Changes in Physiological Indexes of SPDS Transgenic Potato Plants under Low Temperature Stress
2015-12-14JinhuiYANGLixiongHEWanJIANGYongSONG
Jinhui YANG, Lixiong HE, Wan JIANG, Yong SONG,
1. College of Horticulture and Landscape, Hunan Agricultural University, Changsha 410128, China;
2. College of Bioscience and Biotechnology, Hunan Agricultural University, Changsha 410128, China;
3. Hunan Provincial Engineering Research Center for Potato, Changsha 410128, China
Potato (Solanum tuberosum)is an annual herb belonging to the genus Solanum of family Solanaceae, which is an important grain and economic crop with high nutritional value, strong adaptability and high yield. Low temperature stress may cause great harm to the growth of potato plants,resulting in a serious decline in yield. Potato tubers can be damaged at temperatures below 2 ℃;potato seedlings are susceptible to chilling damages at -0.5-0.8 ℃and freezing damages at -2 ℃; potato plants will die at-4 ℃[1].Low temperatures below the critical temperature of potato will cause chilling and freezing damages to plants.Under low temperature stress, physiological mechanisms of SPDS transgenic potato plants may vary to reduce the damage of low temperature to potato plants.Therefore, investigating the changes in physiological indexes of potato plants under low temperature stress to screen effective prevention measures is of important significance for the production of potatoes and breeding of cold-resistant germplasms.
In this study, using SPDS transgenic potato lines 01-6, 01-47 and 01-49 as experimental materials and Atlantic as the control, changes in chlorophyll content, malondialdehyde(MDA) content and superoxide dismutase (SOD) activity of potato leaves under low temperature stress were determined, aiming at providing the basis for the prevention of chilling injury in potatoes.
Materials and Methods
Materials
Potato lines overexpressing spermidine synthase(SPDS)gene,including 01 -6, 01 -47 and 01 -49, were used as experimental materials, with Atlantic as the control. The above materials were provided by Hunan Provincial Engineering Research Center for Potato, College of Horticulture and Landscape, Hunan Agricultural University.
Methods
Potato lines 01-6, 01-47, 01-49 and Atlantic were planed in the greenhouse of Hunan Agricultural University.At the seedling stage,potato plants were transferred into low temperature climatic chamber at 4 ℃. Before the transfer, adequate leaves were collected, ground with liquid nitrogen and persevered in ultra-low temperature refrigerator. After the transfer, potato leaves were collected from each line at 1, 3, 5 d post-treatment, ground with liquid nitrogen and persevered in ultralow temperature refrigerator. After the sampling,various physiological indexes were determined. Chlorophyll content was determined with the method proposed by Zhang et al.[2];MDA content was determined with thiobarbituric acid reaction method; SOD activity was determined using the kit produced by Nanjing Jiancheng Bioengineering Institute.
Data processing
All the experimental data were analyzed using Excel and SPSS statistical software.
Results and Analysis
Changes in chlorophyll content of potato leaves under low temperature stress
As shown in Fig.1, under low temperature stress, leaves of potato plants exhibited no significant change in the color, and chlorophyll content of potato leaves was declined compared with that before treatment. To be specific, chlorophyll content of 01-6 and 01-49 decreased rapidly; at 5 d posttreatment, chlorophyll content of 01-49, 01-6 and Atlantic was reduced by 74%, 57% and 48% compared with that before treatment, respectively;chlorophyll content of 01-47 declined gently, which was only reduced by 25% at 5 d post-treatment compared with that before treatment, indicating that chlorophyll content of 01-47 fluctuated minimally under low temperature stress.
Changes in MDA content of potato leaves under low temperature stress
As shown in Table 1, changes in MDA content of 01-6,01-47 and 01-49 varied significantly compared with Atlantic; before treatment, MDA content of 01-47 and 01-49 varied significantly compared with Atlantic; at 1 d post-treatment,MDA content of 01-47 and 01 -6 varied significantly compared with Atlantic; at 3 d post-treatment, MDA content of 01-47 and 01-49 varied significantly compared with Atlantic; at 5 d post-treatment, MDA content of 01-47 varied significantly compared with Atlantic, while MDA content of 01-6 and 01-49 exhibited no significant change compared with Atlantic. Therefore, MDA content of SPDS-overexpressing potato line 01-47 varied significantly compared with Atlantic under low temperature stress.Moreover, among these four potato lines, MDA content of 01-6 increased first and then declined,which was lower than that of Atlantic under low temperature stress;MDA content of 01-47 showed an upward trend, which was remarkably higher than that of Atlantic under low temperature stress, indicating that 01-47 exhibited more serious membrane damages than Atlantic,while 01 -6 had slighter membrane damages than Atlantic under low temperature stress.
