基于半胱氨酸衍生物的两个手性多核金属簇合物的结构和磁性质
2015-12-15谢黎霞张长丽
徐 鉴 谢黎霞 张长丽
(1南京晓庄学院生物化工与环境工程学院,南京211171)
(2河南农业大学理学院,郑州450002)
基于半胱氨酸衍生物的两个手性多核金属簇合物的结构和磁性质
徐 鉴*,1谢黎霞2张长丽1
(1南京晓庄学院生物化工与环境工程学院,南京211171)
(2河南农业大学理学院,郑州450002)
采用具有手性的半胱氨酸衍生物配体L-硫代脯氨酸(LTP)分别和高氯酸钴、高氯酸锰反应得到配合物[Co(LTP)2]n(1)和[Mn (LTP)2]n(2)。用X-射线衍射对这2个化合物的晶体结构进行了测定,结果表明4个LTP配体采用μ2-N1O2:O3的配位模式将八面体配位构型的金属离子连接起来构成二维结构。磁性测定表明2个化合物中金属离子之间有弱的反铁磁相互耦合作用。
配合物;磁性;手性氨基酸配体
Recently,chiral coordination polymers have become a topic of intense interest due to their intriguing potential application in enantioselective absorption,catalysis,porous materials,nonlinear optical materials and magnetic materials[1-6].To date, numerous fascinating archetypal chiral networks have been obtained,which have been mainly prepared using chiral organic ligands[7-15]or chiral metal complexes[16-17],or by spontaneous resolution from achiral materials[18-20].Though it is already found that the introduction of preferential and extended homochiral interactions are important features for the construction of homochiral coordination polymers,few of the chiral molecules could be resolved spontaneously from the totally achiral species at the supramolecular level.Recent research has proved thatthe most direct and efficient method is the usage of chiral species as reactant precursors toward homochiral solids for it is much easier to control the chirality of the aimed products[21-25].Amino-acids and their derivatives are often used to construct new chiral materials.The chirality of these modifying natural amino acids has been recently exploited for the construction of metal-organic frameworks(MOFs)with more structural diversities,as well as fascinating properties,which are helpful for the synthesis of chiral MOFs with expected functionalities[26-31].Our interest has focused on employment of the derivative of L-cysteine to direct the assembly of chiral metal clusters,and to study the relationship between the chirality and magnetism of the clusters.The ligand L-thioproline(LTP)(Scheme 1)was prepared from L-cysteine and formaldehyde according to the literature[32]. Single crystals of[Co(LTP)2]n(1)and[Mn(LTP)2]n(2) were synthesized in the presence of LTP and characterized by the X-ray single crystal structure an alysis.
1 Experimental
1.1 Materials and measurements
All chemicals were of reagent grade quality obtained from commercial sources and used as received without further purification.Elemental analyses(C,H and N)were carried out on a Perkin-Elmer 240C analytical instrument.Infrared(IR) spectra were recorded on a Bruker Vector22 spectrophotometer with KBr pellets in the 4 000~400 cm-1regions.1H NMR spectral measurements were performed on a Bruker DRX-500 NMR spectrometer at room temperature with TMS as an internal reference.Magnetic susceptibility data on crushed single crystals were collected over the temperature range 1.8~300 K using a Quantum Design MPMS-XL super-conducting quantum interference device (SQUID)magnetometer,and the experimental data were corrected for diamagnetism of the constituent atoms estimated from Pascals constants.
1.2 Preparation of[Co(LTP)2]n(1)
The ligand LTP·HCl(168 mg,1 mmol),and Co (ClO4)3·6H2O(464 mg,1 mmol)were mixed in methanol(20 mL).The triethylamine(0.3 mol·L-1)was added to the resultant solution to adjust the pH value to 4~5.Water was added slowly to the above solution. The solution was heated to obtain a clear solution,and then filtered.The colorless crystals of 1 suitable for X-ray diffraction determination were immediately separated from the mother liquor and then filtered. Red and block-like crystals of 2 were obtained within two weeks upon slow evaporation of the filtrate.Yield: 60%.Anal.Calcd.for C8H12CoN2O4S2(%):C,29.72;H, 3.74;N,8.67.Found(%):C,31.03;H,3.28;N,9.06.
