Comparison of 1,2,3-Trichloropropane reduction and oxidation by nanoscale zero-valent iron, zinc and activated persulfate
2015-12-12LIHuiHANZhantaoMAChunxiaoGUIJianye
LI Hui , HAN Zhan-tao*, MA Chun-xiao, GUI Jian-ye
1Institute of Hydrogeology and Environmental Geology, CAGS, Shijiazhuang 050061, China.
2Key laboratory of groundwater contamination remediation, Shijiazhuang 050061, China.
Abstract: Trichloropropane (TCP) is a chlorinated solvent which derives from chemical manufacturing as a precursor, and it is also an important constituent of solvent formulations in cleaning/degreasing operations. The control and remediation of TCP in polluted sites is a challenge for many conventional remediation techniques due to its refractory behaviour. This challenge in mind, some nano-materials and oxidants were tested to evaluate their effectiveness as in TCP degradation in a laboratory setting. Experimental results indicate that the use of nanoscale zero-valent iron prepared by green tea (GT) as a reductant has negligible degradation effect on TCP under normal temperature and pressure conditions. However, zinc powders of similar size but higher surface reactivity, demonstrated stronger dechlorination capacity in the breakdown of TCP, as almost all of TCP was degraded by carboxymethocel(CMC) stabilized nanoscale zinc within 24 h. Activated persulfate by citric acid (CA) and chelated Fe (Ⅱ) was also used for TCP treatment with a TCP removal efficiency rate of nearly 50% within a 24 h reaction period, and a molar ratio of S2O82-, Fe2+ and CA is 20:5:1. Both the reduction and oxidation reactions are in accordance with the pseudo-first order kinetic equation. These results are promising for future use of TCP for the remediation of polluted sites.
Keywords: 1,2,3-Trichloropropane; Reduction; Advanced oxidation; Nanoparticles; Activated persulfate
Introduction
In our industrial era, 1,2,3-Trichloropropane(TCP) has unfortunately become a widespread chlorinated solvent contaminant in the subsurface environment (Tatnyek P G et al. 2008; Konnecker G et al. 2003). It comes from several sources:Agricultural use of fumigants that contain TCP as an impurity, and chemical manufacturing processes involve TCP as a precursor. Groundwater contaminated with TCP threatens public health due to the solvent’s toxic nature and carcinogenic effects. Even low level exposure to TCP poses a significant human health risk (Kielhorn J et al.2003; Early K O et al. 2000). To support ongoing assessments of the risk from TCP contaminated groundwater, and especially to improve methods for its remediation, a more comprehensive investigation is needed around the processes that control the degradation of TCP.
Nanoparticles, such as nanoscale zero-valent iron (NZVI), are known to reduce most chlorinated solvents (FENG Jing et al. 2008; Kim H et al.2010). Reactivity of TCP may therefore be further improved by using nanoscale particles because their greater surface area to volume ratios and surface sites may have greater intrinsic reactivity.Also, nanoscale particles can be injected by gravity or under pressure directly into a contaminant source zone (Suthersan S S, 2002). Up to and including 2009, there were 44 registered contaminated sites worldwide employing NZVI as a remediation technique, indicating widespread use of NZVI in the environmental remediation field for a variety of contaminants (Karn B et al. 2000).However, it has been shown in batch experiments that the emerging major contaminant TCP is degraded much more effectively by zero-valent zinc (ZVZ) than micro- or nano- zero-valent iron(ZVI) (Salter A J et al. 2010). Zinc has a lower standard redox potential (E0, -0.76 V) than iron(-0.44 V) which represents stronger oxidizing ability (Sarathy V et al. 2009). These preliminary findings encourage us to make further investigations on TCP degradation by NZVI and nanoscale zero valent zinc (NZVZ) to confirm more effective reagents for remediation of TCP-contaminated sites. Furthermore, although many previous investigations on the stability of nanoparticles in suspension have determined that stabilized particles often have improved transport ability in porous media (Johnson R L et al. 2009;Dickson D et al. 2012), suggesting good applicability of nanoparticles in the field, there have been no studies of the stability of NZVZ.
