Biological Characteristics and Pathogenicities of Shewanella algae and Shewanella abalone from Babylonia
2015-12-14ShufangLIJidongZHANGDequanQIUShipingYANGZitongHUANG
Shufang LI, Jidong ZHANG*, Dequan QIU, Shiping YANG, Zitong HUANG
1. Department of Veterinary Medicine, Agricultural College of Guangdong Ocean University, Zhanjiang 524088, China;
2. Fisheries College of Guangdong Ocean University, Zhanjiang 524088, China;
3. Zhuhai Entry-Exit Inspection and Quarantine Bureau, Zhuhai 519015, China
Shewanella is a new genus belonging to Vibrio, and is widely distributed in seawater and sediments[1-2].Shewanella is a class of indigenous intestinal bacteria in some water animals[3-4]. Studies have shown that some species of Shewanella are conditionally pathogenic bacteria to aquatic animals and human[5]. The US Centers for Disease Control and Prevention classified the Shewanella related to human diseases into two major classes, Shewanella putrefaciens and Shewanella alga[5]. In clinics, 77%of the isolates were II-biotype Shewanella putrefaciens[6], and 80% were Shewanella alga[7]. In recent years, it has been found that some species of Shewanella, such as Shewanella putrefaciens, Shewanella alga, She-wanella aquimarina and Shewanella smarisflavi have pathogenicities to Stichopus japonicus, Scophthalmus maximus, Strongylocentyotus internedius, Sciaenops ocellatus and Abramis brama Berg[8-12], indicating that Shewanella is a new class of pathogens to aquaculture animals[8].Wei et al.[13]first isolated Shewanella putrefaciens from commercial fresh pork. Wang et al.[14]isolated Shewanella putrefaciens and Shewanella alga from samples of diarrhea patients, suggesting that food contamination by Shewanella may become a new risk factor for foodborne diseases in human.Khashe et al.[15]found that in mice, Shewanella alga has stronger pathogenicity compared with Shewanella putrefaciens,so they speculated that Shewanella alga is the most important pathogen in Shewanella.Shewanella alga usually infects ears and soft tissues of humans in clinics,and could cause more serious infections, such as bacteremia, meningitis, endocarditis and periostitis[16-17]. In blood,pus,bile,pericardial fluid,urine,sputum and other secretions and trauma tissues of patients, Shewanella alga all could be detected, and in the stool of diarrhea patients and pond water, flies and domestic livestock feces around the patients, Shewanella alga all could be isolated[5]. Currently, the infection caused by Shewanella has attracted widespread concern from clinical medicine,microbiology,aquatic animal breeding,food safety and public health. In this study, the strains of Shewanella algae and Shewanella abalone were isolated from Babylonia suffered from proboscis edema in a farm in Zhanjiang City, Guangdong Province, and their biological characteristics and pathogenicities to mice,chicks and Babylonia were preliminarily studied, thereby providing a reference for researches on pathogenicities of Shewanella to other animals.
Materials and Methods
Source of diseased samples
In October,2011,the disease with symptoms of proboscis edema and burrowing inability occurred in a Babylonia farm in Zhanjiang, Guangzhou.The mortality of Babylonia reached 50% -80% . Total ten ill Babylonia shells were sampled, and they were placed in a sterilized flask. And then,the Babylonia shells were transferred to the laboratory in an ice box.
Main reagents and media
The CHROMagar vibrio colored media were purchased from the Shanghai Central Bio-Engineering Limited Company. The TCBS media were purchased from the Guangdong Huankai Microbial Sci. & Tech. Co.,Ltd. The biochemical reagents for identification were purchased from the Hangzhou Tianhe Microorganism Reagent Co., Ltd. The nutrient broth and nutrient agar were purchased from the Beijing Land Bridge Technology Co., Ltd. The blood agar plates were prepared by the laboratory itself. The MightyAmp®DNA Polymerase kit and DNA Marker were purchased from the TaKaRa Biotechnology (Dalian) Co.,Ltd. The 16SrDNA universal primers were synthesized by the Shanghai Invitrogen Biotechnology Co.,Ltd.
Main apparatus and instruments
In this study,the used main apparatus and instruments included PCR instrument (Biometra Tg PCR), electrophoresis apparatus (DYY-8C,Beijing Liuyi Instrument Factory) and biochemical incubator (SPX-250B-Z,Shanghai Boxun Industry & Commerce Co.,Ltd.).
