The Extraction Process Optimization of Phenolic Compounds from Okra Flower and Its Antioxidant Activities
2015-12-14SongshanQIUYananWANGHongmeiLVCuicuiJIANG
Songshan QIU, Yanan WANG, Hongmei LV, Cuicui JIANG
Development Center of Technology for Fruit & Vegetable Storage and Processing Engineering, Guangdong University of Petrochemical Technology, Maoming 525000, China
Okra is a kind of annual herbaceous plant belonging to Abelmoschus, Malvaceae,and also a kind of health care vegetable for the rich in many compositions such as protein,polyose,flavone and unsaturated fatty acid[1-2]. Okra flower is a kind of androgynous breed,rich in varieties of biology active compounds such as VA, carotene, mu coitin and flavonoid glycoside[3], and it’s proved that okra can suppress the growth of tumour and also exhibit cure effect of gastritis[4-5].Okra flower is in creasingly focused for its special functions and abundant nutrition[6]. Moreover, okra flower contains abundant polyphenol exhibiting stronger capacities of scavenging O2-·and antioxidant activities, studies show that the antioxidant activities are even several times that of VC and VE[7-8].At present,the methods to extract polyphenol in plant are water extraction,organic solvent extraction, supersonic extraction,ultrafiltration, super-critical extraction and microwave extraction[9-11]. Supersonic extraction as one of common use extraction processes can better break cell wall,enhance the extraction rate of polyphenol and enhance extraction efficiency for its comprehensive physical effects.Uniform design is a kind of experiment design methodology that only considers the evenly spread of experiment points in experiment area, which is able to make the optimization experiment simple and flexible, experiment time less and result accurate and reliable[12]. Currently,there are many studies about okra[13-14],however there are fewer studies about the extraction and analysis of polyphenol in okra. Thus, polyphenol in okraflower was extracted by supersonic assisted extraction in the experiment,the optimization of extraction process was studied using uniform design methodology, and the antioxidant properties were also analyzed in order to provide reference for the further development and utilization of okra flower.
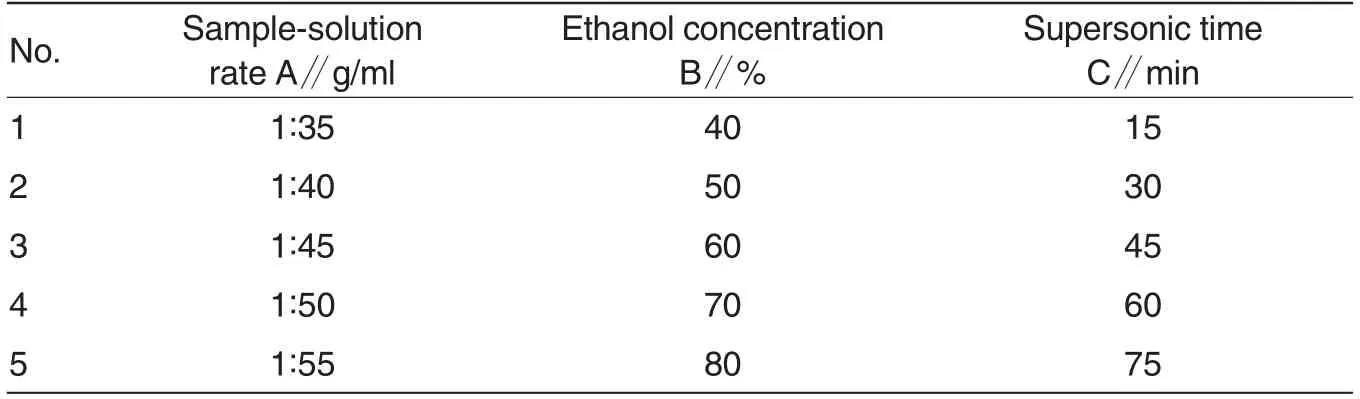
Table 1 Factor and level sheet U5(53)of uniform design methodology
Materials and Methods
Experimental materials and chemicals
Okra flower was purchased from Nongdele Agriculture Cooperative in Maoming, Guangdong Province,China. All chemicals were of analytical grade, including gallic acid,ethanol, VC, K3[Fe(CN)6], TCA, FeCl3,NaH2PO4, Na2HPO4, tris (methyl)amino methane, EDTA, pyrogallol,lead acetate, H3BO3, and distilled water was used in the experiment.