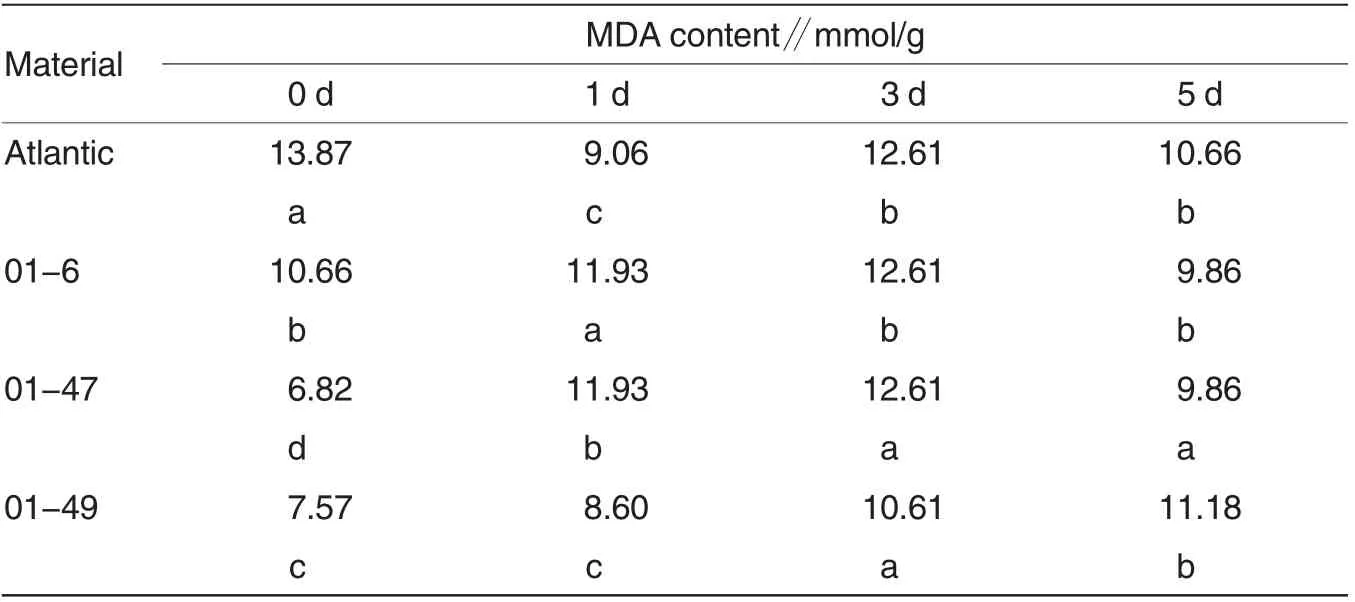
Table 1 Changes in MDA content of potato leaves under low temperature stress
Changes in SOD activity of potato leaves under low temperature stress
The changes in SOD activity of experimental materials under low temperature stress were shown in Fig.2.To be specific, under low temperature stress,SOD activity of Atlantic showed a decreasing trend; SOD activity of 01-6 and 01-47 increased first and then declined and increased subsequently; SOD activity of 01 -49 increased first and then declined. Furthermore, under low temperature stress, SOD activity of 01-6 and 01-47 was significantly higher than that of Atlantic.
Conclusion and Discussion
Low temperature stress exerts various effects on plants and causes a series of physiological and biochemical changes. Under low temperature stress, plants produce excessive reactive oxygen species that damage antioxidant system of plants, thereby resulting in chilling injury[3]. Studies have shown that changes in chlorophyll content, MDA content and SOD activity are responses of plants to lowtemperature stress[4-5].
Chlorophyll is an important pigment involved in photosynthesis in plants. In general, the minimum temperature, maximum temperature and optimal temperature for the formation of total chlorophyll is 2-4 ℃,around 30℃and 40 ℃,respectively. The results indicate that chlorophyll content of experimental varieties shows a decreasing trend at 4 ℃, suggesting that chlorophyll formation in potatoes is inhibited in low temperature environments, which is not conducive to the growth of potatoes under low temperature stress[6-7].
MDA is the final decomposition product of plasma membrane peroxidation, and its content can reflect the extent of damage to plants. Higher MDA content indicates more serious plasma membrane peroxidation and greater damages to plant cell membrane systems[8]. In this study, MDA content of 01-47 is significantly higher than that of Atlantic; MDA content of 01-6 and 01-49 is lower than that of Atlantic after treatment, indicating that cold resistance of 01-6 and 01-49 is slightly higher than that of Atlantic.