Experimental animals
The germ-free Kunming mice(half male and half female), weighing 20±2 g,were purchased from the Experimental Animal Center of Guangdong Medical University. After oneweek conventional farming, the mice were used for artificial infection test.
The one-day-old Sanhuang chicks (cockerels) were purchased from a hatchery in Suixi, Zhanjiang.After one-week conventional farming,they were used for artificial infection test.
The healthy Babylonia shells,with bright shells,size of (3.41±0.14)cm×(2.01±0.20) cm and normal intaking and moving abilities were purchased from a Babylonia farm in Zhanjiang.They were farmed in a plastic tank, of which the bottom was laid with plastic cage. On the cage, sterilized sand in thickness of 4-5 cm was bedded. In the plastic tank, there was filtered clean seawater in height of 15 cm.The seawater was inflated for 24 h continuously. Every two days, one third of the total seawater was replaced. The water temperature was maintained at 22-26.5 ℃. After one-week conventional farming, the artificial infection test was started.
Isolation and purification of bacteria
In a sterile environment,the shells were broken, and the softwares were removed completely. The softwares were rinsed with sterile saline, and then partial hepatopancreases were cut off. The fresh cutting interfaces were inoculated on the blood far plates, which were then placed in an incubator at 35 ℃for 18-24 h. From each of the plates, 3-5 colonies were selected for each sample, and they were inoculated in NaCl nutrient agar(3.5%, pH 7.8) for purified culture respectively. Finally, the strains were conserved for use.
PCR analysis and cultural characteristics of Shewanella
16S rDNA PCR,sequencing and sequence analysis The genomic DNAs were extracted from the newlyconserved strains and used as templates. With the bacterial universal primers (27f: AGAGTTTGATCCTGGCTCAG; 1492r: TACGGCTACCTTGTTACGACTT), the 16S rDNA PCR amplification was performed according to the instructions of MightyAmp®DNA Poiymerase Ver.2.The reaction system was as follows:2× MightyAmp buffer 25 μl, Primer 1 15 pmol, Primer 2 15 pmol,MightyAmp ® DNA Polymerase 1 μl(0.025 U/μl), adding ddH2O to 50 μl.The reaction conditions were as follows: pre-denaturation at 98 ℃for 2 min;denaturation at 98 ℃for 10 s,annealing at 55 ℃for 15 s, extension at 68 ℃for 90 s, 40 cycles; extension at 68 ℃for 90 s.The amplification products were examined by 1% agarose gel electrophoresis. The sequencing was completed by the Shanghai Invitrogen Biotechnology Co., Ltd. The sequencing results were aligned with the nucleotide sequences in NCBI using Blast.
Cultural and morphological characteristics of Shewanella After the 16S rDNA sequence analysis, the Shewanella strains were inoculated inthe blood agar media, TCBS media and CHROMagar vibrio colored media respectively. They were incubated at 35 ℃for 18-24 h. Subsequently, the colony characteristics were observed.In addition, the single colonies in the blood agar media were picked,smeared and subjected to Gram staining. The results were observed under a microscope.
Biochemical reactions of Shewanella The strains above were inoculated in the NaCl nutrient agar(3.5%) respectively and incubated at 35 ℃for 18-24 h. Subsequently, the fresh colonies were picked and inoculated in the biochemical identification tubes for Vibrio,including glucose,sucrose, arabinose, mannose, inositol,VP test, lysine, arginine, 0% NaCl peptone water, 3% NaCl peptone water, 6% NaCl peptone water, 8% NaCl peptone water and 10%NaCl peptone water. The incubation was performed at 35 ℃. According to the instructions,the tests were carried out and the results were observed.
Artificial infection test of Shewanella
Preparation of test strains of Shewanella One strain of each Shewanella algae and Shewanella abalone, of which the 16S rDNA sequence showed 100%of similarity with that of model strain, was selected as the test strain.They were inoculated in NaCl nutrient broth (3.5%, pH 7.8) respectively and incubated at 35 ℃for 18 h. Using plate count method, the concentration of Shewanella algae in the nutrient broth was adjusted to 1.20×108cfu/ml, and of Shewanella abalone was adjusted to 3.8 ×108cfu/ml for artificial infection tests.
