Effect of viscoelasticity on skin pain sensation
2015-11-10FushengLiuChenghaiLiShaobaoLiuGuyGeninGuoyouHuangTianjianLuFengXu
Fusheng Liu,Chenghai Li,Shaobao Liu,Guy M.Genin,Guoyou Huang,Tianjian Lu,∗,Feng Xu∗
aState Key Laboratory for Strength and Vibration of Mechanical Structures,Xi'an Jiaotong University,Xi'an 710049,China
bBioinspired Engineering and Biomechanics Center(BEBC),Xi'an Jiaotong University,Xi'an 710049,China
cDepartment of Neurological Surgery,School of Medicine,Washington University in St.Louis,St.Louis 63130,USA
dDepartment of Mechanical Engineering&Materials Science,Washington University in St.Louis,St.Louis 63130,USA
eThe Key Laboratory of Biomedical Information Engineering of Ministry of Education,Xi'an Jiaotong University,Xi'an 710049,China
Effect of viscoelasticity on skin pain sensation
Fusheng Liua,b,Chenghai Lia,b,Shaobao Liua,b,Guy M.Geninc,d,Guoyou Huangb,c,Tianjian Lua,b,∗,Feng Xub,e,∗
aState Key Laboratory for Strength and Vibration of Mechanical Structures,Xi'an Jiaotong University,Xi'an 710049,China
bBioinspired Engineering and Biomechanics Center(BEBC),Xi'an Jiaotong University,Xi'an 710049,China
cDepartment of Neurological Surgery,School of Medicine,Washington University in St.Louis,St.Louis 63130,USA
dDepartment of Mechanical Engineering&Materials Science,Washington University in St.Louis,St.Louis 63130,USA
eThe Key Laboratory of Biomedical Information Engineering of Ministry of Education,Xi'an Jiaotong University,Xi'an 710049,China
A R T I C L EI N F O
Article history:
Accepted 14 September 2015
Available online 28 November 2015
Viscoelasticity Skin tissue
Pain sensation may appear under long-lasting mechanical stimulation.Although people have the experience that pain sensation generally decreases with time while the stimulation remains,the underlying mechanism remains elusive.We experimentally studied the thermal and strain ratedependent viscoelastic behavior of skin in uniaxial stretch and numerically investigated the effects of temperature and strain rate on pain sensation.The results indicate that the viscosity of skin tissue decreases with increasing temperature and reducing strain rate,which subsequently decreases the discharge frequency of skin nociceptor and thus relieves the pain sensation.The results would contribute to the understanding of pain relief mechanism and optimizing for mechanical treatment.
©2015 Published by Elsevier Ltd on behalf of The Chinese Society of Theoretical and Applied Mechanics. This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/ by-nc-nd/4.0/).
Skin plays very important roles in the body,including sensory,thermoregulatory andhost defense,etc.[1].Uncomfortable feeling or pain sensation may appear when skin is under extreme mechanical and thermal stimulations,where the stimulation may be long-lasting(even hours)in some cases.For instance,in traditional Chinese cupping treatment[2]and furuncle disease[3],we feel pain due to continuous mechanical stimulation in skin tissue.Although people have the experience that uncomfortable feeling or pain sensation generally decreases with time even if the stimulation remains,the underlying mechanism is still elusive. However,it is well-known that skin is a viscoelastic material,therefore we hypothesize that its viscoelastic property may play an important role in pain sensation.
Traditionally,pain can be classified as neuropathic pain,inflammatory pain and nociceptive pain,where the physiology of nociceptive pain has been studied extensively[4].In nociceptive pain,nociceptors transduce a noxious stimulus into ionic current that generates action potentials(discharge).The discharge is subsequently transmitted via nerve fibers from the peripheral sensory site to the synapse in the central nervous system,where the action potentials are converted into neurotransmitter release at thepresynapticterminal[5].Neuroscientistsbelievedischargefrequencyisthemainfactorintheencodingofpain[6],asreflectedby theincreasedpainintensitywithincreasingdischargefrequencyin intimated experiment[7,8].Although several mathematical models have been used to study the thermal and mechanical skin sensation[1,9],the effect of skin viscoelasticity on nociceptive pain sensation has not been explored yet.