In addition to nanometer materials, chemical oxidation technology is a potent soil remedial option that can effectively eliminate an extensive range of contaminants. Recently, persulfate oxidation has been developed as an option for in situ chemical oxidation (HUANG Kun-chang et al.2002; Kusic H et al. 2011). Persulfate is a strong oxidant with a high redox potential of 2.01 V and can be activated by various initiators to form more powerful sulfate free radicals (·SO4-), which have a higher redox potential of 2.40V (LIANG Chen-ju and GUO Yi-yu, 2010). Persulfate oxidation has been applied to degrade various chlorinated solvents, such as trichloroethylene (TCE),1,1,1-trichloroethane (TCA), polychlorinated biphenyls (PCBs) to name a few. However there are few studies that have investigated the treatment of TCP using persulfate oxidation.
In this study, batch experiments were conducted to evaluate the feasibility of TCP-contaminated groundwater remediation using nanoparticle reduction and persulfate oxidation.The main objectives were to (1) determine the optimum stabilizer for NZVZ particles and chelator for Fe (Ⅱ); (2) compare the removal efficiencies of TCP by NZVI, NZVZ and persulfate; (3) determine the kinetics of contaminant removal by the reduction and oxidation reagent.
1 Experimental materials and methods
1.1 Materials
All chemicals used in this work were reagent grade, excepting TCP (>98.0%) and n-hexane which were chromatographic grade. To prepare the nano-iron suspension, green tea was selected as a reductant for Fe (Ⅲ). Nanoscale zinc powder was purchased from Chaowei Nanotechnology Limited of Shanghai, and the sample particles were tested to confirm their purity of 99.9% (Table 1) and a measured mean size of 30-50 nm (Fig. 1).
Soil samples used in experiments were collected at a depth between 12-15 m underground in a site at Tianjin, China, which is contaminated with chlorinated and aromatic hydrocarbons. Once collected, soils were naturally dried, triturated and sealed for later use. Sample grain size consisted mostly of medium grain sand and the organic matter content measured by the Total Organic Carbon (TOC) analyzer was found to be 1.56 g/kg.
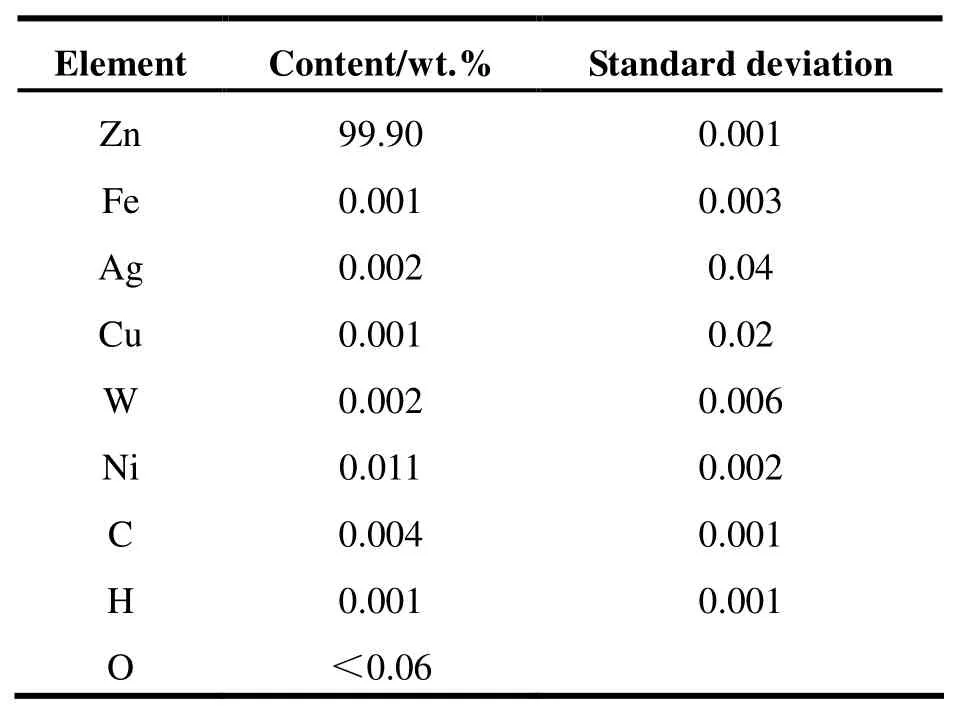
Table 1 Element composition of NZVZ particles

Fig. 1 Transmission electron microscope image of nano zero-valent zinc particles
1.2 Methods
1.2.1 TCP degradation using Green Tea Nano Zero-valent Iron (GT-NZVI)
Preparation and characterization of NZVI suspension.A mixture of alcohol and water at the ratio of 10:1 was prepared as a solvent followed by the addition of GT. The solution was then heated to 80 ℃ and smashed with ultrasonic vibration for 20 minutes to extract the GT. Iron chloride solution(FeCl3·6H2O) of 0.1 mol/L was prepared using the solvent and mixed with the GT extract for NZVI suspension after 20 minutes of continuous agitation. X-ray photoelectron spectroscopy (XPS)and transmission electron microscopy (TEM) were used to evaluate the composition and structure of NZVI particles. To make these microscopic measurements, samples were prepared by dispersing the slurry in ethanol in an ultrasonic bath for 10 minutes. One drop of the obtained suspension was dispersed and dried on copper grid for microscopic analysis by XPS and TEM.
