)Ru(CO)(μ-CO)]2(R=CH2CH2CH3(6),CH(CH3)2(7),C5H9(8),CH2C6H5(9),CH2CH=CH2(10))和[(η5-C9H6)(H3CH2C)CHCH(CH2CH3)(η5-C9H6)][Ru(CO)(μ-CO)]2(11)。通过元素分析、红外光谱、核磁共振氢谱对配合物的结构进行了表征,并用X-射线单晶衍射法测定了配合物6,9,10和11的结构。
茚基;羰基钌;X-射线衍射;结构
0 Introduction
Substituted cyclopentadienyl and related indenyl anions occupy a prominent role in organometallic chemistry,serving as versatile ligands for transition metals.Seemingly subtle changes in either cyclopentadienyl or indenyl ligand substitution can have profound consequences on chemical reactivity. The cyclopentadienyl or indenyl metal complexes have received increasing attention due to their diverse andflexible hapticities and the enhanced reactivity both in stoichiometric and catalytic reactions[1-9].The side chain functionalized cyclopentadienyl or indenyl ligands have usually been used to form intramolecular coordination to a Lewis acidic metal center or to construct oligonuclear metal complexes,which usually show different structures and reactivity[10].In our previous work we studied the reactions of substituted cyclopentadienes with Ru3(CO)12and obtained a series of ruthenium carbonyl complexes involving novel intramolecular C-H activation,allyl isomerization,bridged or normal and including different bonding modes of cyclopentadienyl ligands[11-18].Considering the properties and the reactivity of transition-metal complexes are influenced by the electronic and steric properties of the surrounding ligands,we further studied the reactions of alkyl-substituted indenyl ligands[C9H7R] with Ru3(CO)12,and a series of diruthenium complexes with η5-fashion were obtained.
1 Experimental
1.1 General considerations
All manipulations of air-and moisture-sensitive complexes were performed at an argon/vacuum manifold using standard Schlenk techniques.Solvents were distilled from appropriate drying agents under an atmosphere of nitrogen prior to use.1H NMR spectra were recorded on a Bruker AV 500 instrument,while IR spectra were recorded as KBr disks on a FT IR 8900 spectrometer.X-ray measurements were made on a Bruker Smart APEX diffractometer with graphite monochromated Mo Kα(λ=0.071 073 nm)radiation. Elemental analyses were performed on a Vario ELⅢanalyzer.The ligand precursors[C9H7R](R=CH2CH2CH3(1),CH(CH3)2(2),C5H9(3),CH2C6H5(4),CH2CH =CH2(5))were synthesized according to the literature[19]. Ru3(CO)12were purchased from J&K Scientific Ltd and used without further purification,other reagents were purchased from commercial suppliers.
1.2 Reaction of 1 with Ru3(CO)12in xylene
A solution of ligand precursor C9H7CH2CH2CH3(1)(0.22 g,1.41 mmol)and Ru3(CO)12(0.30 g,0.47 mmol)in 25 mL of xylene was refluxed for 12 h.After removal of solvent under reduced pressure,the residue was chromatographed on an alumina column using petroleum ether/CH2Cl2(6∶1,V/V)as eluent. Only the yellow band was eluted and collected.After vacuum removal of the solvents from the above eluate, the residue was recrystallized from n-hexane/CH2Cl2(1:3,V/V)at room temperature to give complex 6 as orange-yellow crystals(Yield:0.343 g,77.5%).m.p. 184℃(dec.).Anal.Calcd.(%)for C28H26Ru2O4:C, 53.50;H,4.17.Found(%):C,53.12;H,4.33.1H NMR (500 MHz,CDCl3):7.23~7.32(m,6H,C9H6),7.02(d, 2H,J=8.0 Hz,C9H6),5.47(d,2H,J=3.0 Hz,C9H6), 5.08(d,2H,J=2.5 Hz,C9H6),2.65~2.83(m,4H, CH2),1.61~1.74(m,4H,CH2),0.98(t,6H,J=7.5 Hz, CH3).IR(νCO,cm-1):1 938(s),1 790(s).