Artificial infection test in mice The 36 mice were randomly divided into six groups with six mice for each group.The Group 1 and Group 2 were treated as control groups.In Group 1,each mouse was injected abdominally with 0.2 ml of NaCl nutrient broth(3.5%,pH 7.8); while in Group 2, each mouse was injected abdominally with 0.5 ml of NaCl nutrient broth (3.5%, pH 7.8).And then,they were farmed isolatedly.In Group 3, each mouse was injected abdominally with 0.2 ml of Shewanella algae nutrient broth; while in Group 4,each mouse was injected abdominally with 0.5 ml of Shewanella algae nutrient broth. In Group 5, each mouse was injected abdominally with 0.2 ml of Shewanella abalone nutrient broth;while in Group 6,each mouse was injected abdominally with 0.5 ml of Shewanella abalone nutrient broth. After the injection,the activities and mortality of mice were observed every day,and the observation lasted for 7 d.The dead mice were dissected as soon as possible, and pathological changes in their internal organs were observed and recorded; moreover, bacterial isolation was carried out from their livers.If the isolated strain had the completely same cultural and morphological characteristics with the inoculated strain,the death of mouse was considered to be caused by the test strain.
Artificial infection test in chicks
The 36 one-week-old chicks were randomly divided into six groups with six chicks for each group. The Group1 and Group 2 were control groups;Group 3 and Group 4 were Shewanella algae infection groups; Group 5 and Group 6 were Shewanella abalone infection groups. The treatment method was as described above. The incidence and mortality of chicks were observed and recorded,and the observation lasted for 7 d. The pathological examination and bacterial isolation were carried out for test chicks.
Artificial infection test in Babylonia The 60 Babylonia shells were randomly divided into six groups with 10 for each group. The Group1 and Group 2 were control groups; Group 3 and Group 4 were Shewanella algae infection groups;Group 5 and Group 6 were Shewanella abalone infection groups.The bacteria were injected into the foot muscles of the Babylonia shells,and the injection amounts were as described above. After the infection, the climbing, burrowing, intaking and mortality of Babylonia shells were observed, and the observation lasted for 30 d.
Determination of half lethal doses(LD50s)of Shewanella
Preparation of test strains The Shewanella algae strains, as used in the artificial infection tests,were inoculated in NaCl nutrient agar (3.5%, pH 7.8) and incubated at 35 ℃for 18 h.The colonies and lawn were washed with sterilized 3.5%NaCl solution.And then, the bacterial concentration was adjusted to 0.5 McFarland units(equivalent to 1.5×108cfu/ml).Subsequently,the bacterial concentration was diluted gradiently into 1.5×107, 1.5×106, 1.5×105, 1.5×104and 1.5×103cfu/ml, respectively for toxicity determination test.
Determination of half lethal doses
Total 35 clinically healthy mice were divided into seven groups with five mice for each group. The mice in each group were injected abdominally with 1.5×108, 1.5×107, 1.5×106, 1.5×105,1.5×104and 1.5×103cfu/ml of fresh Shewanella algae broth(0.1 ml/mouse),respectively, and the mice in the other group were injected with equivalentamount sterilized 3.5% NaCl solution(control). After the injection, the incidence and mortality of the mice were observed every day, and the observation lasted for 7 d.The half lethal dose(LD50) of the test strain was calculated using the Reed-Muech method.
Results and Analysis
Bacterial isolation and 16S rDNA identification
Total 33 hemolytic strains were isolated from the 10 Babylonia shells.The 16S rDNA PCR amplification products were examined by 1% agarose gel electrophoresis, and a gene fragment in length of 1 490 bp was amplified from all the strains.The 16S rDNA sequence alignment showed that there were 21 strains belonging to Shewanella, 10 strains belonging to Vibrio and 2 strains belonging to Bacillus. The 16S rDNA sequences of the 21 Shewanella strains were aligned with the known sequences in GenBank. The results showed that among the 21 strains, 12 strains showed 100% or 99% of homologies with the model strain OK-1 (registration number NR028673)of Shewanella algae, and 9 strains showed 100%or 99% of homologies with the model strain DW.1 (registration number NR 044134)of Shewanella abalone.
Cultural characteristics of Shewanella
In the 3.5% NaCl nutrient agar media (pH 7.8),the colony characteristics of Shewanella algae and Shewanella abalone were basically thesame. The colonies were all round, white,smooth and moist with neat edges and convex surfaces. On the blood agar plates, the colonies of Shewanella algae and Shewanella abalone were all white, smooth,moist and sticky. Shewanella algae possessed β hemolysis, while Shewanella abalone possessed α hemolysis. On the TCBS plates, the colonies of Shewanella algae and Shewanella abalone were all round, green with neat edges and fat convexes. However, when the incubation time exceeded 24 h, the edges of Shewanella algae colonies turned black,but the centers were still green; the Shewanella abalone colonies were still uniform green. In the CHROMagar vibrio colored media, both Shewanella algae and Shewanella abalone colonies were colorless transparent. However,if observed from the back of the media,the colonies were all light green.