In this paper,we first characterized the viscoelasticity of pig dorsal skin via a uniaxial tensile experiment under different temperatures and strain rates,and analyzed the viscoelastic properties of skin by using the quasi-linear viscoelasticity(QLV)model(Fig.1).Then,combining the QLV model and our previous pain sensation model of skin nociception,we compared the frequency of action potential induced by mechanical stimulation with and without considering skin viscoelastic properties and analyzed the effect of viscoelasticity on skin pain sensation.
The specimen is from the belly of adult pig with dimensions 12 mm×12 mm×2 mm.The viscoelastic behaviors of skin were studied using a computer-controlled uniaxial tensile equipment,wheredetailsconcerningtheexperimentequipmentandmaterials preparation can be found from our previous study[10].In brief,the pig dorsal skin was placed at the central of a chamber filled with buffer solution.The thermal environment was controlled by a circulation system connected to a thermostat.Before starting,preconditioning process was performed at 37°C to ensure a reproducible response.Both ends along the fiber direction were evenly fixed by 18 hooks(Fig.1),a square of nine dots and video camera were marked to record strain in each direction,and inplane loads were measured by temperature compensated force transducers.The fiber direction was loaded under a range of environment temperature T(25°C,37°C,50°C,and 60°C)as well as loading rateγ(5%/min,10%/min,25%/min,and 50%/min),which is equal to strain rate.
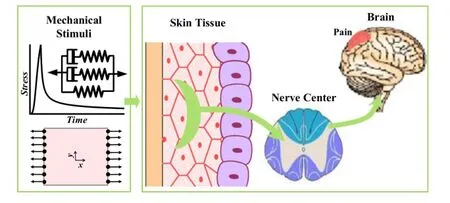
Fig.1.A model of pain sensation in viscoelastic skin.
The QLV model was used to describe the viscoelasticity of skin tissue,which can be obtained as:
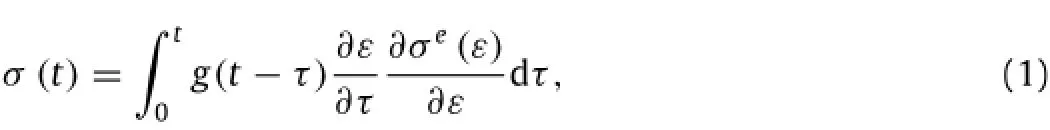
whereσeis the transient stress and g is the relaxation function,which can be approximately described by using the prony series as:

Here,the percentage of stress at the equilibrium state of relaxation process k0+k1+k2=1,andτ1is the long-and short-term relaxation time.The instantaneous response is described by the following expression:

The strain is given by loading rate,as:
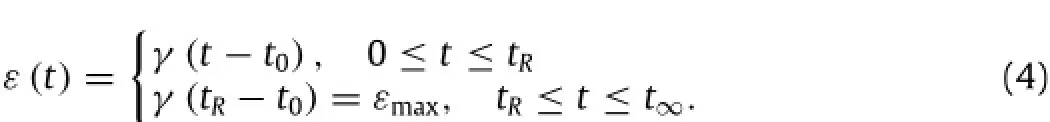
ThestresscanbeexpressedbysubstitutingEqs.(2)-(4)intoEq.(1),as:
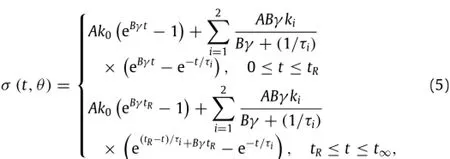
whereθ=θ(A,B,k0,k1,k2,τ1,τ2)is a dummy function.
The signal of pain sensation starts from the opening of ion channels in nociceptors as induced by noxious stimuli.Mechanical stimulus-induced current(Ist)may be calculated as[11]:whereσandσtare the stress and mechanical pain threshold at the location of nociceptor,Cmechis the stress conversion constant(µA/cm2),and H(x)is the Heaviside function.