1.3 Reaction of 2 w ith Ru3(CO)12in xylene
By using a similar procedure to that described above,ligand precursor C9H7CH(CH3)2(2)reacted with Ru3(CO)12in refluxing xylene for 10 h,after chromatography and eluted with petroleum ether/CH2Cl2, complex 7 was obtained(0.309 g,69.7%yield)as orange-yellow solid.m.p.212℃.Anal.Calcd.(%)for C28H26Ru2O4:C,53.50;H,4.17.Found(%):C,53.83; H,4.31.1H NMR(500 MHz,CDCl3):7.50(d,2H,J= 8.0 Hz,C9H6),7.37(t,2H,J=7.5 Hz,C9H6),7.26(t, 2H,J=7.5 Hz,C9H6),6.98(d,2H,J=8.5 Hz,C9H6), 5.46(d,2H,J=2.5 Hz,C9H6),4.72(d,2H,J=2.5 Hz, C9H6),1.31(d,6H,J=6.8 Hz,CH3),1.52(d,6H,J= 6.5 Hz,CH3),3.30~3.40(m,2H,CH).IR(νCO,cm-1): 1 950(s),1 772(s).
1.4 Reaction of 3 w ith Ru3(CO)12in xylene
By using a similar procedure to that described above,ligand precursor C9H7C5H9(3)reacted with Ru3(CO)12in refluxing xylene for 10 h,after chromatography and eluted with petroleum ether/CH2Cl2, complex 8 was obtained(0.383 g,79.9%yield)as orange-red solid.m.p.210℃.Anal.Calcd.(%)for C32H30Ru2O4:C,56.46;H,4.44.Found(%):C,56.73; H,4.20.1H NMR(500 MHz,CDCl3):7.51(d,2H,J= 8.5 Hz,C9H6),7.35(t,2H,J=7.5 Hz,C9H6),7.25(t, 2H,J=7.5 Hz,C9H6),6.97(d,2H,J=8.5 Hz,C9H6), 5.44(d,2H,J=2.5 Hz,C9H6),4.77(d,2H,J=2.5 Hz, C9H6),3.43~3.49(m,2H,C9H6CH),1.71~1.97(m,16H,(CH2)4).IR(νCO,cm-1):1 944(s),1 786(s).
1.5 Reaction of 4 w ith Ru3(CO)12in xylene
By using a similar procedure to that described above,ligand precursor C9H7CH2C6H5(4)reacted with Ru3(CO)12in refluxing xylene for 12 h,after chromatography and eluted with petroleum ether/CH2Cl2, complex 9 was obtained(Yield:0.439 g,86.1%)as orange-yellow solid.m.p.228℃(dec.).Anal.Calcd. (%)for C36H26Ru2O4:C,59.66;H,3.62.Found(%):C, 59.83;H,3.48.1H NMR(500 MHz,CDCl3):7.27~7.35(m,8H,C9H6),7.05~7.25(m,10H,C6H5),5.52 (d,2H,J=3.0 Hz,C9H6),5.20(d,2H,J=3.0 Hz,C9H6), 4.05~4.15(m,4H,C6H5CH2).IR(νCO,cm-1):1 952(s), 1 784(s).
