Mechanism of PEDF promoting the proliferation of lens epithelial cells in human eyes
2015-10-31WenLeiYangLinZhang
Wen-Lei Yang, Lin Zhang
Department of Ophthalmology, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200127,China
Mechanism of PEDF promoting the proliferation of lens epithelial cells in human eyes
Wen-Lei Yang, Lin Zhang*
Department of Ophthalmology, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200127,China
ARTICLE INFO
Article history:
Received in revised form 20 September 2015
Accepted 15 October 2015
Available online 20 November 2015
Human
Lens
Epithelial cells
Eye protein
Vascular endothelial growth factor
Pigment epithelium-derived factor
Objective: To investigate the regulation effect of pigment epithelium-derived factor (PEDF) on the growth of human lens endothelial cells (LECs) and related mechanisms in vivo and in vitro. Methods: In the part of in vivo study, 82 eyes of 82 patients with age-related cataract were included to collect the central lens anterior capsule (diameter at 5.0 to 5.5 mm) with the informed consent of surgery for patients. The selected specimens were divided into the LECs low density group and high density group with 20 specimens for each group based on hematoxylin and eosin staining results. The relative expression level of PEDF mRNA in LECs was detected by reverse transcription PCR. In the part of in vitro study, LEC line (HLEB3) was cultured and 50 ng/mL PEDF was added in media for 72 h in PEDF culture group,while normally cultured cells were used as the control group. The percentage of LECs at G0and S phases and apoptotic rate of cells were assayed by using flow cytometry with annexinⅤ-FITC/7-AAD double staining method. Intracellular expression of vascular endothelial growth factor (VEGF) mRNA was detected by real-time fluorescence quantitative PCR. Results: The central anterior subcapsular LECs density and relative expression level of PEDF mRNA were lower than those of high density group. There were no significant differences between two groups (P=0.168). The apoptotic rate in the PEDF culture group was significantly reduced in comparison with the control group (P<0.001). In addition, the expression level of VEGF mRNA was lower in the PEDF culture group compared with the control group (P<0.001). Conclusions: In human eyes, PEDF may function as cytotropic factor to promote survival of LECs through anti-apoptosis and reducing-expression of VEGF. Decrease of PEDF content in LECs probably modulates the pathophysiological process of lens cells and further cataractogenesis.
Document heading doi:10.1016/j.apjtm.2015.10.009
1. Introduction
The lens epithelial cell (LEC) is one of the key mechanisms on regulating the growth and aging of lens cell and also associated with cataractogenosis and its development. Its biochemical behavior allows molecular regulation by a variety of intracellular and extracellular signal transduction[1-4]. The pigment epithelium-derived factor (PEDF) is a kind of factor with multiple effects widely distributed in embryo and adult[5,6]. The previous research indicated a significantly antagonism action existed between PEDF and vascular endothelial growth factor (VEGF), while both of them had the effect of nutrition protection detected in histiocyte[7,8]. According to the earlier study of our research, the PEDF levels in human aqueous humorand in LECs presented positive correlation with the aging level of body and the degree of cataract attack[9-12]. However, whether PEDF has regulating effect on the growth of LECs and its relevant molecular mechanism has not been reported so far. The present study aimed to evaluate the regulating effect of PEDF on the growth of LECs and its relevant molecular mechanism by in vivo and in vitro experimental study.
2. Materials and methods
2.1. Clinical materials
2.1.1. In vivo experiment
A total of 82 eyes of patients (male: 31 cases of 31 eyes, female: 51 cases of 51 eyes) with cataract treated by operation in our hospital from January 2011 to December 2012 were collected under the following standards: (1) the age ranged from 52 to 92; (2) noncongenital cataract, non-metabolic cataract and non-secondary cataract; (3) without gestational diabetes mellitus, fundus lesions,uveitis and glaucoma; (4) no eye trauma and the history of intraocular surgery; (5) no anterior lens capsules turbidity; (6) no other lens turbidity unless age-related lens. According to LOCSⅡ standard, the turbidity degree of lens cortex, lens nucleus and posterior subcapsular can be divided into from C2 to C5 level, N2 to N3 level and with or without turbidity, respectively. We declared that the study was approved by the ethics committee in our hospital. All subjects in this study knew and understood the content and risk of the research and signed the informed consent.
2.1.2. In vitro experiment
The human LE cell line, HLE-B3, provided by the experiment center of our hospital was cultured in complete medium mixed with 10% of fetal bovine serum for subculture. Then the optical microscope was used to observe the cell growth in good condition. After 70%-90% of cell density, the medium was digested by pancreatin and kept under -80 ℃ or liquid nitrogen for further use.