Morphological and biochemical characteristics of Shewanella
Shewanella algae and Shewanella abalone are all gram-negative bacilli. The Shewanella algae thalli are straight rodshaped,and a few are slightly curved.They are scattered. Both the ends are rounded.The Shewanella abalone thalli stubby rod and club-shaped, and a few are slightly curved. Both the ends are rounded. The thalli are usually scattered, while clubshaped thalli always exist in pairs.
The results of physiological and biochemical tests showed that the 12 Shewanella algae strains exhibited the completely same type of biochemical reactions,and the 9 Shewanella abalone strains also exhibited the completely same type of biochemical reactions. Both Shewanella algae and Shewanella abalone are non-fermenting bacteria. They do not break down glucose,sucrose,arabinose,mannitol or inositol. They are all VP-negative. Shewanella abalone can produce lysine decarboxylase and arginine decarboxylase, while Shewanella algae can only produce arginine decarboxylase. The two kinds of strains all grow well in 3%-6% NaCl peptone water,but cannot grow in salt-free peptone water or peptone water with NaCl concentration higher than 8%(Table 1).
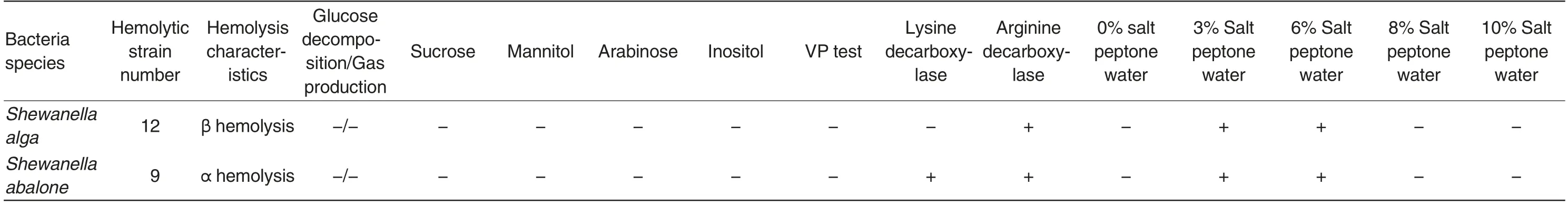
Table 1 Hemolysis and biochemical characteristics of Shewanella
Determination of pathogenicities of Shewanella and toxicity of Shewanella algae
Artificial infection test of Shewanella to mice The mortalities of test animals infected with Shewanella algae and Shewanella abalone were shown in Table 2.There was no death in mice in the control group during the experimental period. The pathological autopsy of the mice that died of Shewanella algae infection showed that their livers, spleens, kidneys, lungs, hearts and other solid organs were all congestive,bleeding and swelling,and the blood clotting was incomplete, which were all typical symptoms of sepsis.The stomachs and intestinal mucosa were all bleeding. There were a lot of gas and bleeding mucus in the intestinal tracts, and the inflation was most obvious in small intestines. The livers and spleens of the mice that died of artificial infection of Shewanella abalone were slightly swelling and bleeding, but there were no significant pathological changes in the other solid organs. The strains that showed the completely same cultural and morphological characteristics with the originally inoculated strains were isolated from the livers of all the infected mice. There were no significant pathological changes in internal organs of mice in the control group,and none bacteria were isolated from their internal organs.
Artificial infection test of Shewanella to chicks The chicks in the two Shewanella algae infection groups all died within 24 h since the infection. In the two Shewanella abalone infection groups,the chicks showed transient depression,feather handstand and appetite loss, which gradually returned to normal after 24-48 h. Total 12 chicks survived 7 d after the infection. In the control groups,the mental state,feeding and activities of the chicks were all normal, and no death was observed.
