靶向时代肿瘤疗效评价标准的探索
2015-11-23王琳综述王馨审校
王琳 综述 王馨 审校
·综述·
靶向时代肿瘤疗效评价标准的探索
王琳 综述 王馨 审校
肿瘤分子靶向药物具有与化疗药物不同的作用机理。目前对肿瘤的疗效评价标准主要是针对化疗药物疗效的评价,从最早的WHO标准发展到RECIST和RECIST 1.1标准,尽管在细节和标准化方面不断完善,但均以测量肿瘤的大小作为评价指标,其原理基于化疗药物直接杀死肿瘤细胞。但是,靶向治疗药物主要是抑制肿瘤细胞增殖,其对肿瘤的作用模式不同于化疗药物,因此近几年出现了对靶向药物疗效评价标准的探索,本文对以CT值作为功能学指标的肿瘤疗效评价标准-Choi标准及其后续的一系列mChoi、SACT、MASS的肿瘤疗效评价标准进行了详述。
肿瘤疗效评价标准 靶向药物治疗
判断治疗前后肿瘤形态的变化是评价治疗疗效的一个手段,肿瘤疗效评价标准在肿瘤学领域起到了标尺的作用。本文对肿瘤疗效评价标准的发展进行综述,简述了WHO、RECIST、RECIST 1.1标准,着重介绍了适合分子靶向药物疗效评价的标准-Choi标准及其后续的一系列(mChoi、SACT、MASS)疗效评价标准。
1 以测量肿瘤大小为指标的疗效评价标准
1.1WHO标准
最早的肿瘤疗效评价标准于1981年由WHO组织首次发表,以肿瘤双径的乘积之和作为评价肿瘤负荷的指标(图1)。WHO疗效评价标准[1]在肿瘤学界和制药业中应用了很久,为国际交流和临床试验的国际合作提供了基础。
1.2RECIST标准
在WHO标准应用几十年后,发现常需调整内容以适应新技术或者补充说明其中不明确的部分,导致各机构之间的结论不够统一,为了解决这些问题,90年代专门成立了一个国际工作组来简化评价标准,由此,2000年推出RECIST标准(the Response Evaluation Criteria in Solid Tumors)[2],它以肿瘤最大径作为肿瘤负荷的指标(图2),就可测量病灶的最小体积、随访的病灶数目(最多10个,每个器官最多5个)等进行了统一的规定,使其可操作性更强,从而
成为最为广泛使用的疗效评价标准(表1)。
图1 双径测量Figure 1Measurement of two diameters
图2 单径测量Figure 2Measurement of one diameter

表1 WHO标准和RECIST标准Table 1WHO criteria and RECIST criteria