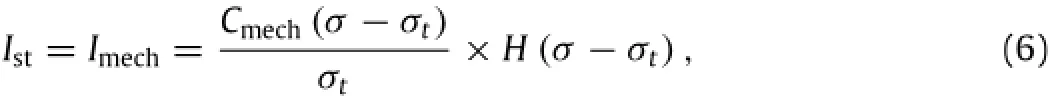
The action potential model in skin nociceptors can be described as[12]:
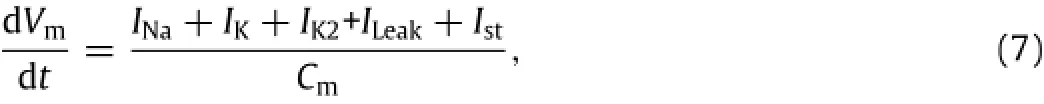
where Vmis membrane potential(mV);Cmis membrane capacity(µF/cm2);INa,IK,IK2and Ileakare sodium,potassium,the second potassium and leakage current components(µA/cm2);Istis stimuli-induced current.
The discharge frequency(f)can be calculated using the following equation[1]:
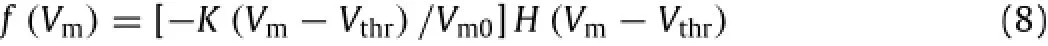
where K is a constant;Vthris the firing threshold potential;Cmech,σtand Vthrare assumed to be 20µA/cm2,0.2 MPa and -55 mV[11].The details of relevant material parameters can be found in our previous studies[12].
The mechanical properties of skin tissue are sensitive to temperature and strain rate during tensile testing[13],which may cause different pain sensation.To characterize the temperaturedependent viscoelastic behavior of skin tissue,we performed uniaxial tensile and relaxation test under different temperatures(25°C,37°C,50°C,and 60°C)and fixed strain rate ofγ=10%/min(Fig.2(a)).Thestressreachesthepeakvaluewhenthestrainis40%,thenthestressrelaxeswithtime.Asacontrol,stressrelaxationwill not occur if viscoelasticity of skin tissue is ignored(Fig.2(a)).We observedthatthestressrelaxesfasterwithincreasingtemperature when temperature is less than 50°C.The stress relaxation had no obvious change around 50-60°C.The final stress level after relaxation is much higher under low temperature than that under high temperature.The possible mechanism is that collagenous fibers,which provide the principal structural and mechanical support for skin tissue[14],were thermally desaturated under hyperthermal temperatures[15],resulting in the decrease of skin mechanical properties.With the similar method,we obtained the tensile and relaxed stress with different strain rates(5%/min,10%/min,25%/min,50%/min)under a constant temperature of 30°C(Fig.2(b)).The higher strain rate induces a higher stress peak value under the same maximal strain 40%,which exhibits the strain rate hardening behavior of skin tissue.However,we did not observe significant effect of strain rate on the final stress after stress relaxation for the same maximal strain of 40%.To quantify the effect of temperature and strain rate on the viscosity of skin tissue,we used the QLV model to fit the experimental data by using the Levenberg-Marquardt algorithms in Origin 9.0.The values of goodness-of-fitχ2were adopted to assess the theoretical results(Table 1),which were over 0.96.The results show that therelaxation time(τ1,τ2)of skin tissue decreases with increasing temperature but increases with increasing strain rate.