The pathological autopsy of the chicks that died of Shewanella algae infection showed that their livers, spleens, kidneys,lungs and brains were all swelling and congestive, and the blood clotting was incomplete, which were all typical symptoms of sepsis. The stomach mucosa and intestinal serosa and mucosa were all bleeding.There were a lot of gas and bleeding mucus in the intestinal tracts. The livers and spleens of the chicks infected with Shewanella abalone were slightly swelling, but not bleeding. From the livers of chicks infected with Shewanella algae or Shewanella abalone, bacterial strains that showed the completely same cultural and morphological characteristics with the originally inoculated strains were isolated.There were no significant pathological changes in the other solid organs.There were no significant pathological changes in various solid organs of chicks in the control groups,and none bac-teria were isolated.
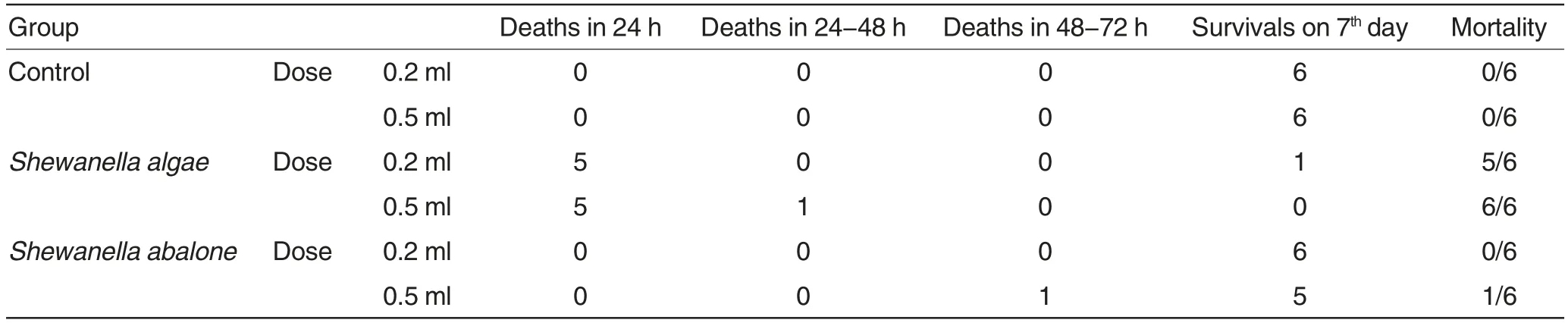
Table 2 Artificial infection test of Shewanella to mice
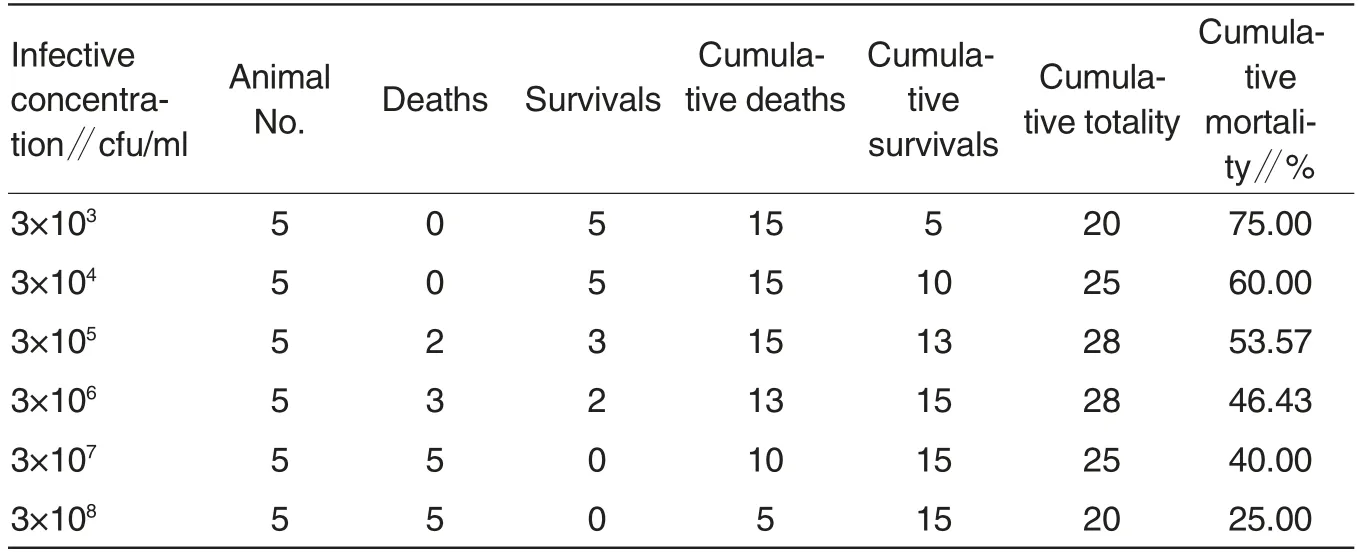
Table 3 Determination of half lethal dose of Shewanella algae(Reed-Muech method)
Artificial infection test of Shewanella to Babylonia After the injection of Shewanella algae to foot muscles, the Babylonia shells in the infection groups all lay on the sand surface in the plastic tank, and the burrowing, climbing and intaking activities were all abandoned. After 24 h, all the Babylonia shells in the infection groups sneaked into the sand and abandoned their climbing and intaking activities, except one Babylonia shell in Group 4 still lying on the sand surface(it died 18 d after the infection; during the period of survival, it made responses to mechanical stimuli, but had no spontaneous activities). On day 3 since the infection, a few Babylonia shells climbed out of the sand, but they still did not take food. On day 7, the 10 Babylonia shells in Group 3 and 9 Babylonia shells in Group 4 all could climb out of the sand themselves, but their exercise capacities were significantly reduced; there were almost no climbing and intaking activities on the sand surface. On day 12, most Babylonia shells in the two infection groups recovered their climbing and burrowing capacities, and were prone to taking food. On day 2 and 3 since the infection, the Babylonia shells infected with Shewanella abalone all could sneak into the sand themselves; and after another two days,they all could climb out and sneak into the sand themselves,and were prone to taking food. After another three days, the climbing and intaking activities recovered to normal,and no death was observed. In the control groups, the Babylonia shells were injected with sterilized 3.5%NaCl nutrient broth, and they all could climb out and sneak into the sand easily 5-6 h after the injection. Till then, their climbing, burrowing and intaking capacities recovered to normal. There was no death in the control groups.Proboscis edema did not occur in Babylonia shells infected with Shewanella algae or Shewanella abalone 30 d after the injection.