自2000年以来,RECIST标准成为国际上通用的标准,随着国际上临床试验合作增多,从实践中得出了修改的证据,2009年European Journal of Cancer上首次发布了新的标准-RECIST 1.1疗效评价标准[3]。
1.3RECIST 1.1标准
RECIST 1.1标准仍是以肿瘤最大径作为测量指标,与RECIST一脉相承。近几年大型临床试验和临床实践已经开始应用RECIST 1.1作为统一的疗效评价标准。
RECIST 1.1主要针对引起广泛争议及模糊不清的内容进行了修改[3],具有简单、最优化、标准化等特点。减少可测量病灶数目、废除疗效的再确认使得临床试验的工作量大大减少。对病理性淋巴结、骨病灶、囊性病灶、既往局部治疗过的病灶、以及病灶太小测量误差太大的等情况下如何处理,进行了清晰的定义[4]。
2 以肿瘤大小为测量指标的肿瘤疗效评价标准的不足
RECIST 1.1中提到可以应用FDG-PET作为确认新发病灶的一种影像学方法,简单涉及到了肿瘤的功能学改变。事实上,近些年肿瘤的治疗取得了巨大的进步,出现了越来越多的靶向治疗药物。传统的化疗药物主要是直接杀死肿瘤细胞,因此WHO标准、RECIST标准、RECIST 1.1标准均关注肿瘤的大小变化,而靶向治疗药物主要是抑制肿瘤细胞增殖,肿瘤大小变化常常不能敏感反映疗效,先于肿瘤大小变化之前,其内部的功能已经发生了变化,化疗一般要2个月左右才能看到疗效,而靶向药物一般只要2~4周[5]。
很多靶向药物使肿瘤稳定而不是缩小,尽管以RECIST标准来看反应率很低,但临床却看到了总生存的延长[6-8]。有时因疗效较佳,肿瘤内部发生液化和坏死,而肿瘤外部形态变化不明显(图3)[9],甚至由于出血或坏死而表现为体积增加(图4)[10],若仅依据外部形态大小改变的RECIST标准则易误判为稳定或者病情进展[5,8]。
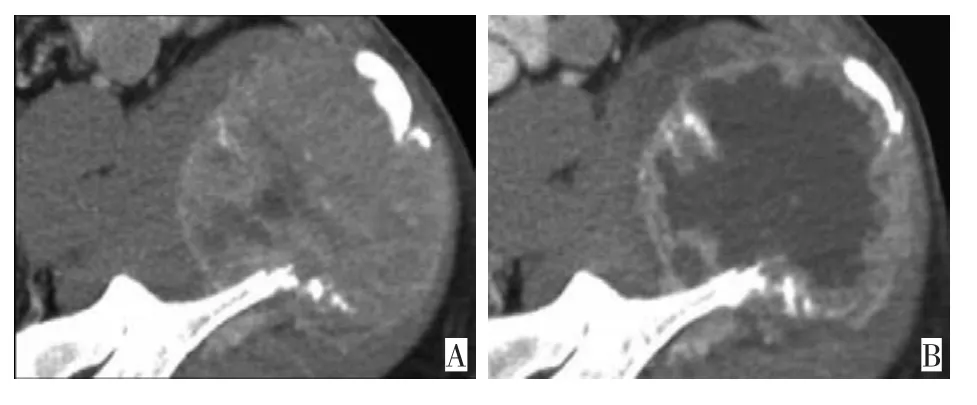
图3 转移性肾癌TKI(酪氨酸激酶抑制剂)治疗后肿瘤中心发生坏死,而体积变化不大Figure 3A.CT images obtained before TK-inhibitor therapy;B.CT images obtained after TK-inhibitor therapy show marked central necrosis and minimal change in size