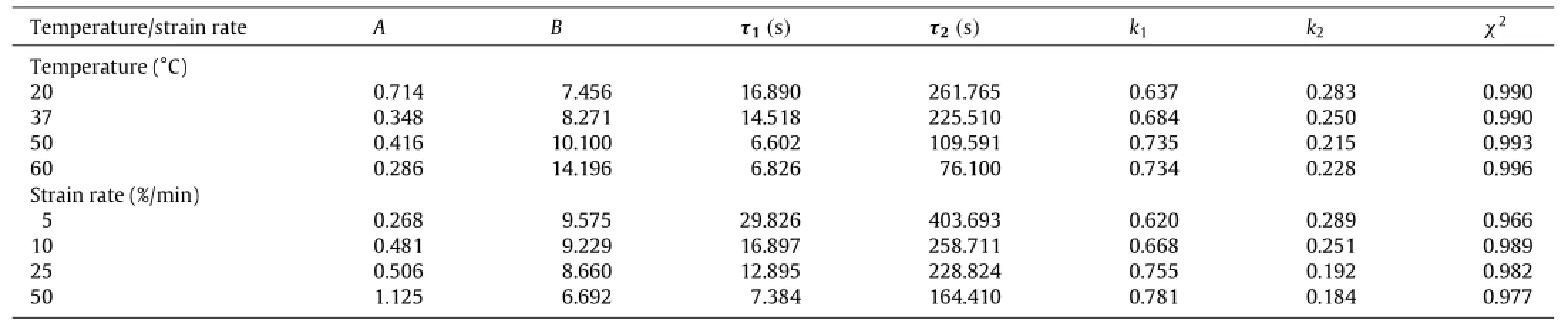
Table 1 Viscoelastic parameters in QLV model of skin with different strain rates and temperatures.
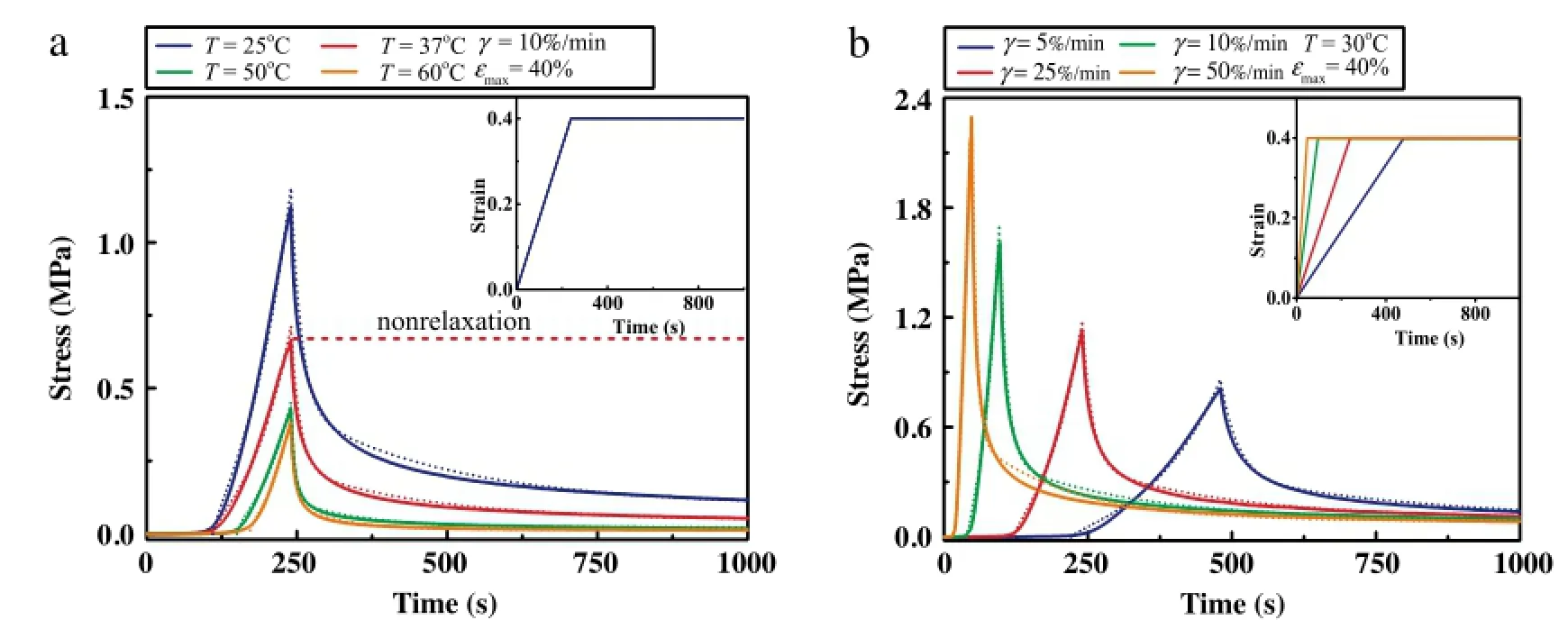
Fig.2.Viscoelastic behaviors of skin under different(a)temperatures and(b)strain rates.Theoretical(dash line)and experimental(solid line)data are compared.The red short dash represents the stress without regard to the skin viscidity.(For interpretation of the references to color in this figure legend,the reader is referred to the web version of this article.)