Toxicity (LD50) determination of Shewanella algae The mortalities of mice in various groups were shown in Table 3. Using the Reed-Muech method, the half lethal dose (LD50) of the representative strains of Shewanella algae was calculated as 10-5.50/0.1 ml.
Discussion
Pathogenicities of Shewanella algae and Shewanella abalone to Babylonia
Since the ill Babylonia was symptomatized by proboscis edema and climbing, burrowing and intaking inability or loss, and the disease was trended to be spread across the farming pond with mortality more than 50%, the disease was speculated to be caused by pathogenic bacterial infection. Therefore, in this study,the hemolytic strains were isolated and identified according to the isolation and culture methods of Vibrio. The bacterial isolation results showed that Shewanella algae and Shewanella abalone are the dominant bacteria in ill Babylonia.However,the artificial infection tests showed that the Shewanella algae or Shewanella abalone infection could not lead to proboscis edema. It suggested that Shewanella algae and Shewanella abalone were not the major or only pathogenic factors of the disease in Babylonia, and they might be cooperative factors for other pathogens or secondary infections.The artificial infection tests also showed that Shewanella algae has obvious pathogenicity to all of Babylonia, mice and chicks. The Babylonia infected with Shewanella algae lost the abilities of burrowing, climbing and intaking for a long time, which might be related to the propagation and invasion of Shewanella algae in Babylonia.Shewanella algae can produce tetrodotoxin(TTX).TTX can selectively bind with receptors of sodium channels on the nerve cell membrane and block the sodium channels, obstructing the associated physiological activities,with symptom of nerve and muscle paralysis[22]. Therefore, the longtime loss of moving and intaking abilities in Babylonia artificially infected with Shewanella algae may be associated with neural inhibition and paralysis caused by TTX.
So far,there have been no reports on isolation and pathogenicity of Shewanella abalone in aquatic animals. In this study, Shewanella abalone was isolated from the ill Babylonia, and the representative strains of Shewanella abalone caused the short-term loss of moving and intaking abilities in Baby-lonia, which recovered to normal finally.It indicates that the pathogenicity of Shewanella abalone is not strong to Babylonia, and was not major pathogen for the incidence.