1.6 Reaction of 5 w ith Ru3(CO)12in xylene
By using a similar procedure to that described above,ligand precursor C9H7CH2CH=CH2(5)reacted with Ru3(CO)12in refluxing xylene for 12 h.The solvent was removed under reduced pressure,and the residue was placed in an Al2O3column.Elution with CH2Cl2/ petroleum ether developed two bands.The first band gave 0.268 g(60.9%)of 10 as orange-red crystals, and the second band yielded 0.047 g(10.7%)of 11 as orange-red crystals.Data for 10:m.p.174℃.Anal. Calcd.(%)for C28H22Ru2O4:C,53.84;H,3.55.Found (%):C,53.50;H,3.78.1H NMR(500 MHz,CDCl3): 7.23~7.31(m,8H,C9H6),5.79(d,2H,J=2.5 Hz,C9H6), 4.92(d,2H,J=2.5 Hz,C9H6),5.67(d,4H,J=3.0 Hz, CH2),5.46~5.48(m,2H,CH),2.73~2.87(m,4H,CH2). IR(νCO,cm-1):1 942(s),1 784(s).Data for 11:m.p. 225℃.Anal.Calcd.(%)for C28H24Ru2O4:C,53.67;H, 3.86.Found(%):C,53.95;H,3.65.1H NMR(500 MHz,CDCl3):7.55(d,2H,J=8.5 Hz,C9H6),7.42(t, 2H,J=7.5 Hz,C9H6),7.33(d,2H,J=8.5 Hz,C9H6), 7.22(t,2H,J=7.5 Hz,C9H6),6.16(d,2H,J=3.0 Hz, C9H6),5.46(d,2H,J=3.0 Hz,C9H6),2.88(s,2H,CH), 1.75~1.61(m,4H,CH2),0.57(d,6H,J=7.5 Hz,CH3). IR(νCO,cm-1):1 977(vs),1 938(m),1 776(s).
1.7 Crystal structure determ ination