Extraction of polyphenol in okra flower
Okra flower was firstly dried in electro-thermostatic blast oven (DGG-9140A, Qixin Science Instrument Cooperation,Shanghai)for 24 h in 70 ℃.Dried okra flower was ground in a high-speed and multi-function mill(YB-400A, Yongkang Sufeng Industry and Trade Cooperation, Zhejiang) and then filtered using 40 mesh sieve to obtain fine powder. Secondly the pretreated dried powder was mixed with ethanol solution of given concentrations ranging from 40%to 80%and the ratios of sample to solution were changed from 1∶35 to 1∶55. Lastly, the mixed solution was extracted using supersonic assisted extraction for a period ranging from 15 min to 75 min.There are references[16-17]indicating that the extraction of polyphenol in 80W supersonic power can get higher extraction rate and keep more antioxidant activity. So supersonic power of 80W was selected for the supersonic assisted extraction.
Isolation and determination of polyphenol
The extraction slurry was centrifuged at 4 200 rpm/min for 20 min to collect the supernatant. Then the supernatant was incorporated and concentrated using a rotary evaporator(RE52AA,Yarong Biochemical Instrument Factory, Shanghai) at 55 ℃under vacuum. The resulting solution was mixed with dehydrated ethanol solution and kept overnight at 4 ℃.The solution was centrifuged at 4 200 rpm/min for 20 min, washed six times with dehydrated ethanol, and the precipitate was collected as crude extract.The extract was air-dried at 50 ℃until its weight was constant, and then was weighted with a balance (BS2202S,SARTORIUS, Germany). The extraction rate of polyphenol in okra flower was calculated according to the following equation.
Extraction rate of polyphenol(%)=Polyphenol mass(g)/Sanple mass(g)×100%
Gallic acid was used to make the standard curve by Folin-Ciocalteu colorimetry[15].The O.D was measured under 760nm, and the results were employed to make standard curve with the concentration of gallic acid as Xaxis and the O.D as Y-axis. The regression equation of standard curve was as follows: y=0.002 3x+0.000 4,R2=0.999 5, and the curve nearly exhibited a straight line in the range of 50-250 μg/ml.
Optimization of extraction process
After determining the preliminary range of the extraction variables through single-factor test, a uniform design with three independent variables (X1, sample-solution rate; X2,ethanol concentration; X3, supersonic time)at five levels was performed.The range of independent variables and their levels was presented in Table 1.
Analysis of antioxidant properties
Reducing power was measured by K3[Fe(CN)6]reduction method, and computed according to the standard curve of Vc[17]; the rate of scavenging O2-·was measured by pyrogallol oxidation method[18], and computed according to the equation shown by(1).
Where Acrepresented the O.D of blank tube under 325 nm and As represented the O.D of sample tube under 325 nm.
Result and Analysis
The effect of sample-solution rate on polyphenol extraction
The ratio of sample to solution is a factor that would influence the extraction efficiency and selectivity of the fluid. The effect of ratios of sample to solution on extraction rate of polyphenol was shown in Table 1. Extraction was carried out at different ratios of sample to solution conditions while other extraction parameters were as follows: ethanol concentration of 70%and supersonic time of 60 min.
It was shown in Table 2 that ratio of sample to solution had great impact on extraction rate of polyphenol in okra flower. Extraction rate of polyphenol reached the peak of 4.14% from 3.53%when ratio of sample to solution ranged from 1∶10 to 1∶30, then the extraction rate started to decrease, at last as ratio of sample to solution was 1 ∶50, the extraction rate dropped to 3.72%. The reason may be that more solution has bigger extraction capacity and contributes to the extraction of polyphenol, however, overmuch solution decreases polyphenol concentration, increases the difficulty of recollection and also leads to severe waste.Therefore, the ratio of sample to solution of 1∶30 was considered to be the best ratio of sample to solution in present experiment.
The effect of ethanol concentration on polyphenol extraction
The effect of ethanol concentration on extraction rate of polyphenol was shown in Table 3. Extraction was carried out at different ethanol concentration conditions while other extraction parameters were as follows:ratio of sample to solution of 1∶30 andsupersonic time of 60 min.
Table 3 showed that the extraction rate reached the peak of 4.12%as ethanol concentration was 50%. Then extraction rate started to decrease, at last extraction rate dropped to 2.52%as ethanol concentration was 80% .The reason may be that more ethanol concentration contributes to break cell wall and enhance extraction rate; but excessive ethanol concentration leads to decrease the polarity of solution and increase the volatility. Therefore,ethanol concentration of 50% was the optimal ethanol concentration in present experiment.