Low temperature conditions can not only improve the level of reactive oxygen species, but also induce the establishment of plant defense systems.SOD is a very important antioxidant enzyme in plants, which can effectively remove O2-to reduce or prevent damages of reactive oxygen species to plants[9-11]. According to the results, SOD activity of 01-6 and 01-47 increases first and then declines,which is higher than that of Atlantic after treatment, suggesting that 01 -6 and 01-47 are more adaptive to low temperature stress than Atlantic.
In this study, SPDS transgenic potato lines 01-6 and 01-47 exhibit superior physiological performance to Atlantic under low temperature stress.Therefore, 01-6 and 01-47 can be further cultivated to breed new coldresistant potato varieties.
[1]ZHANG Z (张珍). Early-maturing coldpreventing cultivation technique of potato(马铃薯早熟防寒栽培技术)[J]. Agriculture of Henan (河南农业), 2013(3):46.
[2]ZHANG ZA(张治安), ZHANG MS(张美善), WEI RH (蔚荣海). Experimental Guide for Plant Physiology(植物生理学实验指导)[M].Beijing:China Agricultural Science and Technology Press(北京:中国农业科学技术出版社),2004.
[3]BACK KH, SKINNER DZ. Alteration of antioxidant enzyme gene expression during cold acclimation of near-isogenic wheat lines[J].Plant Science,2003,165:1221-1227.
[4]WANG HT(王洪涛),XI Z(希珍),ZHENG T (郑搪), et al. Effects of graft on lipid peroxidation and antioxidative enzyme activities of Capsicum annum seedlings under low temperature and weak light intensity(嫁接对低温弱光下辣椒幼苗膜脂过氧化及抗氧化酶活性的影响) [J].Chinese Journal of Applied Ecology (应用生态学报),2010,21(5):1289-1294.
[5]SUN F (孙富),YANG LT (杨丽涛),XIE XN (谢晓娜), et al. Effect of chilling stress on physiological metabolism in chloroplasts of seedlings of sugarcane varieties with different chilling resistance (低温胁迫对不同抗寒性甘蔗品种幼苗叶绿体生理代谢的影响)[J]. Acta Agronomica Sinica(作物学报),2012,38(-4):732-739.
[6]PAN RC(潘瑞炽).Plant Physiology(植物生理学) [M]. Beijing: Higher Education Press(北京:高等教育出版社),2004.
[7]ZENG SX(曾昭西),WANG YR(王以柔),LIU HX (刘鸿先). Enzymatic reactions related to the reduction in chlorophyll content of cucumber cotyledons under low temperature and light stress(低温光照下与黄瓜子叶叶绿素降低有关的酶促反应)[J].Plant Physiology Journal(植物生理学报),1991,17(2):177-182.
[8]LI F(李飞),LIU J(刘杰),DUAN SG(段绍光), et al. Physic-biochemical changes related to the freezing tolerance during cold acclimation in potato seedlings (马铃薯幼苗在冷驯化期间的生理生化变化)[J].Chinese Potato Journal (中国马铃薯),2008,22(5):257-260.
[9]BUCHANAN BB, BALMER Y. Redox regulation:a broadening horizon[J].Annu Rev Plant Biol,2005,56:187-220.
[10]LUO Y (罗娅), TANG HR (汤浩茹),ZHANG Y(张勇).Effect of low temperature stress on activities of SOD and enzymes of ascorbate-glutathione cycle(低温胁迫对草莓叶片SOD 和AsAGSH 循环酶系统的影响)[J]. Acta Horticulturae Sinica (园艺学报),2007,34(6):1405-1410.
[11]SUN XC (孙学成),TAN QL (谭启玲),HU CX ( 胡承孝), et al. Effects of molybdenum on antioxidative enzymes in winter wheat under low temperature stress (低温胁迫下钼对冬小麦抗氧化酶活性的影响)[J]. Scientia Agricultura Sinica (中国农业科学),2006,39(5):952-959.
猜你喜欢
杂志排行
Agricultural Science & Technology的其它文章
- Effect of Tree Species and Dosage of Rhizomorph Wood on Asexual Propagation of Wild Gastrodia elata.Bl.f.glauca S.Chow in Ganzi
- In vitro Maturation of Tan Sheep Oocytes
- Biological Characteristics and Pathogenicities of Shewanella algae and Shewanella abalone from Babylonia
- Research on Physiological Characteristics of Tall Fescue under Nitrogen Stress
- Cloning and Characterization of Phytochrome A Gene FaPHYA from Tall Fescue
- Effects of Different Application Times of Tillering Fertilizer on Grain Yield and Population Development of Double-cropping Rice Transplanted by Machine