Crystals of the complexes 6,9,10 and 11 suitable for X-ray diffraction were isolated from the slow evaporation of hexane-dichloromethane solution.Data collection were performed on a Bruker SMART APEX-CCD detector with graphite monochromated Mo Kα (λ=0.071 073 nm)radiation using the φ-ω scan technique.The structures were solved by direct methods and refined by full-matrix least-squares procedures based on F2using the SHELX-97 program system[20]. Hydrogen atoms were included in calculated positions riding on the parent atoms and refined with fixed thermal parameters.Crystallographic data and experimental details of the structure determinations are given in Table 1.
CCDC:937499,6;972340,9;944111,10;970734, 11.
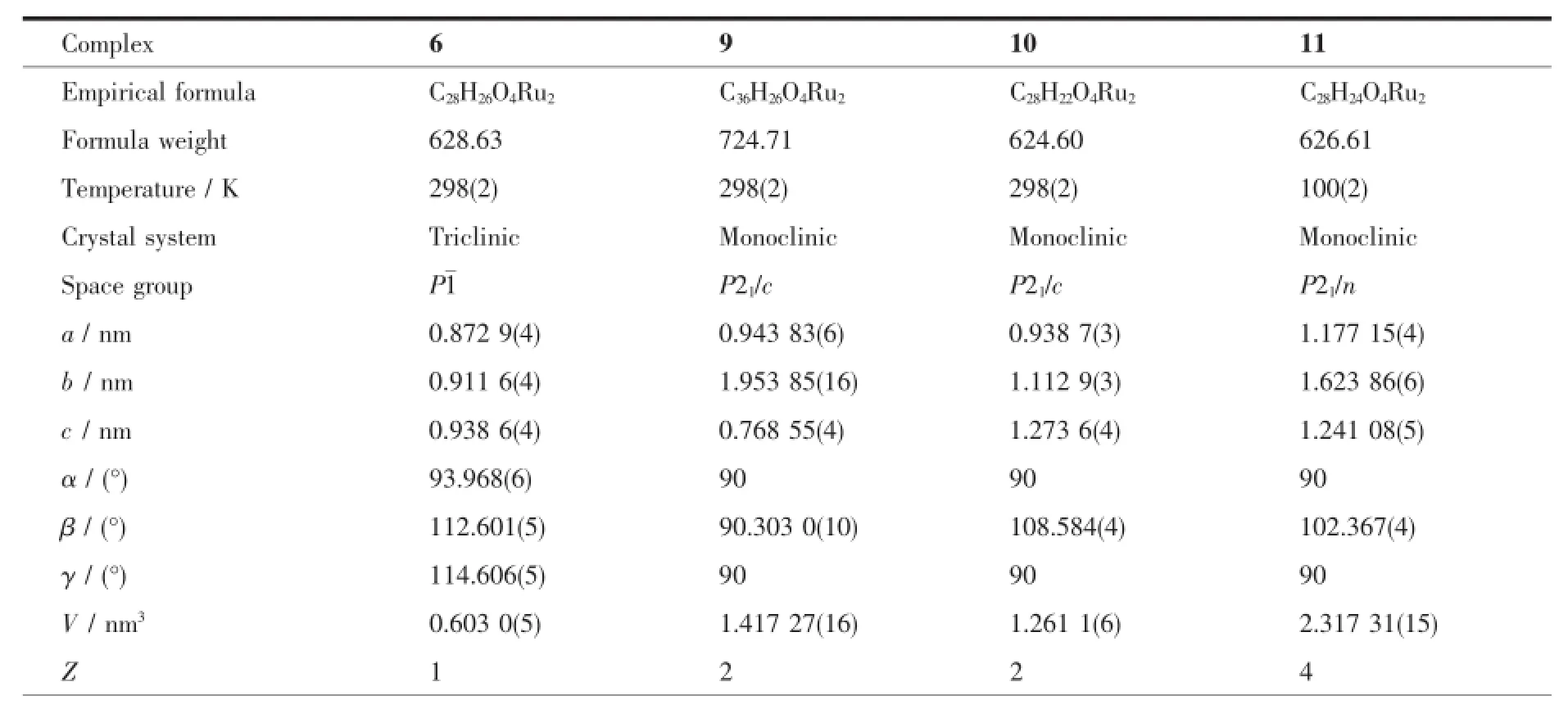
Table1 Crystal data and structure refinement parameters for complexes 6,9,10 and 11
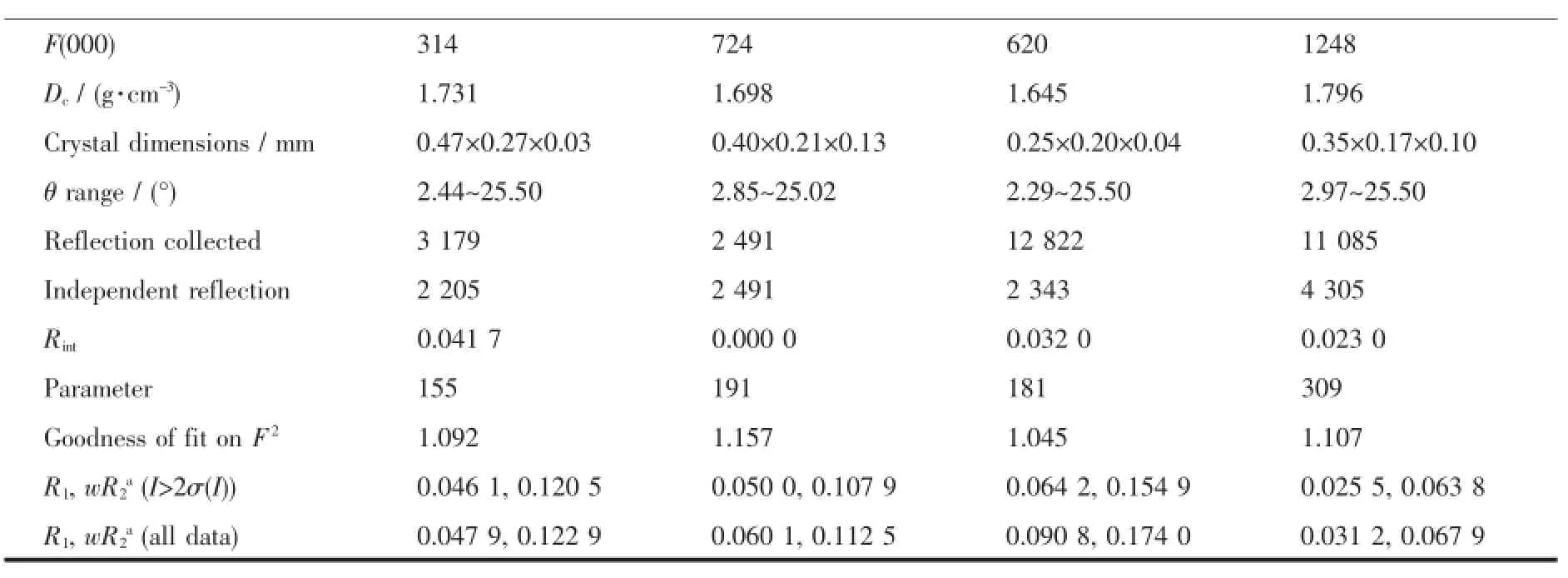
Continued Table 1
2 Results and discussion
Reactions of ligand precursors C9H7R(R=CH2CH2CH3(1),CH(CH3)2(2),C5H9(3),CH2C6H5(4))with Ru3(CO)12in refluxing xylene afforded the corresponding products 6(77.5%),7(69.7%),8(79.9%)and 9(86.1%), respectively(Scheme 1).When[C9H7R](R=CH2CH= CH2(5))was treated with Ru3(CO)12in refluxing xylene, the diruthenium complex 10(60.9%)and the bridged product 11(10.7%)were obtained(Scheme 2).When heptane was chosen as the solvent instead,the corresponding product was 6(10.5%),7(9.21%),8(20.8%), 9(22.3%)and 10(14.1%),respectively.The products were the same,but the total yield was much lower than that in xylene.