目前,对靶向药物临床试验的疗效评价仍采用RECIST标准,导致其可能低估了靶向药物的客观有效率[11],从而低估了其疗效,导致敏感性不足。甚至在Ⅱ期临床试验时,一些可能带来临床获益、值得进一步研究的靶向药物因为RECIST标准低估了其疗效,导致达不到统计学意义,而不能进入Ⅲ期临床试验,错失了可能有效的药物。在临床应用中,RECIST标准在最初评价为SD的时候,由于敏感性不足,无法将下次评价时为PD与PR的患者区分开,以便及时地
改变治疗策略[12]。
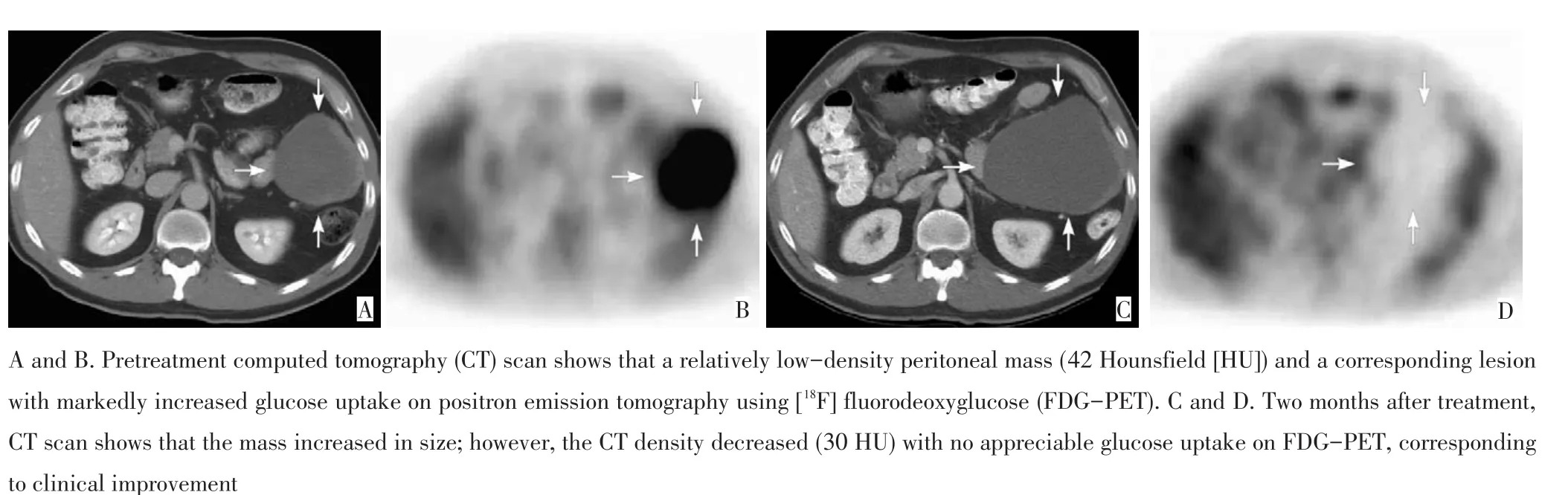
图4 腹膜转移的胃肠间质瘤患者伊马替尼治疗前后肿瘤影像学变化Figure 4CT and FDG-PET images obtained before and after treatment of imatinib mesylate on a gastrointestinal stromal tumor patient with recurrent peritoneal metastases.
3引入CT值为测量指标的肿瘤疗效评价标准
RECIST标准以肿瘤大小作为指标难以准确反映分子靶向药物的治疗疗效,有学者倡议不应再使用[13],后续不少学者都尝试改变思路,探索引入功能学改变的指标。
3.1Choi标准
在功能学指标的疗效评价标准中,值得关注的是Choi标准[10],因为其与RECIST标准均建立在CT检查的基础上,方法简便、成熟、经济、易于推广,并与RECIST标准有一定的延续性。Choi标准将CT值作为肿瘤最大径外的另一项指标引入到肿瘤疗效评价标准中,基于CT值反映组织的密度,而肿瘤密度的改变间接反映了代谢功能的改变,以增强CT上的CT值来描述,单位是Hounsfield Units(HU)。PR被定义为最大径减少≥10%,或增强CT检查CT值下降≥15% HU,而PD被定义为最大径增大≥10%且CT值变化不符合PR标准[10](表2)。
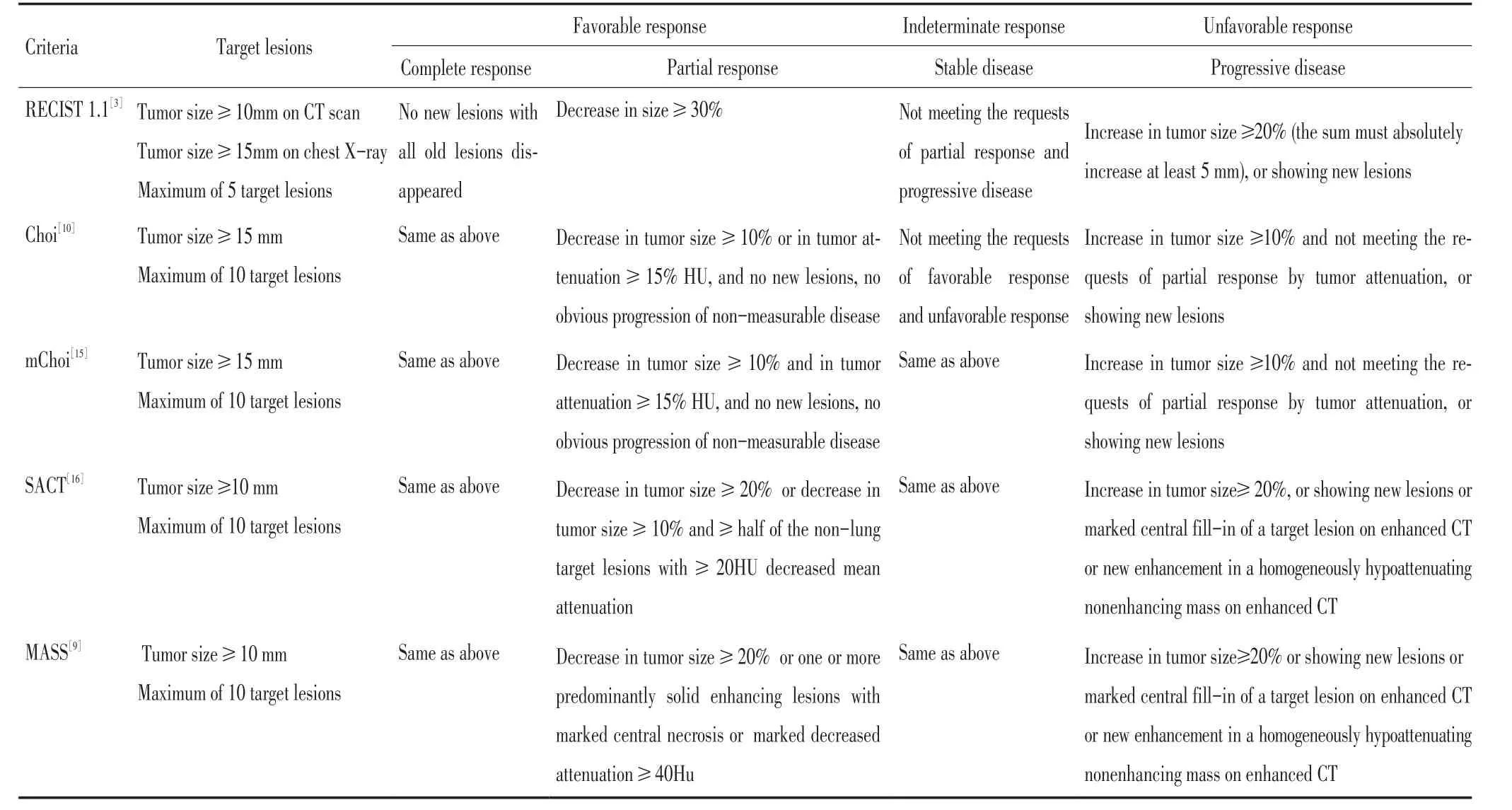
表2 主要疗效评价标准对比Table 2Comparison of main tumor response evaluation criteria
Choi等[10]选取伊马替尼治疗转移性胃肠间质瘤40例,满足RECIST要求的172个病灶。在治疗前及治疗后2个月行CT及FDG-PET检查。对CT上肿瘤的大小及密度(HU)和FDG-PET的SUVmax结果进行多变量分析,其疗效评价标准的分界点是基于同一病灶在FDG-PET上SUVmax减少70%。