1.3 Preparation of[Mn(LTP)2]n(2)
The ligand LTP·HCl(168 mg,1 mmol),and Mn (ClO4)3·6H2O(460 mg,1 mmol)were mixed in methanol(20 mL).The triethylamine(0.3 mol·L-1)was added to the resultant solution to adjust the pH value to 6~7.An equal volume of water was added slowly to the above solution.The solution was heated to obtain a clear solution.The colorless crystals of 1 suitable for X-ray diffraction determination were immediately separated from the mother liquor and then filtered. Yield:80%.Anal.Calcd.for C8H12MnN2O4S2(%):C, 30.10;H,3.79;N,8.77.Found(%):C,29.94;H,3.63; N,9.14.
1.4 Crystal structure determ ination
Crystallographic data collections for 1 and 2 were carried out on a Siemens SMART-CCD diffractometer with graphite-monochromated Mo Kα(λ=0.071 073 nm) at 20(2)℃using the SMART and SAINT programs[33]. 45 frames of data were collected at 298 K with an oscillation range of 1°per frame and an exposure time of 10 s per frame.Indexing and unit cell refinement were based on all observed reflections from those 45 frames.The structures were solved by direct method and refined on F2by full-matrix least-squares methods with SHELXTL[34].All non-hydrogen atoms except the disordered solvent molecules were refined anisotropically.Hydrogen atoms of the organic ligands were added geometrically and refined using the riding model,whereas hydrogen atoms of the coordination water molecules and non-disordered water molecules were found from difference Fourier map,however andrefined by fixed its O-H distances of 0.085 nm,and the isotropic thermal parameters being fixed 1.2 times of the atoms they attached.Hydrogen atoms of the disordered solvent molecules were not refined.The crystal data and structure determination summaries for these complexes are listed in Table 1.The selected bond length and angles are listed in Table 2.
CCDC:1042934,1;1042935,2.
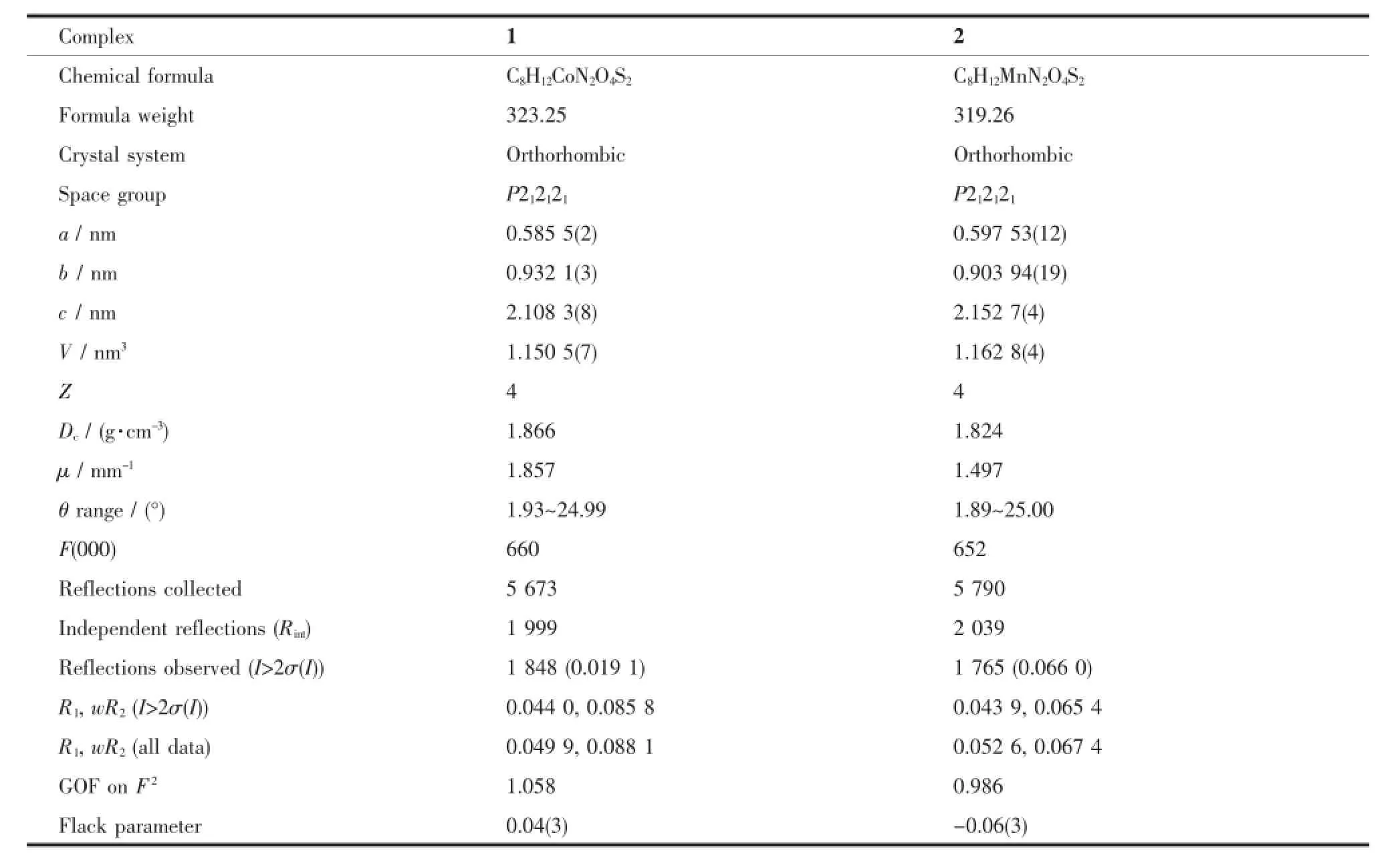
Table1 Crystallographic data for 1 and 2
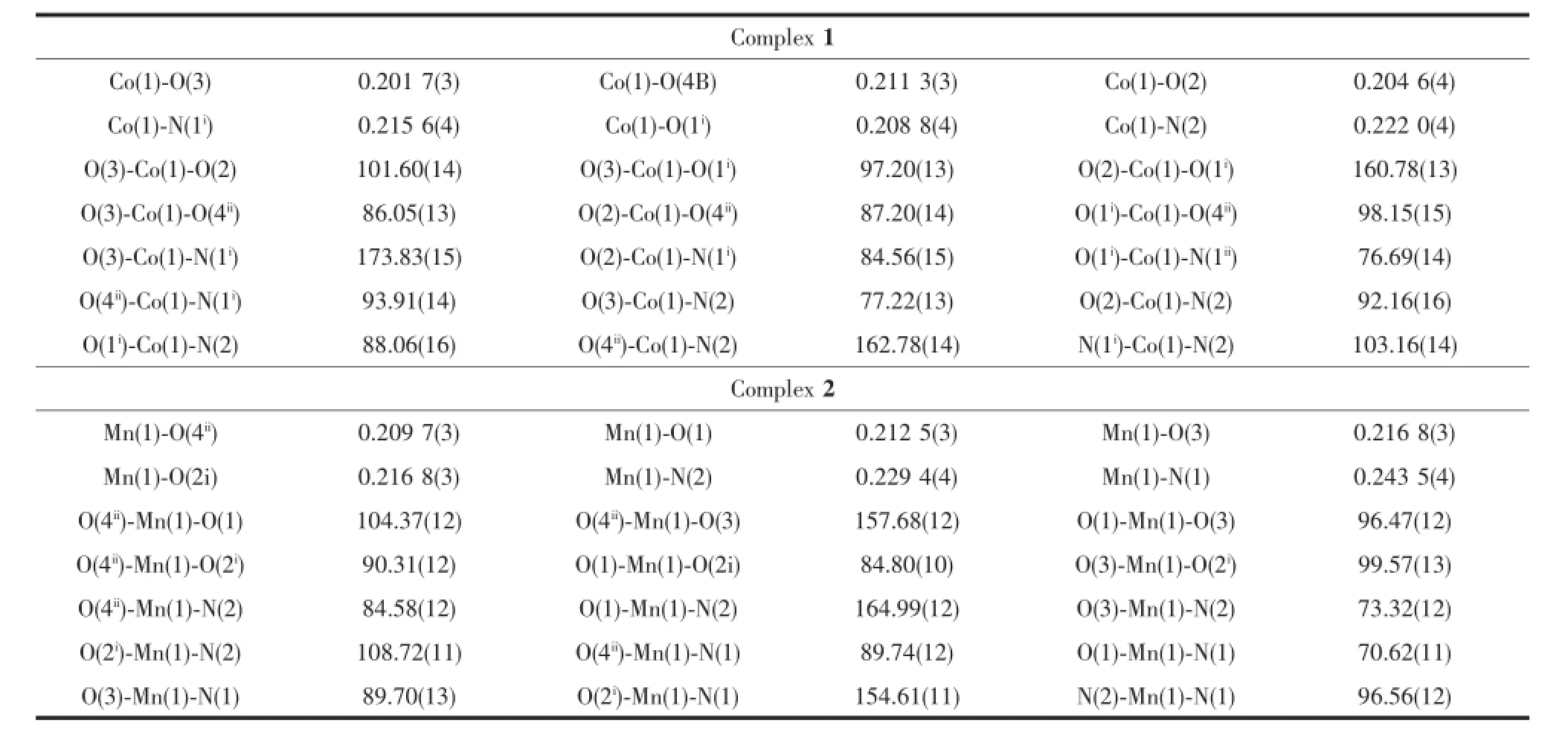
Table2 Selected bond lengths(nm)and angles(°)for 1 and 2
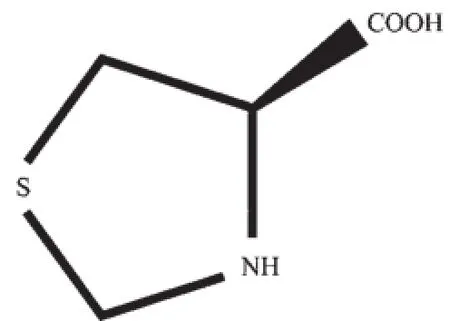
Scheme 1 Structure of LTP
2 Results and discussion
2.1 Description of the crystal structures
Reaction of Co(ClO4)3·6H2O and LTP·HCl,in the presence of triethylamine gave complex 1.Singlecrystal structure analyses reveal that complex 1 is made up of carboxylate-bridged[Co(LTP)]moieties and adopts a 2-D polymer structure.As shown in Fig. 1,each Coion is coordinated by two thiazolidine nitrogen atoms and four carboxylate oxygen atoms from four LTP ligands.The Co coordination sphere can be described as a distorted octahedral coordination with the Co-N bond distance varying from 0.215 6(4) to 0.222 0(4)nm.Co-O bond distances range from 0.201 7(3)to 0.211 3(3)nm.The ligand LTP forms a five-membered ring upon chelation to the metal ion. In the 2-D structure,each octahedral metal ion is linked to another four metal ions by four LTP ligands adopting a μ2-N1O2:O3coordination mode.Complex 2 is similar to 1 in the coordination mode and network structure and also crystallizes in the orthorhombic crystal system.