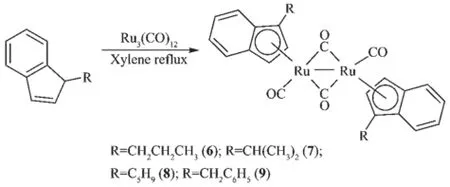
Scheme 1 Synthesis of complexes 6~9
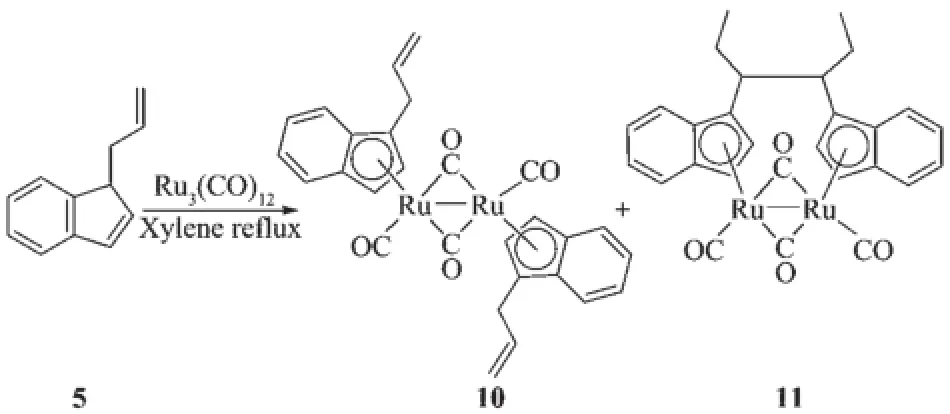
Scheme 2 Synthesis of comp lexes 10 and 11
Based on their1H NMR and IR spectra,6~10 were assigned as the normal Ru-Ru single bonded dinuclear complexes.The IR spectra of 6~10 all exhibited a strong terminal carbonyl absorption at 1 938~1 952 cm-1and a strong bridging carbonyl absorption at 1 772~1 790 cm-1,which are comparable to other metal-metal bond spectra found in other single substituted indenyl ruthenium dimers.The1H NMR spectra of the normal indenyl diruthenium complexes 6~10 are similar,and they all show peaks at 7.55~6.97 for the C6-ring protons of the indenyl ligands and two doublets at 6.16~4.72 for the C5-ring protons of the indenyl ligands.Different from 6~10,complex 11 shows two terminal and one bridging carbonyl absorptions at 1 977(vs),1 938(m)and 1 776(s)cm-1in its IR spectrum.The1H NMR spectra of 11 shows one singlet at 2.88 for the methyne protons,one multiplet at 1.75~1.61 for the methylene protons,and one doublet at 0.57 for the methyl protons.This illustrated that the coupling of the allyl pendant group took place,which agrees with the single crystal X-ray diffraction analysis results.