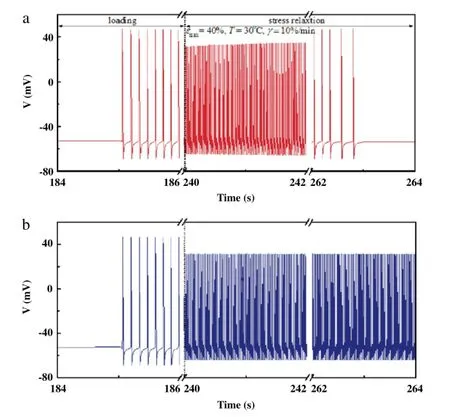
Fig.3.Action potential of skin nociceptor(a)with and(b)without considering skin viscidity.
The pain sensation will appear when the stress in skin tissue surpasses the mechanical threshold of nociceptor(~0·2 MPa)[16].To investigate the effect of viscoelasticity on skin pain sensation,we first simulated the action potential(AP)by applying the stress relaxation using the QLV model as obtained from the experimental study as the stimulus to our model of skin pain sensation under the temperature of 37°C and strain rate of 10%/min(Fig.3).We found that when viscoelasticity is considered,the frequency of action-potential discharge of skin nociceptors increases with increasing stress,and then significantly decreases with stress relaxation(Fig.3(a)).However,the frequency of action potential in skin nociceptors is nearly unchanged when ignoring the influence of stress relaxation(Fig.3(b)).This result indicates that skin viscoelasticity may play a very important role in releasing pain sensation.To assess this,we calculated the action potential discharge frequency of skin nociceptor under different temperatures and strain rates.We observed that the discharge frequency decreases with increasing temperature during uniaxial stretch and relaxation process(Fig.4(a)).As previously discussed,the viscoelastic properties of skin tissue decreased under higher temperature due to denaturation of collagen fibers,which results in lower stress and more obvious stress relaxation under the same maximal tensile strain,making the local nociceptor bear lower mechanical stimuli.This may explain the mechanism that the heat cupping usually gives more comfortable feeling than the cold cupping in traditional Chinese medicine.However,with increasing strain rate,the maximum tensile stress in skin increases(Fig.2(b)),the discharge frequency of skin nociceptor is higher and the time of discharge is earlier(Fig.4(b)).This indicates that a lower loading rate may give a more comfortable feeling in mechanical treatment.