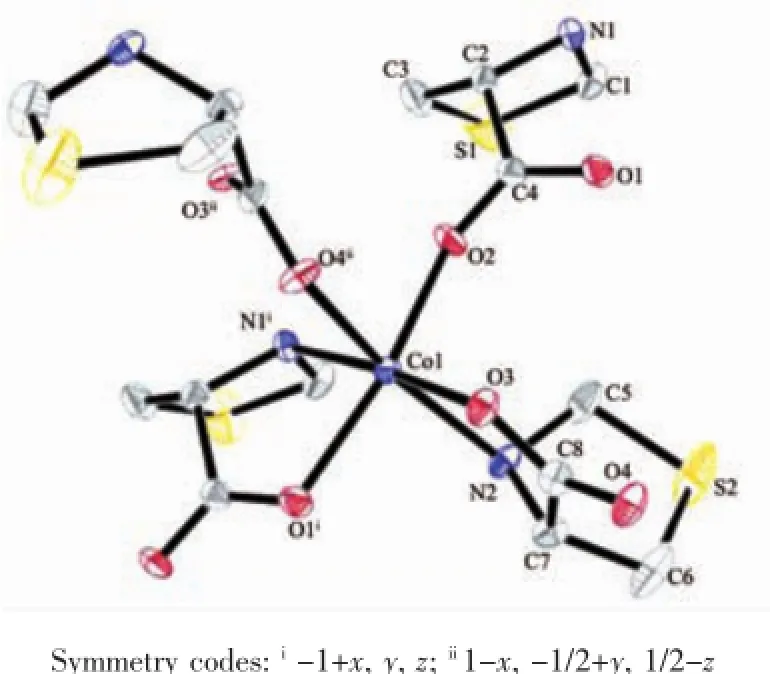
Fig.1 Coordination environment of Co(30%displacement ellipsoids)
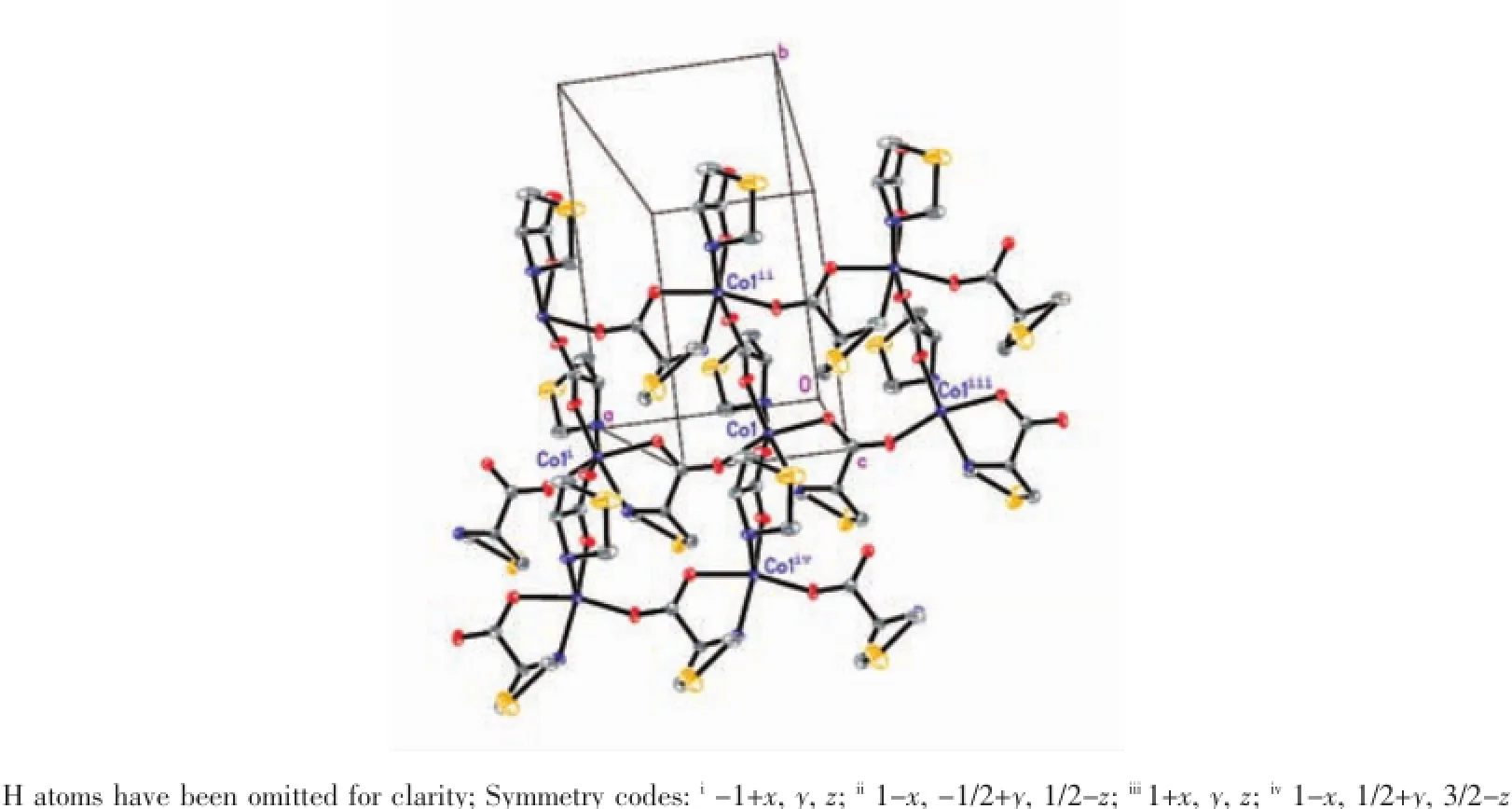
Fig.2 Fragment of the 2D plane of comp lex 1(30%disp lacement ellipsoids)
2.2 M agnetic properties for 1 and 2
Thetemperature-dependentmagnetic susceptibility data of complexes 1 and 2 have been collected for polycrystalline samples in the temperature range of 1.8~300 K.