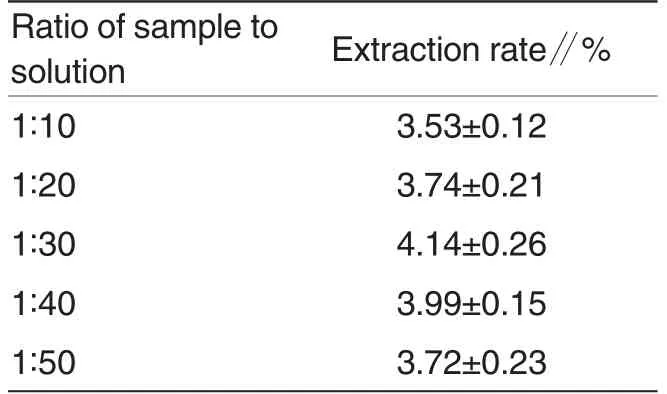
Table 2 Effect of the ratio of samplesolution on the extraction rate
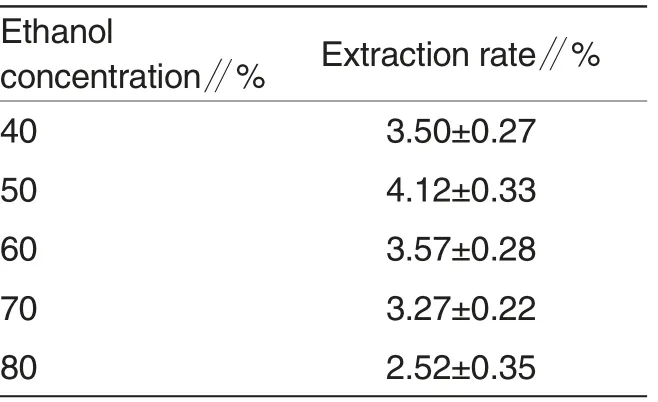
Table 3 Effect of ethanol concentration on the extraction rate
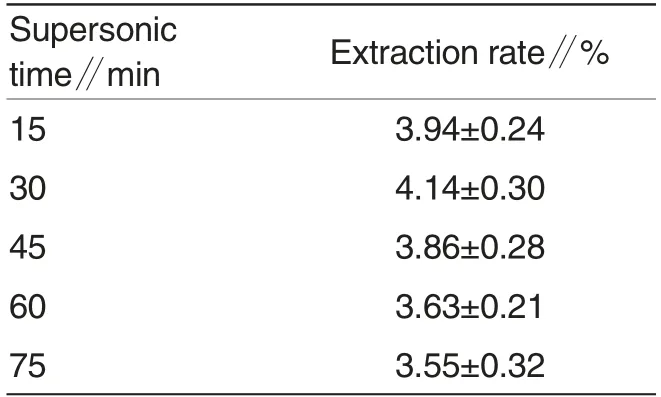
Table 4 Effect of supersonic time on the extraction rate
The effect of supersonic time on polyphenol extraction
Supersonic time works in the exposure of polyohenol to release medium where the liquid penetrated into the dried powder, dissolved the polyphenol and subsequently diffused out from the powder. While other extraction parameters were as follows: samplesolution rate of 1∶30 and ethanol concentration of 50%, the extraction rate of polyphenol was shown in Table 4 with the change of supersonic time.
Different supersonic times were shown in Table 4 to have different extraction rates of polyphenol.Extraction rate reached the peak of 4.14% after extracting 30min,and then in following time extraction rate started to fall sharply until 3.55%. The reason may be that supersonic will break chemical structure of polyphenol. Therefore, 30 min was considered to be the optimal supersonic time in present experiment.
Optimization of extraction process by uniform design methodology
Uniform design methodology is more advantageous than the traditional single parameter optimization in that it saves time, space and raw material.There were a total of 5 runs for optimizing the three individual parameters in the current uniform design. Table 5 showed the experimental conditions and results of extraction rate of polyphenol according to the factorial design.
By applying multiple regression analysis on the experimental data, the response variable and the test variables were related by the following second-order polynomial equation:
The multiple coefficients of correlation r =1.000 0 suggested a very close agreement between experimental value and predicted value. The coefficient of determination (r2) of predicted model was 0.995 4,indicating a good fit, and the predicted model seemed to represent the observed values. Therefore, the response was sufficiently explained by the predicted model. The significance of coefficients in the equation was determined using the Student t-test and P-value. The corresponding variables will be more significant if the absolute t-value becomes larger and the P-value becomes smaller. The t-values of coefficients in regression equation presented the result of P <0.01, which revealed that all coefficients exhibited significant meaning. Regression equation revealed that the optimal process was following: A=1 ∶35, B=55.34, C=21.5,and predicted extraction rate was 4.28% in regression equation. Because of experiment practicability, the process was finally ensured: A=1:35,B=55, C=22, namely sample-solution rate of 1:35, ethanol concentration of 55%and supersonic time of 22 min.