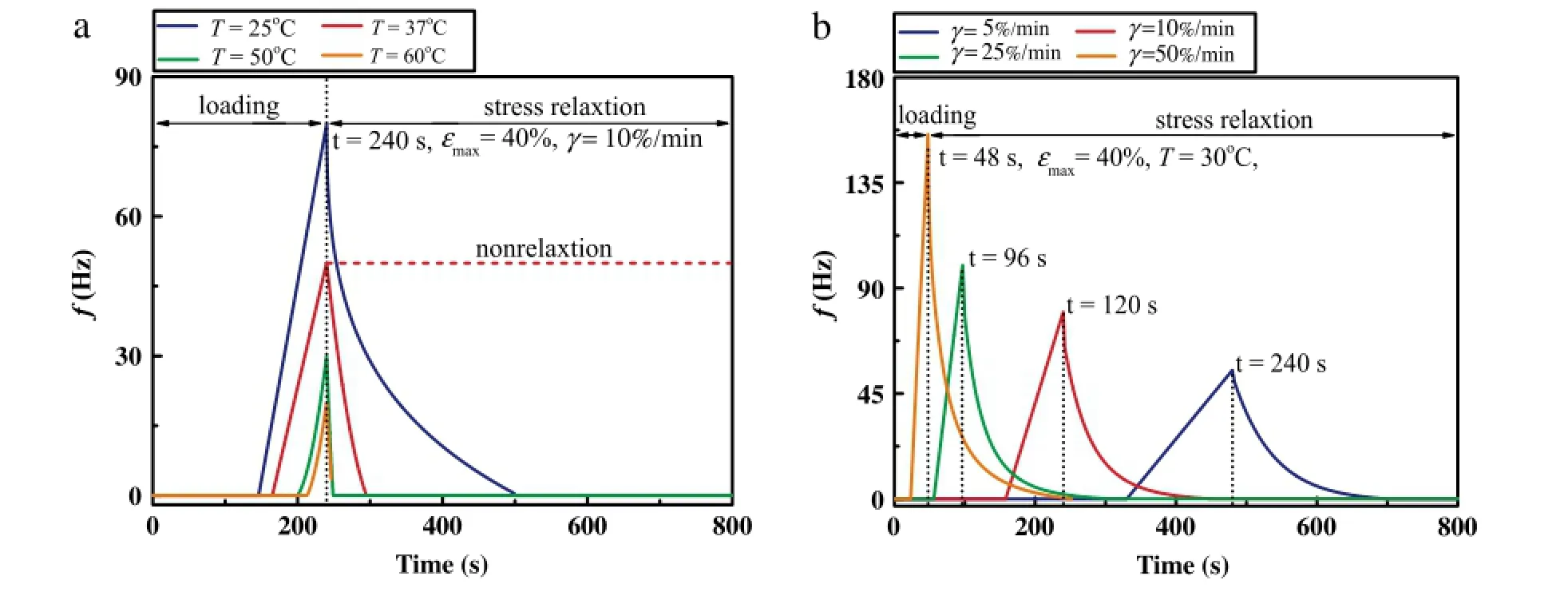
Fig.4.Dischargefrequency of skin nociceptorunder(a)different temperatures and(b)strainrates.Thered shortdash in plot(a)represents thedischargefrequency without regard to viscoelasticity.(For interpretation of the references to color in this figure legend,the reader is referred to the web version of this article.)
In previous modeling,we did not consider the effect of thermally-induced pain sensation.In fact,a higher temperature which is more than thermal pain threshold(~43°C)will induce the discharge of skin nociceptors and generate the thermal pain sensation[1].To estimate the thermal effect,we introduce the influence of thermal stimuli current Iheatinto the action potential model(see Ref.[1]for details).The results(Fig.5)show that the discharge frequency of thermomechanical stimuli(Imech+Iheat)is significantly higher than that of mechanical stimulation(Imech)alone,which is even more obvious under higher temperature(60°C).This indicates the thermomechanical stimuli will be more obvious than mechanical stimulation and the remission effect of skin viscoelasticity on pain sensation is not less meaningful when the temperature is more than thermal pain threshold.
We focus here on the mechanical factors contribution to pain relief.Furthermore,physiological factor is also responsible for the nociceptive pain relief.For instance,ion channel in nociceptor(i.e.TRPA1)controls the action potential and receptor(i.e.C nociceptors)releases the neuropeptides and expresses the c-Ret neurotrophin receptor,which has identified a host of potential therapeutic targets[17]by drug for treatment of persistent pain. Obviously,answers to pain relief mechanism will require the combined use of mechanical,physiological method.In future research,we need to comprehensively consider the effect of them on pain relief.
Pain sensation is largely affected by the viscoelastic behaviors:when suffering long-lasting noxious mechanical stimuli,stress relaxation of skin tissue reduces nociceptor stress level.That will reduce the frequency of action-potential discharge in nociceptor and relieve the pain sensation;an increased strain rate has a tendency of hardening and leads to a sharp pain.Higher temperature can increase extensibility of skin tissues,relieves the pain sensation when temperature is less than the threshold 43°C.
Acknowledgments
This research was supported by the National Natural Science Foundation of China(11372243)and the International Science and Technology Cooperation Program of China(2013DFG02930).