Pathogenicities of Shewanella to mice and chicks
In this study, the isolated Shewanella algae possess β-hemolysis,and the half lethal dose (LD50) of the representative strains is 10-5.50/0.1 ml.It can cause mice and one-week-old chicks to die of sepsis,suggesting that Shewanella algae isolates have stronger pathogenicity, and hemolysis may be a major pathogenic factor for sepsis in mice and chicks. Whether the TTX and hemolysis produced by Shewanella algae is the same substance still needs to be confirmed by further studies. There have been rare reports on pathogenicity of Shewanella algae to aquatic animals,livestock and poultry. Zhang et al.[10]reported that Shewanella algae is the pathogen for kidney swelling in Sciaenops ocellatus.Chen et al.[19]found that Shewanella algae is the pathogen for ulcer disease in Sciaenops ocellata. So far, there have been no reports on incidence of Shewanella algae in livestock and poultry. This study first confirmed that Shewanella algae has pathogenicity to chicks. In coastal areas, the Shewanella algae-contaminated feed, addition of Shewanella algae-contaminated fish meal and shell meal in feed are all likely to cause illness and death in livestock and poultry. Therefore, the pathogenicity of Shewanella algae to livestock and poultry deserves the attention by related areas.
Role of Shewanella algae in food hygiene inspection and public health
TTX is a highly toxic alkaloid, and it was once considered as one of the largest-toxicity neurotoxins in the nature.The toxicity of TTX is higher than that of sodium cyanide by 1 250 times,and only a trace amount (0.5 mg)can cause death of human. TTX has thermal stability. Neither salting nor sunlight can destroy its toxicity. TTX can only be broken down by more than 30 min of high temperature heating or under basic conditions. It can be completely destroyed by 20 -60 min of heating at 220 ℃.The incubation period of TTX poisoning is very short. The short is 10-30 min,while the long is 3-6 h.The onset is acute,and once poisoned, human may die as soon as within 10 min, as late as within 4-6 h.Studies have shown that some species of Vibrio and Shewanella in seawater can produce TTX. Although the yield is low to the level of ng, TTX can still be enriched and accumulated in marine organisms that take the chlorella and small conch adsorbed by TTX. Vibrio and Shewanella are common bacteria in marine animals.When certain diseases occur in animals or body resistance is weakened, TTXproducing bacteria may become the dominant bacteria. Thus the TTX production is increased, leading to TTX poisoning in humans and terrestrial animals that eat seafood. The cooking processes of fish, shrimp and other seafood are usually shorter, so the TTX cannot be completely destroyed by heating, leading to food poisoning.Therefore, aquatic product contamination by Shewanella algae may become another important risk factor for foodborne infections in coastal areas.
[1]SATOMI M,VOGEL BF,VENKATESWARAN K,et al.Description of Shewanella lacialipiscicola sp. Nov. and Shewanella algidipiscicola sp.Nov.,isolated from marine fish of the Danish Baltic Sea, and proposal that Shewanella affinis is alater heterotypic synonym of Shewanella colwelliana [J]. Int. J. Syst.Evol.Micr.,2007,7:347-352.
[2]XIAO X,WANG P,ZENG X,et al.Shewanella psychrophila sp, Nov. and Shewanella piezotolerans sp. Nov., isolated from west Pacific deep-sea sediment[J]. Int. J. Syst. Evol. Micr., 2007,57:60-65.
[3]ZHANG WJ(张文姬),HOU HM(侯红漫),ZHANG GL(张公亮),et al.Study on diversity of intestine cultivable microorganisms from Apostichopus japonicas(仿刺参肠道可培养微生物多样性研究)[J].Science and Technology of Food Industry (食品工业科技), 2011, 32(9):149-155.
[4]GU JD (顾继东), FAN YZ (范延臻),MITCHELL RALPH. Biological control of Zebra mussels by indigenous pathogenic bacteria and their extracellular products(土著致病菌及其胞外物在生物防治斑马贝中的作用)[J]. Chin J Appl Environ Biol (应用与环境生物学报),2001,7(6):572-576.
[5]YANG JC(杨晋川),GUO H(郭惠),XU JJ( 许静静), et al. Study on biological characteristics of Shewanella(希瓦菌生物学特性的实验研究)[J].Chinese Journal of Zoonoses(中国人兽共患病学报),2009,25(7):699-700.
[6]SHIDEH KH. Biochemical and pathogenic properties of Shewanella alga and Shewanella putrefaciens[J].Clin Microbiol,1998,36(3):783-787.
[7]WANG YL(汪永禄),WANG DC(王多春),ZHAN SW (詹圣伟). Advances in research on pathogenicity of Shewanella(施万氏菌致病性的研究进展)[J]. Chinese Journal of Zoonoses (中国人兽共患病学报),2011,27(5):444-446.