Three experiments were used to verify the optimal process. Eventually,the mean extraction rate was 4.26%,which was very close to the predicted value in uniform design regression model and evidently higher than others in the regression model.
Determination of polyphenol
Analysis of the crude extraction was carried out using Folin-Ciocalteu colorimetry, and the content of polyohenol was found to be 98.2%. Therefore, it’s foundamentally clear that the crude extraction was polyphenol.
Analysis of antioxidant properties
The O.D of polyphenol under 700 nm was measured by K3[Fe(CN)6]reduction method,and the absorbance related with the mass concentration of Vitamin C was shown by Fig.1.
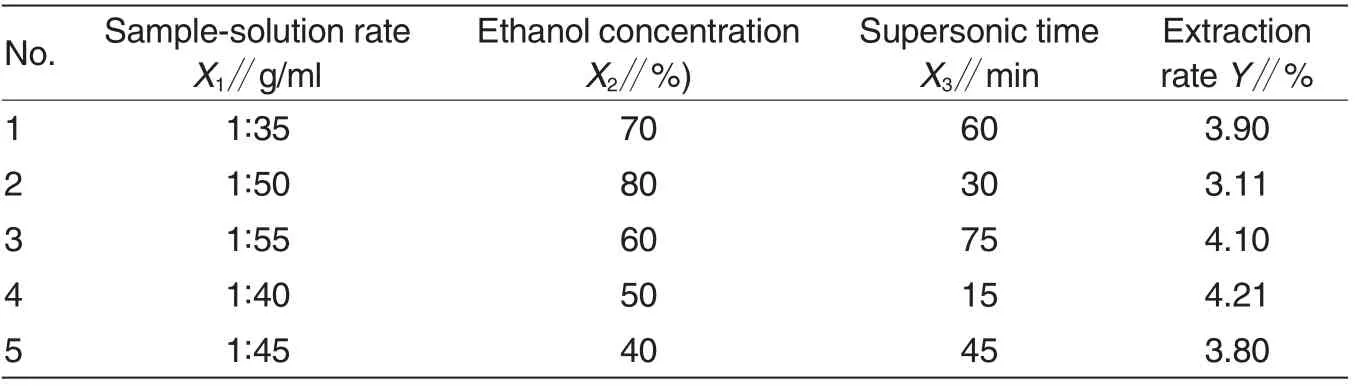
Table 5 The results of uniform design
The data above was analyzedby regression analysis, and the standard curve of Vc reducing power was as follows: y=0.004 1x-0.004 6, R2=0.998 7. The curve exhibited superior liner relationship in the range of 25-200 μg/ml. The reducing power of polyphenol in okra flower measured by the same method was 0.672. Therefore,the reducing power of polyphenol of 0.2 mg/ml in okra flower was 0.672,equal to that of Vc of 165 μg/ml. The capacity of scavenging O2-·was investigated by pyrogallol oxidation method. The rate of scavenging O2-·was 13.13%.
Conclusion
In order to enhance extraction efficiency and value of utilization and development of polyphenol in okra flower, the optimization of supersonic assisted extraction process was studied by uniform design methodology.The optimal extraction process of polyphenol in okra flower was as follows: sample-solution rate of 1 ∶35,ethanol concentration of 55% and supersonic time of 22 min,and extraction rate of polyphenol was 4.28%accordingly. The okra flower polyphenol exhibited stronger antioxidant properties,total reducing power of 0.2 mg/ml was 0.672 and the rate of scavenging O2-·was 13.13%. Therefore, polyphenol in okra flower is worth for further study and development for its stronger antioxidant properties.
[1]ZHENG W, ZHAO T, FENG WW, et al.Purification, characterization and immunomodulating activity of a polysaccharide from flowers of Abelmoschus esculentus [J]. Carbohydr Polymers,2014,106:335-342.
[2]PHONGLOSA A, BHATTACHARYYA K, RAY K, et al. Integrated nutrient management for okra in an inceptisol of eastern India and yield modeling through artificial neural network[J].Scientia Horticulturae,2015,187:1-9.
[3]ADELAKUN OE, OYELADE OJ, ADEOMOWAYE BI,et al.Chemical composition and the antioxidative properties of Nigerian Okra Seed(Abelmoschus esculentus Moench) Flour [J]. Food and Chemical Toxicology, 2009, 47 (6):1123-1126.