Degradation experiments.A TCP solution of 100 mg/L was prepared for testing. A 10 mL volume of the NZVI suspension and 0.4 mL of TCP solution were placed in a serum bottle which was sealed under a pressurization valve and shaken in an oscillator at 175 rpm. The sample was drawn from the bottle and filtered through a 0.2 µm micron filtration membrane at a specific time interval to determine the concentration of TCP using a gas chromatograph (GC) machine.
1.2.2 TCP degradation using Nano Zero-valent Zinc (NZVZ)
Stability of NZVZ suspension.To prepare the NZVZ suspension, 0.2 g of NZVZ powder and 0.2 g carboxymethocel (CMC) or polystyrolsulfon(PSS) were dispersed in 100 mL KCl solution of 1 mMol/L. After ultrasound vibration, the solution is left to sit for some time and the precipitation conditions of the suspension were observed and recorded using a camera. A second test of the suspension adjusted the CMC/PSS concentration to investigate its influence on the stability of NZVZ suspension. The suspended concentration of NZVZ was measured by ultraviolet spectrophotometer (UV) at 600 nm. Results proved a better surfactant for NZVZ stability would be chosen as the stabilizer in the following tests.
Degradation experiments.The experimental process for degradation experiments were conducted identical to the TCP degradation with NZVI experiment, except that CMC-stabilized NZVZ was substituted for NZVI as a reductant.
1.2.3 TCP degradation using activated persulfate
Determination of an optimum chelator and oxidant/activator dosage.Tests were conducted in 150 mL serum bottles. Firstly, 100 mL TCP solution of 300 mg/L was prepared for the tests,and then a calculated amount of FeSO4×7H2O and chelator, as well as quantitative Na2S2O8, were added in TCP solution to make sure a 20:5:1:1 molecular ratio of, Fe (II), chelator and TCP.The serum bottles were shaken in an oscillator and residual concentration of TCP was measured at special times. To determine the appropriate dosage of oxidant and activator for field applications, the concentration of Na2S2O8and Fe (II) was adjusted to 100:5:1, 20:5:1 and 20:1:1 molecular ratio to TCP with constant dosage of chelator.
Influence of organic matter.For practical applications, there is potential for the utilization ratio of oxidants for pollutant degradation to decline due to excess consumption of oxidants by organic matter in groundwater and soil. A group of experiments were designed in light of these considerations. The experiment process for these experiments mirrored the procedures for the determination of an optimum chelator and oxidant/activator dosage experiments, except that 20 g of contaminated soil was added to the serum bottle for a 5:1 mass ratio of water and soil.Sampling frequency properly decreased to once a day because of organic matter existence.
2 Results and discussion
2.1 TCP degradation with GT-NZVI
Fig. 2 is the XPS image of the GT-NZVI suspension. This image illustrates that the iron element was the main component of the prepared sample of GT-NZVI aside from the substrate elements of C and O. Comparison with the XPS database and peaking results obtained from relevant software, confirmed Fe (0) and Fe (Ⅲ) as the primary valence of iron in GT-NZVI suspension, and their mass fraction is 55.6% and 44.4% respectively. The TEM image of GT-NZVI is shown as Fig. 3. The NZVI particles prepared through the GT extracting solution for reducing Fe(Ⅲ) displayed uniform morphology and favorable dispersibility, with a mean size of 30-50 nm.