应用Choi标准和RECIST标准识别FDG-PET影像上有变化者(CR和PR)的敏感性、特异性分别为97%、100%和52%、100%。2个月后应用Choi评价标准判定有变化者比无变化者具有更长的TTP(time to progression),其结果有统计学意义(P=0.01),且Choi评价标准比RECIST标准与DSS(disease-specific survival)有更好的相关性。并在后续的伊马替尼治疗转移性胃肠间质瘤患者中再次得到印证[14]。
在舒尼替尼治疗转移性肾癌的患者中,也发现了Choi疗效评价标准有益于早期识别可以从舒尼替尼的治疗中获益的患者,比RECIST标准对无进展生存(progression-free survival,PFS)和总生存(overall survival,OS)具有更好的预测价值[12]。
3.2基于Choi标准的后续疗效评价标准(mChoi、SACT、MASS标准)
在借鉴Choi疗效评价标准的思路后,陆续有不同的学者通过研究转移性肾癌靶向治疗的资料,提出了一系列的疗效评价标准,如mChoi疗效评价标准(Modified Choi criteria[15]、SACT疗效评价标准(size and attenuation CT criteria)[16-18]、MASS疗效评价标准(morphology,attenuation,size and structure criteria)[19]都显示出比RECIST标准更高的敏感性,同时保持了特异性。
mChoi疗效评价标准和Choi的不同是,PR的评价需要满足最大径缩小10%且CT值下降15%,即大小和功能的变化都要满足标准(而不是其中一项满足标准),才能判定为PR,从而调高了分界点(表2)。比较RECIST、Choi、mChoi疗效评价标准,mChoi标准的中位TTP曲线距离最大[15]。
SACT疗效评价标准中,满足以下任何一种情况即可定义为PR:1)最大径减少≥20%;2)最大径减少≥10%且一半的非肺病灶平均CT值下降20HU。且发现了一些特殊的CT增强方式(起初中心坏死的部分出现明显的增强;原本均匀一致的无增强低CT值的区域出现新的增强)与进展相关,当存在这些CT增强方式的时候,也定义为进展(表2)。当基线CT值较低时,治疗后早期就可以下降15%,从而降低了疗效评价的准确性,因此,使用CT值下降的绝对值作为分界点可能是SACT标准比Choi及mChoi标准更有利的原因[16]。