The crystal structures of 6,9,10 and 11 were determined by X-ray diffraction analysis.Selected bond lengths and angles are given in Table 2.The molecular structures of 6,9,10 and 11 are presented in Fig.1~4,respectively.
Complexes 6,9 and 10 are diruthenium complexesand have similar structures,in which two indenyl ligands coordinate with two ruthenium atoms through their indenyl ring in η5-bonding.Similar to the cyclopentadienyl analogue trans-[(η5-Cp)Ru(CO)(μ-CO)]2(where Cp=cyclopentadienyl ligand),all the structures are trans form,and all the complexes have two forms of coordinated carbonyl ligands,terminal and bridging CO.The two indenyl ring planes are parallel.The Ru-Ru bond distances(0.273 99(10)nm for 6,0.275 73(16) nm for 9 and 0.273 54(14)nm for 10)are close to that of analogous complexes[{(η5-C9H7)Ru(CO)2}2] (0.274 12(5)nm)[21],[η5-(MeC5H3N)CH2CMe2(C9H6)Ru (CO)]2(μ-CO)2(0.274 43(13)nm),[η5-(C5H4N)CH2(C9H6) Ru(CO)]2(μ-CO)2(0.273 89(15)nm)[22]and[(η5-C9H6nBu)2Ru2(CO)4(0.275 5(2)nm)[23].For 11,single crystal X-ray diffraction analysis showed that C(8)-C(9),C(9)-C(10),C(10)-C(11),C(12)-C(13),C(13)-C(14),C(8)-C(9) and C(9)-C(12)are all single bonds,and this illustrated that the coupling of the allyl pendant group took place,which was evidenced by its1H NMR spectrum. Complex 11 is an unexpected product and the structure is unusual.We obtained the products of the allyl isomerization,but our team has never observed similar reaction of substituted tetramethylcyclopentadienes with metal carbonyl complex.After consulting some literatures[24-26],we proposed the following mechanistic pathway shown in Scheme 3.When ligand 5 reacted with Ru3(CO)12under refluxing xylene for 12 h,the ruthenium hydride complex a was firstly obtained,then the isomerization of allyl pendant group took place to give the intermediate species b. Subsequent homolysis of the Ru-H formed the free radical c and hydrogen free radical,then free radical addition created d.At last the coupling reaction of d generated the dinuclear species 11.