[1]F.Xu,T.J.Lu,K.Seffen,Skinthermalpainmodeling—aholisticmethod,J.Therm. Biol.33(2008)223-237.
[2]C.C.Ji,L.P.Huang,G.Q.Yang,etal.,Clinicalvalueofcuppingspoteffect,Chinese Acupunct.Moxib.34(2014)1217-1220.
[3]A.D.Pickering,T.G.Pottinger,Stress responses and disease resistance in salmonid fish:effects of chronic elevation of plasma cortisol,Fish Physiol. Biochem.7(1989)253-258.
[4]L.Werhagen,K.Borg,Analysis of long-standing nociceptive and neuropathic pain in patients with post-polio syndrome,J.Neurol.257(2010)1027-1103.
[5]E.W.McCleskey,M.S.Gold,Ion channels of nociception,Annu.Rev.Physiol.61(1999)835-856.
[6]S.D.Waldman,Atlas of Interventional Pain Management,Elsevier Health Sciences,2009.
[7]D.M.Cain,S.G.Khasabov,D.A.Simone,Response properties of mechanoreceptors and nociceptors in mouse glabrous skin:an in vivo study,J.Neurophysiol. 85(2001)1561-1574.
[8]R.C.Coghill,D.J.Mayer,D.D.Price,The roles of spatial recruitment and discharge frequency in spinal cord coding of pain:a combined electrophysiological and imaging investigation,Pain 53(1993)295-309.
[9]F.Xu,T.J.Lu,K.A.Seffen,Modeling of nociceptor transduction in skin thermal pain sensation,Trans.ASM J.Biomech.Eng.130(2008)041013.
[10]F.Xu,T.J.Lu,K.A.Seffen,Effect of thermal damage on compressive behavior of skin tissue,J.Mech.Med.Biol.9(2009)81-104.
[11]C.Belmonte,F.Cervero,Neurobiology of Nociceptors,Oxford University Press,USA,1996.
[12]F.Xu,T.J.Lu,Introduction to Skin Biothermomechanics and Thermal Pain,Springer,2011.
[13]P.Y.Chen,J.McKittrick,M.A.Meyers,Biological materials:Functional adaptations and bioinspired designs,Prog.Mater.Sci.57(2012)1492-1704.
[14]H.Vogel,Agedependenceofmechanicalandbiochemicalpropertiesofhuman skin.I:Stressstrain experiments,skin thickness and biochemical analysis,Bioeng.Skin 3(1987)67-91.
[15]B.Zhou,F.Xu,C.Q.Chen,et al.,Strain rate sensitivity of skin tissue under thermomechanicalloading,Philos.Trans.R.Soc.A:Math.368(2010)679-690.[16]D.Barefield,S.Sadayappan,Phosphorylation and function of cardiac myosin binding protein-C in health and disease,J.Mol.Cell.Cardiol.48(2010)866-875.
[17]A.I.Basbaum,D.M.Bautista,G.Scherrer,et al.,Cellular and molecular mechanisms of pain,Cell 139(2009)267-284.
31 August 2015
s.
E-mail addresses:tjlu@mail.xjtu.edu.cn(T.Lu),fengxu@mail.xjtu.edu.cn(F.Xu).
http://dx.doi.org/10.1016/j.taml.2015.11.002
2095-0349/©2015 Published by Elsevier Ltd on behalf of The Chinese Society of Theoretical and Applied Mechanics.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Temperature Strain rate
Pain
*This article belongs to the Biomechanics and Interdiscipline
杂志排行
Theoretical & Applied Mechanics Letters的其它文章
- Anomalous friction of graphene nanoribbons on waved graphenes
- Combined modeling of cell aggregation and adhesion mediated by receptor-ligand interactions under shear flow
- The critical pressure for driving a red blood cell through a contracting microfluidic channel
- Mechanical responses of the bio-nano interface:A molecular dynamics study of graphene-coated lipid membrane
- Effects of humidity on shear behavior of bamboo
- Review on structural fatigue of NiTi shape memory alloys:Pure mechanical and thermo-mechanical ones