[4]WANG H,CHEN G,REN DD,et al.Hypolipidemic activity of okra is mediated through inhibition of lipogenesis and upregulation of cholesterol degradation[J]. Phytotherapy Research, 2014, 28(2):268-273.
[5]CHEN Y, ZHANG JG, SUN HJ, et al.Pectin from Abelmoschus esculentus:Optimization of extraction and rheological properties[J].International Journal of Biological Macromolecules, 2014, 70:498-505.
[6]AMEENA K,DILIP C,SARASWATHI R,et al. Isolation of the mucilages from Hibiscus rosasinensis linn, and Okra(Abelmoschus esculentus L.)and studies of the binding effects of the mucilages [J]. Asian Pacific Journal of Tropical Medicine,2010,3(7):539-543.
[7]CZYZOWSKA A, KLEWICKA E, NOWAK A. Polyphenols, vitamin C and antioxidant activity in wines from Rosa canina L. And Rosa rugosa Thunb[J].Journal of Food Composition and Analysis,2015,39:62-68.
[8]LIU Y, MA SS, IBRAHIM SA, et al.Identification and antioxidant properties of polyphenols in lotus seed epicarp at different ripening stages[J].Food Chemistry,2015,185:159-164.
[9]NAIMA R, OUMAM M, HANNACHE H,et al. Comparison of the impact of different extraction methods on polyphenols yields and tannins extraction from Moroccan Acacia mollissima barks[J].Industrial Crops and Products, 2015,70:245-252.
[10]KARAKASHOV B, GRIGORAKIS S,LOUPASSAKI S,et al.Optimisation of polyphenol extraction from Hypericum perforatum (St. John’s Wort) using aqueous glycerol and response surface methodology[J]. Journal of Applied Research on Medicinal and Aromatic Plants,2015,2(1):1-8.
[11]CAPRIOTTI AL,CAVALIERE C,CRESCENZI C, et al. Comparison of extraction methods for the identification and quantification of polyphenols in virgin olive oil by ultra-HPLC-QToF mass spectrometry [J]. Food Chemistry,2014,158:392-400.
[12]LIANG YZ, FANG KT, XU QS. Uniform design and its applications in chemistry and chemical engineering [J].Chemometrics and Intelligent Laboratory Systems,2001,58(1):43-57.
[13]KARTHIK P, VENUGOPAL S, MURTHY KD,et al.Bioefficacy,phytotoxicity,safety to natural enemies and residue dynamics of imidacloprid 70 WG in okra (Abelmoschus esculenta (L)Moench) under open field conditions[J].Crop Protection,2015,71:88-94.
[14]ALBA K, LAWS AP, KONTOGIORGOS V. Isolation and characterization of acetylated LM-pectins extracted from okra pods[J].Food Hydrocolloids,2015,43:726-735.
[15]VZQUEZ CV,ROJAS MGV,RAM REZ CA,et al.Total phenolic compounds in milk from different species. Design of an extraction technique for quantification using the Folin-Ciocalteu method[J]. Food Chemistry, 2015, 176: 480-486.
[16]PORTO CD, PORRETTO E, DECORTI D. Comparison of ultrasound-assisted extraction with conventional extraction methods of oil and polyphenols from grape (Vitis vinifera L.)seeds [J]. Ultrasonics Sonochemistry,2013,20(4):1076-1080.
[17]ZHANG YL,SHEN YX,ZHU YC, et al.Assessment of the correlations between reducing power, scavenging DPPH activity and anti-lipid-oxidation capability of phenolic antioxidants[J].Food Science and Technology, 2015,63(1):569-574.
[18]FANDEZ M, ROJAS M, BOHLE P, et al. Pyrogallol red oxidation induced by superoxide radicals: Application to evaluate redox cycling of nitro compounds[J].Analytical Biochemistry,2011,419(2):284-291.
杂志排行
Agricultural Science & Technology的其它文章
- Effect of Tree Species and Dosage of Rhizomorph Wood on Asexual Propagation of Wild Gastrodia elata.Bl.f.glauca S.Chow in Ganzi
- Effects of Different Application Times of Tillering Fertilizer on Grain Yield and Population Development of Double-cropping Rice Transplanted by Machine
- Biological Characteristics and Pathogenicities of Shewanella algae and Shewanella abalone from Babylonia
- Research on Physiological Characteristics of Tall Fescue under Nitrogen Stress
- Cloning and Characterization of Phytochrome A Gene FaPHYA from Tall Fescue
- Changes in Physiological Indexes of SPDS Transgenic Potato Plants under Low Temperature Stress