Fig.3(a)shows the χmT and 1/χmversus T plots for complex 1.The χmT value gradually decreases from 2.87 cm3·K·mol-1at room temperature to 2.64 cm3· K·mol-1at 100 K;then,it rapidly decreases to 1.76 cm3·K·mol-1at 1.8 K,indicating a dominant antiferromagnetic interaction between the magnetic centers.At 300 K,the effective magnetic moment per Co atom(4.79μB)is higher than the expected spin-only value for S=3/2(3.87μB),attributed to the orbital contribution of Coion.The susceptibility data between 1.8 and 300 K follows the Curie-Weiss law with a Weiss constant θ=-7.56 K.The negative Weiss constant also confirms antiferromagnetic exchange between the Co.
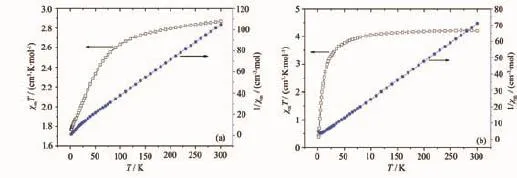
Fig.3 Temperature dependence of the magnetic susceptibility of complex 1(a)and comp lex 2(b)in the form of χmT and 1/χmvs T
Fig.3(b)shows the χmT and 1/χmversus T plots for complex 2.The room temperature effective magnetic moment(μeff)per Mnatom,determined from the equation μeff=2.828(χmT)1/2,is 5.80μB,which is close to the value expected for an isolated system of S=5/2( μeff=5.92μBfor g=2).On cooling from room temperature,the χmT value decreases smoothly.Below 40 K,χmT decreases abruptly until it reaches a minimum of 0.37 cm3·K·mol-1at 1.8 K,indicating that antiferromagnetic couplings are mediated between the Mnions.This is confirmed by a negative Weiss constant of-9.6 K determined by the Curie-Weiss law in the temperature range of 1.8~300 K.
3 Conclusions
In summary,two com plexes based on L-thioproline(LTP)have been obtained.X-ray diffraction analysis reveals that each octahedral metal ion is linked to another four metal ions by four LTP ligands adopting a μ2-N1O2:O3coordination mode.Moreover, the complexes at solid state exhibit weak antiferromagnetic couplings between the ions.