Table2 Selected bond distances(nm)and angles(°)for com p lexes 6,9,10 and 11
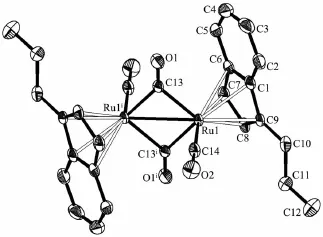
Fig.1 Molecular structure of comp lex 6

Fig.2 Molecular structure of complex 9
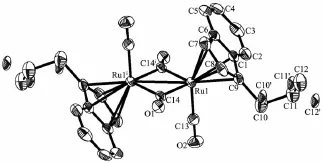
Fig.3 Molecular structure of complex 10
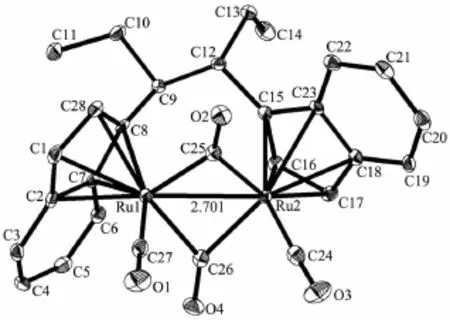
Fig.4 Molecular structure of complex 11
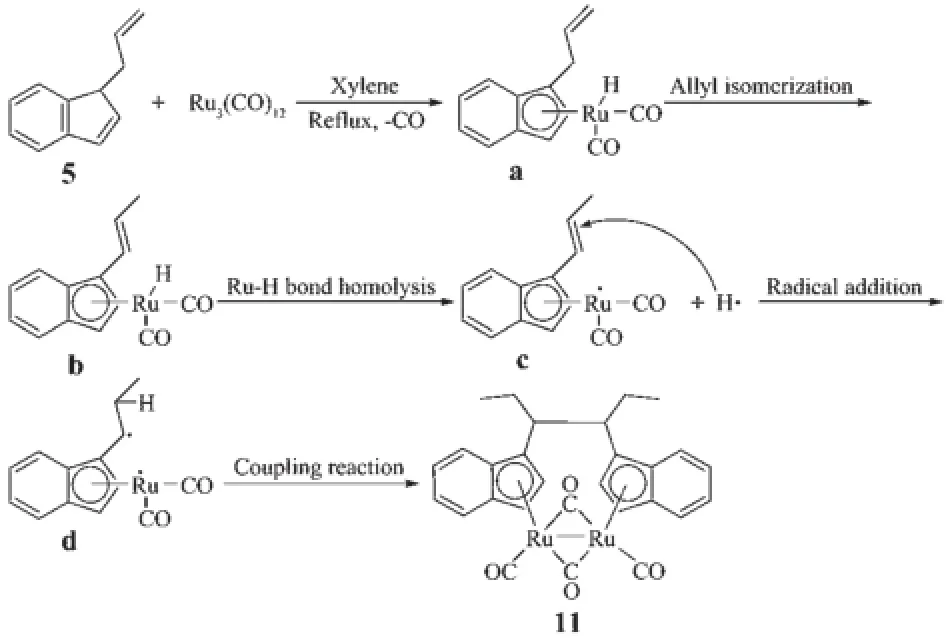
Scheme 3 Possible mechanistic pathway of 11
3 Conclusions
Reactions of alkyl-substituted indenes with Ru3(CO)12in refluxing xylene or heptane were investigated,respectively.Six new metal carbonyl complexes were obtained and the structures of four of them were determined by single-crystal X-ray diffraction.The results clearly reveal that the coordination mode of indenyl diruthenium complexes is η5and substituent group variations display some influence on the Ru-Ru bond length.The solvent also has a significant effect on the reaction yield.
[1]Borah M,Bhattacharyya P K,Das P.Appl.Organometal. Chem.,2012,26:130-134
[2]Stanowski S,Nicholas K M,Srivastava R S.Organometallics, 2012,31:515-518
[3]Do Y,Han J,Rhee Y H,et al.Adv.Synth.Catal.,2011,353: 3363-3366
[4]Kuninobu Y,Uesugi T,Kawata A,et al.Angew.Chem.Int. Ed.,2011,50:10406-10408
[5]Mutseneck E V,Petrovskii P V,Kudinov A R.Russ.Chem. Bull.,2004,53:2090-2091
[6]Zargarian D.Coord.Chem.Rev.,2002,233:157-176
[7]Calhorda M J,Félix V,Veiros L F.Coord.Chem.Rev.,2002, 230:49-64
[8]Stradiotto M,Mcglinchey M J.Coord.Chem.Rev.,2001,219-221:311-378
[9]Resconi L,Cavallo L,Fait A,et al.Chem.Rev.,2000,100:1253-1346
[10]Fischer P J,Krohn K M,Mwenda E T V,et al.Organometallics,2005,24:1776-1779
[11]Lin J,Zhao M X,Ma Z H,et al.J.Chem.Crystallogr.,2009, 39:642-645
[12]Lin J,Ma Z H,Li F,et al.Transition Met.Chem.,2009,34: 797-801
[13]Lin J,Ma Z H,Li F,et al.Transition Met.Chem.,2009,34: 855-859
[14]Liu X H,Ma Z H,Tian L J,et al.Transition Met.Chem., 2010,35:393-397
[15]Tian L J,Ma Z H,Han Z G,et al.Transition Met.Chem., 2011,36:151-156
[16]Ma Z H,Liu X H,Han Z G,et al.Transition Met.Chem., 2011,36:207-210
[17]GUO Kai-Ming(郭凯明),MA Zhi-Hong(马志宏),LI Su-Zhen (李素贞),et al.Chinese J.Inorg.Chem.(无机化学学报), 2012,28:643-646