SACT标准中未纳入肿瘤治疗后出现明显坏死这一现象,事实上,这表示肿瘤治疗非常有效,与较长的PFS相关,在MASS标准中,已将其纳入到PR的诊断标准中(存在一个或多个增强后瘤体显著坏死)(表2)。MASS标准包括了各种形态、大小、CT值、增强模式的定义,因此比SCAT、mChoi、Choi更精确。并且,MASS评价标准在不同的检测者之间显示出了很高的一致性[9]。
3.3Choi标准及其后续标准的特点及应用
近几年,陆续有学者在Choi标准及其后续的一系列(mChoi、SACT、MASS)疗效评价标准发表以后,应用其中的一些概念来分析形态学改变与PFS、TTP、OS、DSS等的关系,甚至与治疗后手术病理改变的关系,以期望找到合适的肿瘤疗效评价方法,这些概念主要包括两个方面(表2):1)肿瘤密度(即肿瘤在增强CT上治疗前后CT值的变化)的改变;2)肿瘤在增强CT上表现的提示疗效的某些特殊增强模式。这些概念在胃肠间质瘤和转移性肾癌的靶向治疗中应用较多,较为成熟,陆续还涉及到神经内分泌癌[17]、结肠癌[18-22]、血管外皮细胞瘤和恶性孤立性纤维瘤[23-24]、脊索瘤[25]、肝癌[26-27]、胸腺瘤[28]、恶性黑色素瘤[29]、滤泡淋巴瘤[30]。并且靶向治疗之外的治疗领域也开始尝试应用这些概念进行疗效评价,如局部治疗[17,21-22]、化疗[18-20],包括一些新药的Ⅱ期临床试验[25]等。
4 结语
肿瘤靶向治疗的时代已经到来,肿瘤靶向治疗后的影像学表现与既往单一化疗有较大差别,随着对肿瘤的各种信号通路的了解更为深入,我们应该重新定义“标尺”,用符合肿瘤靶向治疗后表现的疗效评价标准进行判定。
1Miller AB,Hoogstraten B,Staquet M,et al.Reporting results of cancer treatment[J].Cancer,1981,47(1):207-214.
2Therasse P,Arbuck SG,Eisenhauer EA,et al.New guidelines to evaluate the response to treatment in solid tumors[J].J Nati Cancer Inst,2000,92(3):205-216.
3Eisenhauer EA,Therasse P,Bogaerts J,et al.New response evaluation criteria in solid tumours:revised RECIST guideline(version 1.1)[J].Eur J Cancer,2009,45(2):228-247.