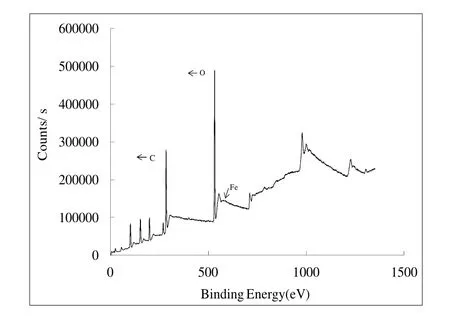
Fig. 2 XPS image of GT-NZVI suspension
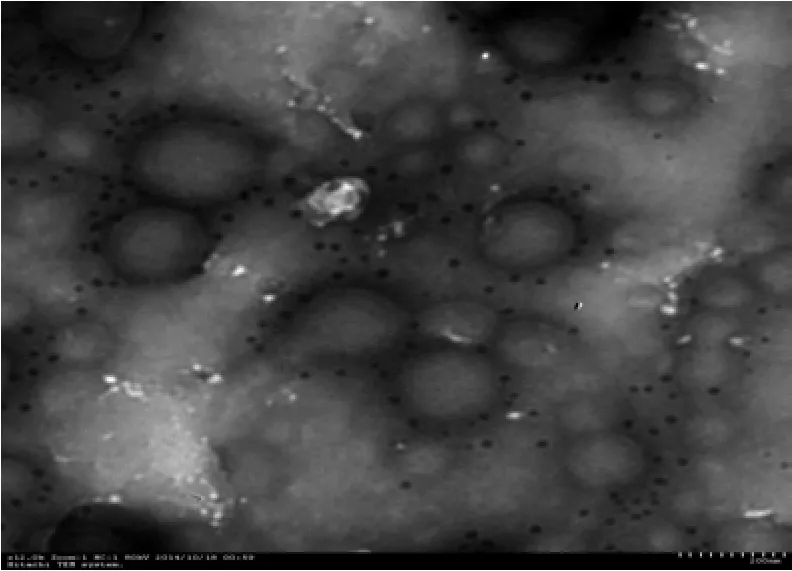
Fig. 3 TEM image of GT-NZVI
The degradation of TCP is visible in Fig. 4,where C/C0is the ratio of residual and initial concentration of TCP. The results demonstrate that in a 32 hour period there was no further removal of TCP by GT-NZVI reduction than in the control test.Compared with other common chlorinated hydrocarbons, it seems that reductive dechlorination of TCP is far more difficult and a reducing material with higher surface reactivity is necessary.Sarathy et al. (2009) demonstrated some improvement in the reaction rate of TCP degradation by variform nanoscale zero-valent iron.However, the value of the surface normalized rate constant (Ksa) was much smaller than the required effective rate for use in in-situ remediation.Similarly, results in Fig. 4 also indicate that the decline of TCP concentration was so negligible that the reduction of GT-NZVI could be regarded as invalid.
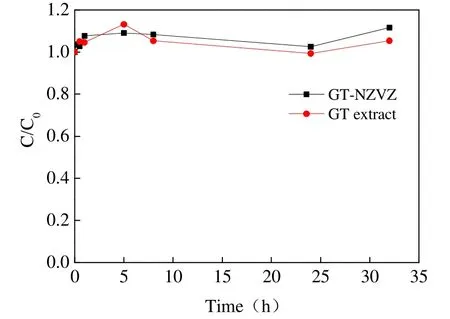
Fig. 4 Degradation curves of TCP by GT-NZVI
2.2 TCP degradation using Nano Zero-valent Zinc
Fig. 5 displays the suspended status of NZVZ modified by CMC and PSS, and the suspension capability of CMC modified NZVZ was clearly superior to that of PSS as a dispersant. After the mixing of NZVZ and CMC and a 24 hour settling period, a considerable portion of the suspended nanoparticles could be observed in the suspension.On the contrary, PSS modified particles started to subside after 3 hours of settling, the process lasting for 6 hours until all the nanoparticles precipitated,demonstrating unsatisfactory effectivity of PSS for NZVZ suspension.
As typical surfactants, CMC and PSS can be adsorbed on the surface of NZVI by nonpolar radicals of -CH2CH2COO-and, which reinforce the repulsion of the nanoparticles resulting in less agglomeration and enhanced suspension stability (Phenrat T et al. 2009).Furthermore, CMC possesses viscous characteristics for colloid particle suspensions and numerous reactive functional groups of -COOH with ability to combine the dispersant with iron nanoparticles by electrostatic force. Therefore, compared with PSS, CMC has clearly superior suspension power for NZVI.