2.1.3. Main reagents and apparatus
In this study, PEDF, Trizol Reagent, RT-PCR reagent, the primers of PEDF, VEGFM and β-actin, Phosphatidylserine kit, fluorescence quantitative PCR reagent, ultraviolet spectrophotometer, flow cytometry and real-time PCR were used in this study.
2.2 .Methods
2.2.1. Detection of PEDF mRNA expression by RT-PCR method
The central lens anterior capsules containing epithelial cells under the sac with diameter at 5.0 to 5.5 mm were obtained by emulsified continuous curvilinear capsulorhexis emulsification during cataract extraction. The obtained anterior capsules samples were divided into two parts, one of which was fixed by adding 4% paraformaldehyde for the later detection of cell density, and the other of which was kept by immerging to Trizol Reagent and at -20 ℃ for the test of mRNA expression.
To roll out the samples, after hematoxylin eosin staining, the detection of cell densities of them was conducted under three different visual angles. The mean of results was calculated as the cell density of LEC. Then the samples were divided into the low density group (<4 000/mm2) and the high density group (>4 500/ mm2) with 20 samples for each group. Each 5 samples of each group were combined into 1 sample for test of the mRNA expression,thus both groups contained 4 samples. The RNA of the lens capsule membrane tissue was extracted and synthesized into cDNA by using RT-PCR kit. According to literature, the sequences of PEDF sense primer and reverse primer, internal reference β-actin sense primer and its reverse primer were5'-TGTGCAGGCTTAGAGGGACT-3',5'-G T T C A C G G G G A C T T T G A A G A-3', 5'-G G T G G C T T T TA G G AT G G C A A G-3 a n d 5'-ACTGGAACGGTGAAGGTGACAG-3', respectively. By adopting the semi-quantitative reverse transcription PCR technology to increase mRNA and after the electrophoretic imaging, the amplified fragment was analyzed and calculated for the relative expressions of PEDF and mRNA. This experiment was repeated 5 times.
2.2.2. Annexin Ⅴ method for detection of LECs growth and apoptosis
HLE-B3 was cultured in DMEM complete medium containing 10% fetal calf serum and 50 ng/mL PEDF in the culture group for 72 h, and in the control group, the HLE-B3 was cultured for 72 h by DMEM complete medium only containing 10% fetal calf serum. The cell cycles and circumstances of apoptosis of both groups were detected by using annexinⅤ-FITC/7-AAD double-staining method. About 0.5×106-1×106of suspension cells were collected in two groups, respectively. One extractive was added in 100 μL buffer solution and 100 μL annexin Ⅴ-FITC for the incubation avoiding light. The other extractive was added in 400 μL buffer and 5 μL 17-ADD solution and quantitatively assayed for its cell cycle and apoptosis condition by using flow cytometry. The suspension cell without adding annexinⅤ-FITC and 7-AAD was used in the negative control group. This experiment was repeated 5 times.
2.2.3. Real-time fluorescence quantitative PCR for detection of VEGF and mRNA expressions
The cell suspension solution of the PEDF group and the control group was extracted, respectively, and added in the Trizol Reagent for extracting RNA. The experiment was continuously, strictly conducted under operation instruction of real-time fluorescence quantitative PCR kit. According to literature, the sequences of VEGF sense primers and reverse primers were 5'-TTCAGAGCGGAGAAAGCATT-3' and 5'-GAGGAGGCTCCTTCCTGC-3', respectively. The size of amplified fragment was 166 bp, whileβ-actin, as internal reference, was 161 bp. By using the real-time fluorescence quantitative PCR, theparameters were set as follows: pre-degeneration at 95 ℃ for 5 min, denaturation at 95 ℃ for 10 s, annealing at 60 ℃ for 20 s and extension at 72 ℃ for 20 s, reaction circulation for 40 times. The results were analyzed by adopting CT method. This experiment was repeated 5 times.
2.3. Statistical analysis
The data during research were analyzed by using SPSS13.0 and the measurement data were expressed as mean ±SD. The differences between groups were tested by t-test and when P<0.05, it was considered as having statistical differences (α=0.05).
3. Results
3.1. Comparison of age, LECs density and relative transcript level of PEDF mRNA between low density group and high density group
The comparison of age between both groups revealed no significant difference (P=0.168). The LECs density of the high density group was significantly higher than that of the low density group(P<0.001). Relative transcript level of PEDF mRNA of the high density group was significantly higher than that of the low density group (P<0.05) (Table 1).