[1]Gingras M.Chem.Soc.Rev.,2013,42:1051-1095
[2]Ribó J M,Crusats J,Sagués F,et al.Science,2001,292: 2063-2066
[3]Prins L J,Huskens J,Jong,F,et al.Nature,1999,398:498 -502
[4]Yeung C S,Dong V M.Angew.Chem.,Int.Ed.,2011,50: 809-812
[5]Liu Y,Xuan W M,Cui Y.Adv.Mater.,2010,22:4112-4135
[6]Janiak C,Vieth J K.New J.Chem.,2010,34:2366-2388
[7]Zhang P,Li D S,Zhao J,et al.J.Coord.Chem.,2011,64: 2329-2339
[8]Nawal K A,Ian S T,Stephen P A.J.Am.Chem.Soc., 2008,130:11641-11649
[9]Mahata P,Prabu M,Natarajan S.Inorg.Chem.,2008,47: 8451-8463
[10]Gemma A,Josefina P,Jordi G A,et al.Aust.J.Chem., 2009,62:475-485
[11]Zhang L,Zhang J,Li Z J.Chem.Eur.J.,2009,15:989-1000
[12]Zhang X M,Tong M L,Chen X M.Angew.Chem.,Int.Ed., 2002,41:1029-1031
[13]Borsari M,Cascarano G,Giacovazzo C.Polyhedron,1999,18:1983-1989
[14]Yang E C,Chan Y N,Liu H.Cryst.Growth Des.,2009,9: 4933-4944
[15]Noriko C K,Rie Y,Yoshitaka S,et al.Inorg.Chem.,2012, 51:1640-1647
[16]Xu J,Yuan Q,Bai Z S.Inorg.Chem.Commun.,2009,12: 58-61
[17]Bauer C A,Timofeeva T V,Settersten T B.J.Am.Chem. Soc.,2007,129:7136-7144
[18]Hu T L,Tao Y,Chang Z.Inorg.Chem.,2011,50:10994 -11003
[19]Lan Y Q,Li L S,Wang X L,et al.Chem.Eur.J.,2008,14: 9999-10006
[20]Janiak C,Chamayou A,Royhan Uddin A K M,et al.Dalton Trans.,2009:3698-3709
[21]Ferey G.Chem.Soc.Rev.,2008,37:191-214
[22]Kristina G,Irena S,Igor A B,et al.Inorg.Chem.,2010,49: 4440-4446
[23]Sahoo S C,Kundu T,Banerjee R.J.Am.Chem.Soc.,2011, 133:17950-17958
[24]Saha R,Biswas S,Mostafa G.CrystEngComm,2011,13: 1018-1028
[25]Huang Q,Yu J C,Gao J K,et al.Cryst.Growth Des., 2010,10:5291-5296
[26]Wang M,Xie M H,Wu C D,et al.Chem.Commun.,2009, 2396-2398
[27]Rebilly J N,Bacsa J,Rosseinsky M J.Chem.Asian J., 2009,4:892-903
[28]Tang X L,Wang W H,Dou W,et al.Angew.Chem.Int. Ed.,2009,48:1-5
[29]Yang X L,Xie M H,Zou C,et al.CrystEngComm,2011,13: 6422-6430
[30]Kamiyama A I,Konno T.Dalton Trans.,2011,40:7249-7263
[31]He R,Song H H,Wei Z,et al.J.Solid State Chem.,2010, 183:2021-2026.
[32]Howard-Lock H E,Lock C J L,Martins M L,et al.Can.J. Chem.,1986,64:1215-1219
[33]SMART and SAINT,Area Detector Control and Integration Software,Siemens Analytical X-ray Systems,Inc.,Madison, WI,1996.
[34]Sheldrick G M.SHELXTL Ver5.1,Software Reference Manual,Bruker AXS,Inc.,Madison,WI,1997.
Structures and Magnetic Properties of Two Chiral Polynuclear M etal Clusters w ith Derivative of L-cysteine
XU Jian*,1XIE Li-Xia2ZHANG Chang-Li1
(1School of Biochemical and Environmental Engineering,Nanjing Xiaozhuang University,Nanjing 211171)
(2College of Sciences,Henan Agricultural University,Zhengzhou 450002)
An antiferromagnetic complexes based on Coor Mnand chiral ligand L-thioproline(LTP)with formula of[Co(LTP)2]n(1)and[Mn(LTP)2]n(2)were synthesized and structurally characterized.The ligand LTP forms a five-membered ring upon chelation to the metal ions.In the 2-D structure,each octahedral metal ion is linked to another four metal ions by four LTP ligands adopting a μ2-N1O2:O3coordination mode.Complexes 1 and 2 are same in the coordination mode and network structure,and crystallize in the orthorhombic crystal system. Magnetic measurements show that a weak antiferromagnetic coupling occurs in the complexes.CCDC:1042934, 1;1042935,2.
coordination comp lex;magnetic behavior;chiral am ino acid ligand
0614.81+2;O614.71+1
A
1001-4861(2015)04-0807-06
10.11862/CJIC.2015.102
2014-10-17。收修改稿日期:2015-01-15。
国家自然科学基金(No.21201057),河南省青年骨干教师(No.2013GGJS-042),江苏省自然科学基金(No.BK20140090)资助项目。*