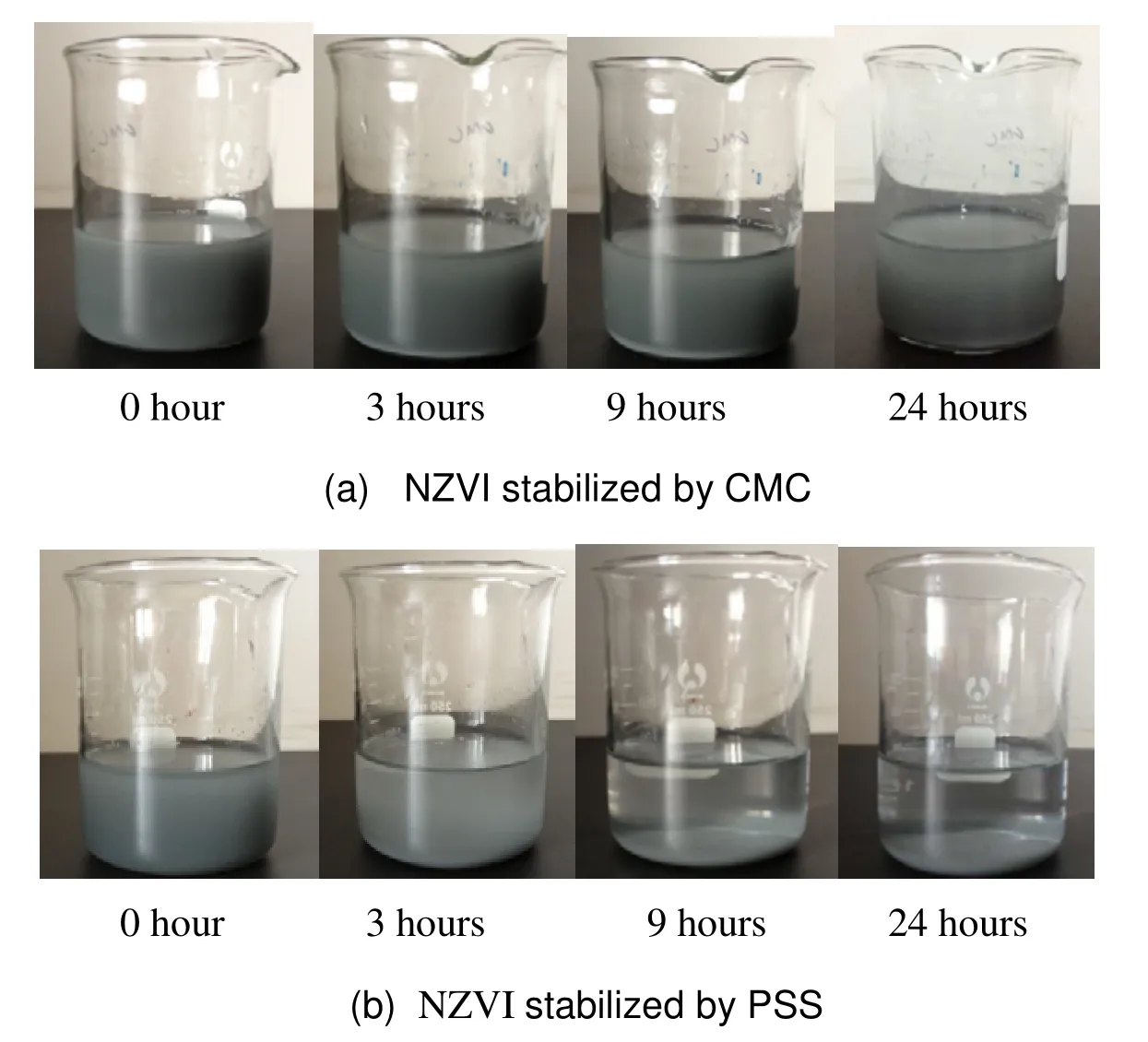
Fig. 5 Suspension images of NZVZ by CMC and PSS as stabilizer
Surfactant concentration has considerable effects on the stability of nanoparticles. As shown in Fig. 6, the suspended concentration of NZVZ slightly decreased when mixed with a small volume of surfactants, but subsequently increased as surfactant concentration increased. Theoretically,the addition of a polymer can improve the stability of nanoscale colloid particles due to enhanced electrostatic repulsion. However, several other researchers have found that a small amount of polymer may immediately induce flocculation and sedimentation of colloid particles (SHEN Zhong,2007), which is due to the revolve and movement of adsorbed macromolecular chains. The results shown in Fig. 6 once again reinforce the suspending power of CMC for NZVZ was much stronger than that of PSS.