[8]QIN L(秦蕾),ZHANG XJ(张晓君),BI KR(毕可然). A new pathogen of gibel carp Carassius auratus gibelio-Shewanella putrefaciens(一种新的异育银鲫病原-腐败希瓦氏菌)[J]. Acta Microbiologica Sinica(微生物学报), 2012, 52(5): 558-565.
[9]GAO XT (高晓田),XIAO GH (肖国华).Isolation and phylogenic analysis of pathogen of red mouth disease in Scophthalmus maximus (大菱鲆红嘴病的病原分离鉴定与系统发育分析)[J].Hebei Fisheries(河北渔业),2011,4:8-10.
[10]ZHANG JT(张继挺),ZHU WY(朱文渊),WANG GL (王国良). Pathogeny and pathogenicity of kidney intumesce of Sciaenops acellatus (美国红鱼肾肿大症的病原及其致病性研究)[J]. Journal of Ningbo University (Science and Technology)(宁波大学学报(理工版)),2013,26(1):6-11.
[11]LI H, QIAO G, LI Q, et al. Biological characteristics and pathogenicity of a highly pathogenic Shewanella marisflavi infecting sea cucumber, Apostichopus japonicas [J]. J. Fish Dis.,2010,33:865-877.
[12]CHENG P(成沛),ZHANG FP(张凤萍),LI SZ(李胜忠),et al.Isolation,identification and drug sensitive test on pathogens of skin ulcer from European bream (东方欧鳊腐皮病病原菌的分离鉴定及药敏试验的研究)[J].Journal of Aquaculture (水产养殖),2013,34(10):1-4.
[13]WEI C(魏超),GUO LA(郭灵安),LIU W(刘炜), et al. Isolation, identification and susceptibility testing of Shewanella spp.in fresh pork (猪肉中腐败希瓦菌的分离鉴定与药敏试验)[J].Southwest China Journal of Agricultural Sciences (西南农业学报), 2013, 26(5):2099-2012.
[14]WANG YL (汪永禄),WANG DC (王多春),ZHAN SW(詹圣伟),et al.Isolation and identification of Shewanella algae and Shewanella abalone from patients suffered from food poisoning(从食物中毒患者标本中分离鉴定海藻和腐败施万菌)[J]. Chinese Journal of Epidemiology(中华流行病学杂志),2009,30(8):836-840.
[15]KHASHE S, JANDA JM. Biochemical and pathogenic properties of Shewanella alga and Shewanella putrefaciens [J].J Clin Microbiol,1998,36(3):783-787.
[16]FANG SB (方四倍). Identification and clinical analysis of two cases of bloodstream infection by Shewanella (希瓦菌血流感染2 例实验室诊断和临床分析)[J].Journal of Huaihai Medicine (淮海医药),2013,31(2):139.
[17]DHAWAN B,CHAUDHRY R,MISHRA BM, et al. Isolation of Shewanella putrefaciens from a rheumatic heart disease patient with infective endocarditis[J].J Clin Microbiol,1998,36(8):2394.
[18]WU SJ (吴韶菊).Preliminary research on tetrodotoxin production of Shewanella alga (海藻希瓦氏菌(Shewanella alga)产河豚毒素(Tetrodotoxin)初步研究)[D].Qingdao:Ocean University of China (青岛: 中国海洋大学),2005.
[19]CHEN C (陈偿), HU CQ (胡超群),CHEN XY(陈晓燕),et al.Identification and characterization of Shewanella algae as a novel pathogen of ulcer disease of fish(新发现的红拟石首鱼溃疡病病原海藻施万氏菌的分离和分子鉴定)[J].Oceanologia et Limnologia Sinica(海洋与湖沼),2003,34(1):1-8.
猜你喜欢
杂志排行
Agricultural Science & Technology的其它文章
- Effect of Tree Species and Dosage of Rhizomorph Wood on Asexual Propagation of Wild Gastrodia elata.Bl.f.glauca S.Chow in Ganzi
- In vitro Maturation of Tan Sheep Oocytes
- Changes in Physiological Indexes of SPDS Transgenic Potato Plants under Low Temperature Stress
- Research on Physiological Characteristics of Tall Fescue under Nitrogen Stress
- Cloning and Characterization of Phytochrome A Gene FaPHYA from Tall Fescue
- Effects of Different Application Times of Tillering Fertilizer on Grain Yield and Population Development of Double-cropping Rice Transplanted by Machine