4van Meerten ELV,Gelderblom H,Bloem JL.RECIST revised:implications for the radiologist.A review article on the modified RECIST guideline[J].Eur Radiol,2010,20(6):1456-1467.
5Figueiras RG,Padhani AR,Goh VJ,et al.Novel oncologic drugs:what they do and how they affect images[J].Radiographics,2011,31(7):2059-2091.
6Escudier B,Eisen T,Stadler WM,et al.Sorafenib in advanced clear-cell renal-cell carcinoma[J].N Engl J Med,2007,356(2):125-134.
7Hudes G,Carducci M,Tomczak P,et al.Temsirolimus,interferon
alfa,or both for advanced renal-cell carcinoma[J].N Engl J Med,2007,356(22):2271-2281.
8van der Veldt AAM,Meijerink MR,van den Eertwegh AJM,et al. Targeted therapies in renal cell cancer:recent developments in imaging[J].Target Oncol,2010,5(2):95-112.
9Smith AD,Shah SN,Rini BI,et al.Morphology,Attenuation,Size,and Structure(MASS)criteria:assessing response and predicting clinical outcome in metastatic renal cell carcinoma on antiangiogenic targeted therapy[J].Am J Roenthenol,2010,194(6):1470-1478.
10 Choi H,Charnsangavej C,Faria SC,et al.Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate:proposal of new computed tomography response criteria[J].J Clin Oncol,2007,25(13):1753-1759.
11 Van Cruijsen H,Van der Veldt AA,Hoekman K.Tyrosine kinase inhibitors of VEGF receptors:clinical issues and unanswered questions[J].Front Biosci,2008,14(2248-2268):266.
12 van der Veldt AAM,Meijerink MR,van den Eertwegh AJM,et al. Choi response criteria for early prediction of clinical outcome in patients with metastatic renal cell cancer treated with sunitinib[J].Br J Cancer,2010,102(5):803-809.
13 Benjamin RS,Choi H,Macapinlac HA,et al.We should resist using RECIST,at least in GIST[J].J Clin Oncol,2007,25(13):1760-1764.
14 Choi H.Response evaluation of gastrointestinal stromal tumors[J]. Oncologist,2008,13(Supplement 2):4-7.
15 Nathan,PD,Yinayan,A,Stott,D,et al.CT response assessment combining reduction in both size and arterial phase density correlates with time to progression in metastatic renal cancer patients treated with targeted therapies[J].Cancer Biol Ther,2010,9(1):15-19.
16 Smith AD,Lieber ML,Shah SN.Assessing tumor response and detecting recurrence in metastatic renal cell carcinoma on targeted therapy:importance of size and attenuation on contrast-enhanced CT[J].Am J Roenthenol,2010,194(1):157-165.
17 Neperud J,Mahvash A,Garg N,et al.Can imaging patterns of neuroendocrine hepatic metastases predict response yttruim-90 radioembolotherapy[J]?World J Radiol,2013,5(6):241-247.
18 Chun YS,Vauthey JN,Boonsirikamchai P,et al.Association of computed tomography morphologic criteria with pathologic response and survival in patients treated with bevacizumab for colorectal liver metastases[J].JAMA-J Am Med Assoc,2009,302(21):2338-2344.
19 Shindoh J,Loyer EM,Kopetz S,et al.Optimal morphologic response to preoperative chemotherapy:an alternate outcome end point before resection of hepatic colorectal metastases[J].J Clin Oncol,2012,30(36):4566-4572.
20 Shindoh J,Chun YS,Loyer EM,et al.Non-Size-Based Response Criteria to Preoperative Chemotherapy in Patients With Colorectal Liver Metastases:The Morphologic Response Criteria[J].Curr Colorectal Cancer Rep,2013,9(2):198-202.
21 Tochetto SM,Rezai P,Rezvani M,et al.Does Multidetector CT Attenuation Change in Colon Cancer Liver Metastases Treated with 90Y Help Predict Metabolic Activity at FDG PET[J].Radiology,2010,255(1):164-172.