The experiments of TCP degradation with bare NZVZ and CMC-NZVZ suspension were carried out and the removal efficiency of TCP is shown in Fig. 7. It can be seen that the concentration of TCP declined below the detection limit in 10 hours,which indicates that TCP was essentially completely degraded by NZVZ during this time.However, the use of CMC-NZVZ in the reaction system as the reducer, led to a decrease in the reaction rate that prolonged the reaction time to 36 hours. These results can be explained by the adsorption mechanism of CMC. As CMC adheres to the surface of nanoparticles the number of reactive sites on the surface area of the nanoparticles is reduced. Thus valid contact with the pollutant is hindered and the reaction rate consequently decreases. The dynamic fitting curves of TCP degradation with NZVZ and CMC-NZVZ are shown in Fig. 8. These figures indicate that the reduction reaction can be described by pseudo-first order kinetics and their calculated reaction rate constant was 0.45 h-1and 0.10 h-1respectively.
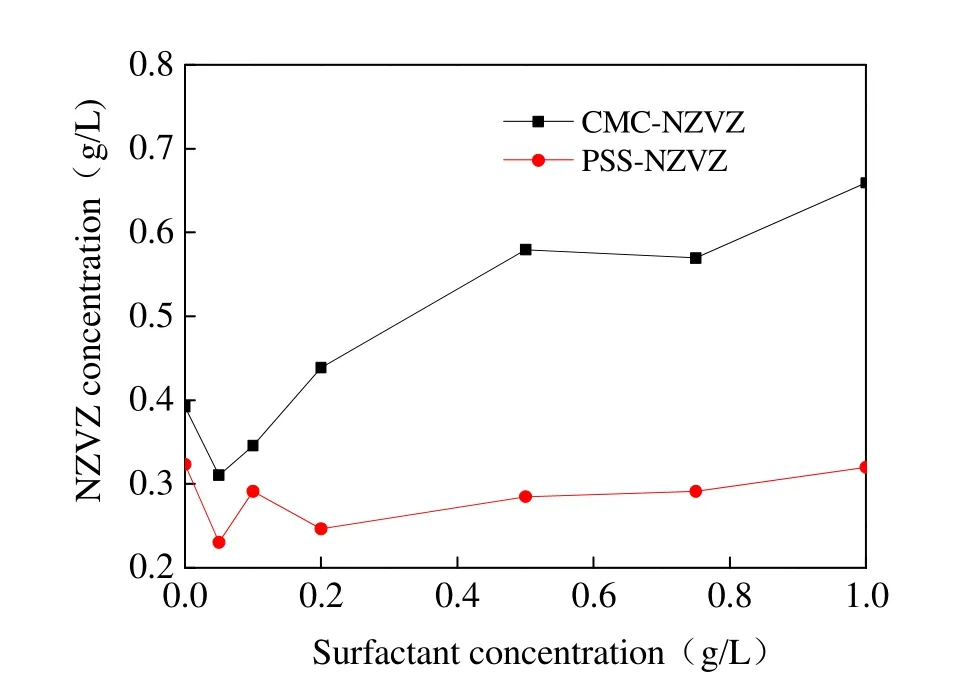
Fig. 6 Influence of surfactant concentration on NZVZ suspension
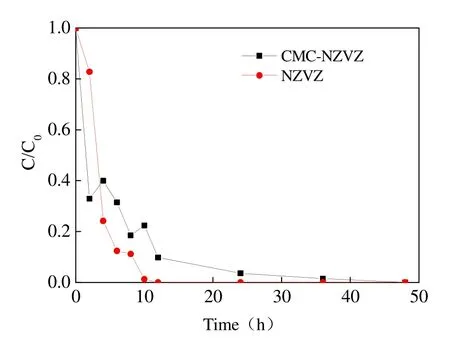
Fig. 7 Degradation curves of TCP by bare by and CMC-stabilized NZVZ
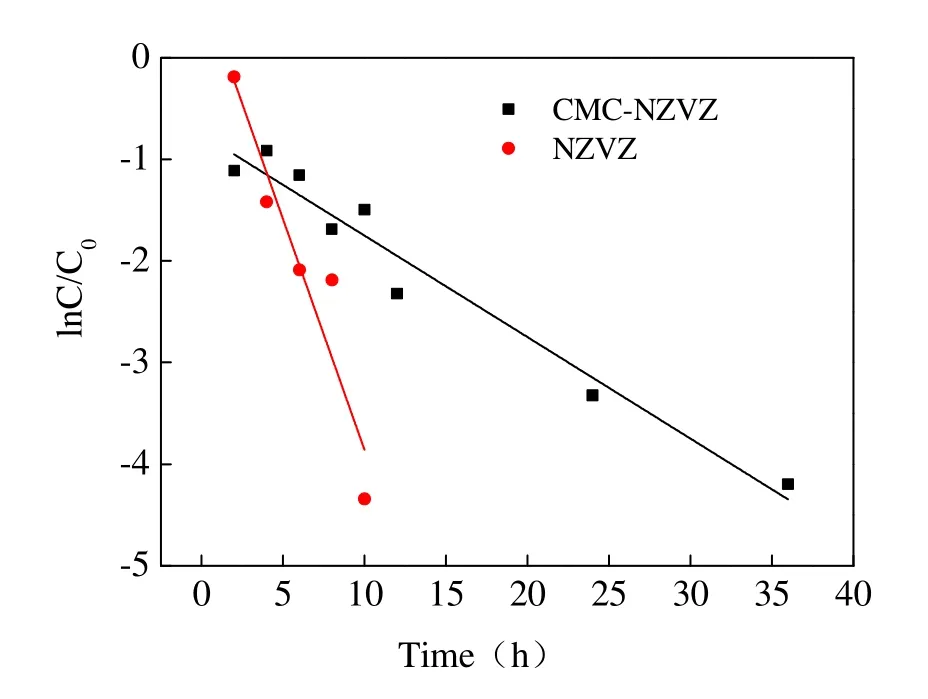
Fig. 8 Kinetics fitting curves of TCP degradation bare and CMC-stabilized NZVZ
2.3 TCP degradation with activated persulfate
Citric acid (CA), ethylene diamine tetraacetic acid (EDTA) and lactic acid (LA) were used as chelator for Fe2+, and their influences on TCP degradation were investigated in our experiments.Fig. 9 illustrates degradation curves and a removal efficiency of TCP was 61.4%, 45.2% and 42.7%respectively over a 24 hour period, demonstrating that the chelate between CA and Fe2+was superior.Chelation can control the concentration of Fe2+to reduce the consumption ofin reaction system,thereby effectively improving the removal rate of pollutant through chelation. LIANG Chen-ju et al.