[18]MA Zhi-Hong(马志宏),WANG Na(王娜),GUO Kai-Ming (郭凯明),et al.Chinese J.Inorg.Chem.(无机化学学报), 2013,29:1882-1886
[19]Grimmer N E,Coville N J,de Koning C B,et al.J.Organomet.Chem.,2000,616:112-127
[20]Sheldrick G M.SHELX-97,Program for the Refinement of Crystal Structures,University of Göttingen,Germany,1997.
[21]Sridevi V S,Leong W K.J.Organomet.Chem.,2007,692: 4909-4916
[22]Chen D F,Xu S S,Song H B,et al.Eur.J.Inorg.Chem., 2008:1854-1864
[23]MA Zhi-Hong(马志宏),LI Fang(李放),LIU Xiao-Huan(刘晓焕),et al.Chinese J.Org.Chem.(有机化学),2009,29: 1294-1297
[24]Royo E,Acebrón S,Mosquera M E G,et al.Organometallics, 2007,26:3831-3839
[25]Sivaramakrishna A,Mushonga P,Rogers I L,et al.Polyhedron, 2008,27:1911-1916
[26]Lai C H,Cheng C H,Chou W C,et al.Organometallics, 1993,12:3418-3425
Ruthenium Carbonyl Com p lexes Containing Alkyl-Substituted Indenyl Ligands:Syntheses and Structures
LIU Ying-Chun1MA Zhi-Hong2SHANG Cheng-Xi3HAN Zhan-Gang1ZHENG Xue-Zhong1LIN Jin*,1
(1The College of Chemistry&Material Science,Hebei Normal University,Shijiazhuang 050024,China)
(2College of Basic Medicine,Hebei Medical University,Shijiazhuang 050017,China)
(3Chengde Nursing Vocational College,Chengde,Hebei 067000,China)
Reactions of the alkyl-substituted indenyl ligands[C9H7R](R=CH2CH2CH3(1),CH(CH3)2(2),C5H9(3), CH2C6H5(4),CH2CH=CH2(5))with Ru3(CO)12in refluxing xylene or heptane gave the responding dinuclear metal carbonyl complexes[(η5-C9H6R)Ru(CO)(μ-CO)]2(R=CH2CH2CH3(6),CH(CH3)2(7),C5H9(8),CH2C6H5(9),CH2CH= CH2(10)),respectively,as well as the bridged diruthenium complex[(η5-C9H6)(H3CH2C)CHCH(CH2CH3)(η5-C9H6)] [Ru(CO)(μ-CO)]2(11).These complexes have been characterized by elemental analysis,IR,and1H NMR spectroscopy.The molecular structures of 6,9,10 and 11 were determined by X-ray diffraction analysis.CCDC: 937499,6;972340,9;944111,10;970734,11.
indenyl;ruthenium carbonyl;X-ray diffraction;structure
O614.82+1
A
1001-4861(2015)04-0774-07
10.11862/CJIC.2015.088
2014-12-11。收修改稿日期:2015-01-14。
国家自然科学基金(No.21372061),河北省自然科学基金(No.B2013205025,B2014205018)和河北师范大学重点基金(No.L2012Z02)资助项目。*