22 Tochetto SM,Töre HG,Chalian H,et al.Colorectal Liver Metastasis After 90Y Radioembolization Therapy:Pilot Study of Change in MDCT Attenuation as a Surrogate Marker for Future FDG PET Response[J].Am J Roentgenol,2012,198(5):1093-1099.
23 Park MS,Patel SR,Ludwig JA,et al.Activity of temozolomide and bevacizumab in the treatment of locally advanced,recurrent,and metastatic hemangiopericytoma and malignant solitary fibrous tumor[J].Cancer,2011,117(21):4939-4947.
24 Park MS,Ravi V,Conley A,et al.The role of chemotherapy in advanced solitary fibrous tumors:a retrospective analysis[J].Clin Sarcoma Res,2013,3(1):7-14.
25 Stacchiotti S,Tamborini E,Vullo SL,et al.Phase II study on lapatinib in advanced EGFR-positive chordoma[J].Ann Oncol,2013,24(7):1931-1936.
26 Ronot M,Bouattour M,Wassermann J,et al.Alternative Response Criteria(Choi,European association for the study of the liver,and modified Response Evaluation Criteria in Solid Tumors[RECIST])Versus RECIST 1.1 in patients with advanced hepatocellular carcinoma treated with sorafenib[J].Oncologist,2014,19(4):394-402.
27 Heo J,Reid T,Ruo L,et al.Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer[J]. Nat Med,2013,19(3):329-336.
28 Marotta V,Ramundo V,Camera L,et al.Sorafenib in advanced iodine-refractory differentiated thyroid cancer:efficacy,safety and exploratory analysis of role of serum thyroglobulin and FDG-PET[J].Clin Endocrinol,2013,78(5):760-767.
29 Uhrig M,Hassel JC,Schlemmer HP,et al.Therapy Response Assessment in Metastatic Melanoma Patients Treated with a BRAF Inhibitor:Adapted Choi Criteria Can Reflect Early Therapy Response Better than Does RECIST[J].Acad Radiol,2013,20(4):423-429.
30 Spira D,Vogel W,Sökler M,et al.Size and attenuation CT(SACT)of residual masses in patients with follicular Non-Hodgkin Lymphoma:More than a status quo[J]?Eur J Radiol,2012,81(7):1657-1661.
(2014-12-29收稿)
(2015-03-12修回)
(编辑:郑莉)
Exploration of tumor response evaluation criteria in the era of targeted therapy
Lin WANG,Xin WANG
Lin WANG;E-mail:wangling_linda82@163.com
The era of targeted molecular therapy for cancer has arrived.The mechanisms of targeted molecular drugs are different from those of chemotherapy drugs.The tumor response evaluation criteria that we currently use are the summary of experiences from evaluating the effect of chemotherapy drugs.Despite continuous improvement and standardization of the details,tumor response evaluation criteria,from the earliest WHO criteria to the improved RECIST and RECIST 1.1 criteria,are all based on measurement of tumor size,as chemotherapy drugs directly kill tumor cells.The aforementioned criteria are outlined in this review.However,targeted molecular drugs mainly inhibit tumor cell proliferation.The effect of targeted molecular drugs on tumors is different from that of chemotherapy drugs.Thus,tumor response evaluation criteria suitable for targeted molecular drugs have been developed in recent years.This review introduces Choi criteria that use CT attenuation coefficient(Hounsfield unit[HU])to describe tumor density.The criteria have measured indicators that include tumor size and tumor density.This review also introduces the following criteria derived from the Choi criteria for Modified Choi(mChoi)criteria size and attenuation CT(SACT),and morphology,attenuation,size,and structure(MASS).
response evaluation criteria in solid tumors,targeted molecular therapy

10.3969/j.issn.1000-8179.20142149
①厦门大学附属中山医院肿瘤科(福建省厦门市361004)
王琳wanglin_linda82@163.com
Department of Oncology,Zhongshan Hospital Affiliated to Xiamen University,Xiamen 361004,China
王琳专业方向为消化道及乳腺恶性肿瘤、恶性肿瘤的综合治疗。
E-mail:wanglin_linda82@163.com