(2004) also found that the rate of TCE dechlorination was much higher when CA was used as chelator instead of sodium tripolyphosphate and EDTA. Therefore, CA was chosen as the optimum chelating agent for use in the following tests.
To determine the dosage of oxidant and activator, an additional test was conducted to explore the effect ofand Fe2+doses on TCP degradation. When the molar ratio ofand CA was 100/5/1, 20/5/1 and 20/1/1 respectively, the removal efficiency of TCP increased alongside the dosage ofand Fe2+,despite the fact there were less differences of C/C0overall (Fig. 10). In light of both cost and efficiency consideration, the molar ratio of 20/5/1 was suggested as a relatively suitable oxidant and activator dosage.
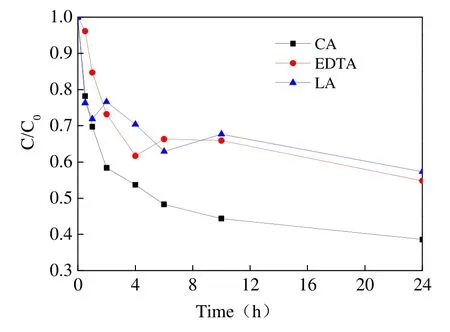
Fig. 9 Influence of different chelators on TCP reduction
Contaminated soil was added to the reaction to investigate the influence of oxidant consumption by soil organic matter on TCP degradation. The concentration curve in Fig. 11 shows that the removal efficiency of TCP was only 8.3%,decreasing by around 86.5% when there was no soil in the system. In the subsequent reaction, TCP concentration dropped continuously and nearly 85.8% of TCP was removed after an 18 day reaction period. These results demonstrate that soil organic matter will take advantage of partial oxidants, which only decreases the reaction rate rather than the final removal efficiency. The dynamic fitting curves for this reaction are shown in Fig. 12. It can be concluded that the process is expressed by pseudo-first-order kinetic equation.The overall rate constant and half-life of the reaction was 0.11 days-1and 6.3 days respectively.
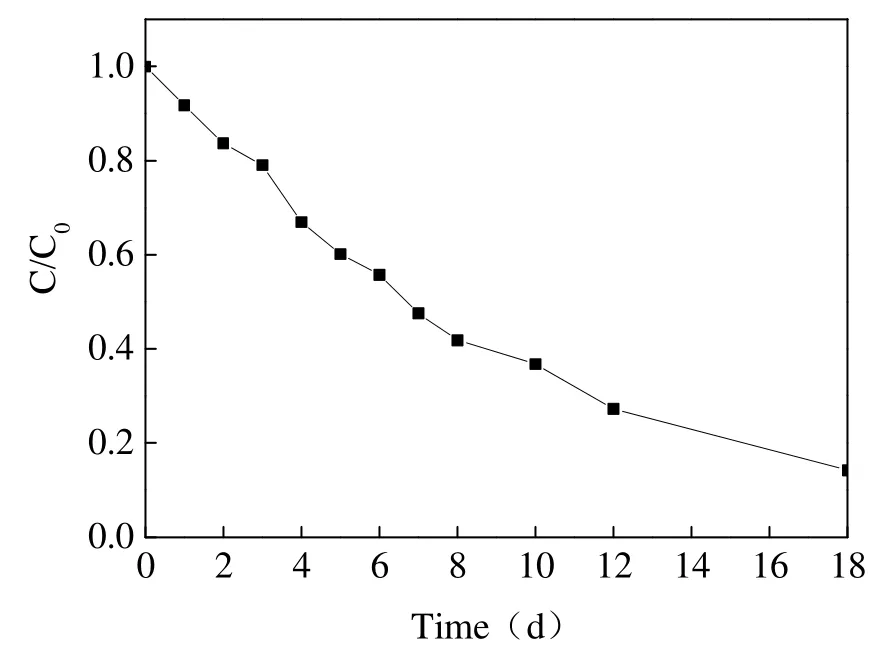
Fig. 11 Degradation curves of TCP by persulfate

Fig. 12 Kinetics fitting curves of TCP degradation by persulfate
3 Conclusions
(1) NZVI with a diameter of 10-20 nm can be prepared by employing green tea extract to reduce Fe (Ⅲ). Nanoparticles prepared this way present better dispersability in an alcohol-water mixture.However, there is little removal of TCP observed in batch degradation experiments with an NZVI suspension, indicating that a reductant with higher surface reactivity is required for effective dechlorination of TCP under natural conditions.
(2) CMC can be used for stabilizing the NZVZ particles in water, as the stability of suspension increases with CMC dosage. Moreover, TCP is confirmed to be degraded by NZVZ effectively,even though the addition of CMC will slightly decrease the rate of the reaction conforming with the pseudo-first-order kinetic equation.
(3) Advanced oxidation technology of Fe (II)activating persulfate is valid for TCP degradation.CA has ideal chelation with Fe (II) compared with other polymers such as EDTA and LA. The removal efficiency of TCP is nearly 50% over a 24 hour reaction period when the molar ratio ofFe2+and CA is 20:5:1. However, efficiency significantly declines with the presence of soil organic matter, and the calculated half-life of the reduction reaction is 6.3 days.
In conclusion, both methods for NZVZ reduction and advanced oxidation show good results for the removal of TCP in laboratory conditions. However, the optimum technology for TCP removal and practical remediation of genuine contaminated sites must take into account engineering considerations as well as site specific economic and environment factors.
Acknowledgements
This study is supported by Basal Science Research Fund from the Chinese Academy of Geological Sciences (Grant No. YWF201405). We greatly appreciate the efforts of HAN Zhantao for his interest in and contributions to our research.
杂志排行
地下水科学与工程(英文版)的其它文章
- Analysis of the negative effects of groundwater exploitation on geological environment in Asia
- Specific yield of phreatic variation zone in karst aquifer with the method of water level analysis
- Application of remote sensing technique to mapping of the map series of karst geology in China and Southeast Asia
- Compilation of Groundwater Quality Map and study of hydrogeochemical characteristics of groundwater in Asia
- Evaluation on water resources carrying capacity of Changchun-Jilin Region
- Governance of protected areas: Kara Kara National Park, Victoria