3.2. Circumstance of proliferation and apoptosis of LECs after cultivating with PEDF intervention
The HLE-B3 cells were cultured by adding PEDF for intervention,and the cellular morphology of them was observed under optical microscope after 24 h, 48 h, and 72 h, respectively. Comparing with the cellula morphology of the control group by normally cultivating,no significantly morphological alteration, and nofibrosis cell were found. After HLE-B3 was cultured by PEDF intervention for 72 h,the ratio of cell in the control group at G2+S phase was significantly lower than that of the PEDF culture group (P<0.001). The apoptosis rate of the control group was significantly higher than that of the PEDF culture group after PEDF intervention culture for 72 h(P<0.001). Therefore, PEDF was considered as having the inhibition effect for HLE-B3 cell apoptosis (Figure 1 and Table 2).
3.3. Relative expression level of VEGF mRNA
The relative expression level of VEGF mRNA in the PEDF culture group was significantly lower than that of the control group and the relative expression level decreased 75.3%. There was a significant difference of this parameter between two groups (P<0.001)(Table 3).

Table 1 Comparison of age, LECs density and relative transcript level of PEDF mRNA between low density group and high density group.

Table 2Apoptosis rate and cell ratio at G2+ S phase in PEDF culture group and control group.
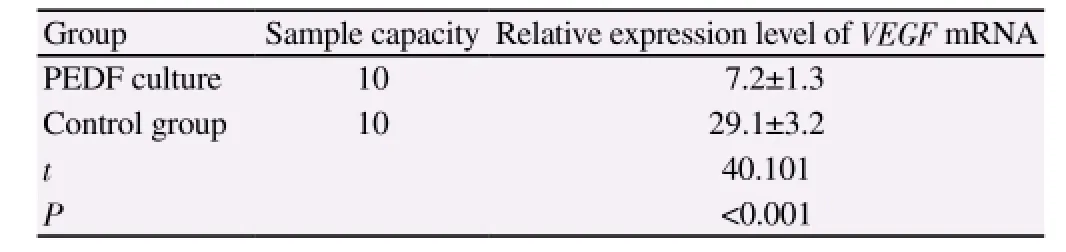
Table 3 Comparison of relative expression level of VEGF mRNA.
4. Discussion
The PEDF synthesis starts in a variety of eye cells of 7 wks of human embryo and PEDF gene and proteins were widely distributed in adult intraocular, retina, ciliary body, cornea, choroid and intraocular fluid[13-15]. We generally consider that PEDF in eyes mainly functions for protecting retina and promoting its differentiation[16,17], and the effect of PEDF for inhibiting the production of abnormal vascular was proved by relative research[18]. The research of Golan et al revealed that there existed the PEDF expression in LECs of mouse eyes[19]. Huang et al. detected the PEDF gene in LECs of human eyes by applying the cDNA sequence technique and proved that it was related to the cataract[20]. The earlier study stage of this research revealed that the PEDF level of LECs in human aqueous humor and under anterior capsules was negatively correlated with the age of patients and circumstance of cataract attack. However, there are no relative researches reported regarding the correlation between PEDF and the metabolism of LECs. It has been known that PEDF has the significant effect for maintaining the lens without vascularization, while the problems on whether it also has some other effects for the proliferation, differentiation and senescence of LECs and cellular morphological characters or not,how are the mechanisms of production of these effects and how they affect during cataracts attack and prognosis, are all worth further exploring. The results of this study revealed that in a group, if the LECs were in low density, its PEDF level would decrease at the same time. The in vitro experiment showed that PRDF could inhibit the apoptosis of LECs and decrease the expression of VEGF.
PEDF is a kind of cytokines with multiple effects containing cell protection and nutrition, anti-tumor, antioxidant and anti angiogenesis, etc. These effects of PEDF have duality for which can not only inhibit cell division and induce cell apoptosis, but promote cell proliferation and resist the cell premature aging[14,21,22]. The biological effects of PEDF are sensitive to many factors including cell type, isomer formed by transcription or translation,the distinction of receptor, signal pathway and environment,etc[23,24]. It can be speculated by the results of this research that PEDF participated in the process of growth and development of lens by secretion and/or paracrine and it played a role in adjusting the LECs proliferation differentiation, maintaining LECs activity and biological activity, inhibiting LECs oxidation and apoptosis. The decreased level of PEDF in LECs maybe one of the factors in cataractogenosis and its development.
The aging of body was mainly characterized by the abnormal expressions of multiple genes[25]. The LECs, as a carrier for playing a key role in lens structure maintaining, metabolism and function, the degree of its senility is one of the pathogenic decisive factors[26]. The expressions of PEDF in the early phase with cDNA-1mutiplication, in aging cells and tissues drop significantly. As the cytokine in relation to some life-span of cells, PEDF is the specific genes reflecting cell multiplication capacity at the G0phase[27-29]. Some studies have been reported that PEDF has a certain effect during occurrence and development of some age-related diseases[30]. With the human LECs as the research subject, this study investigated the effects of PEDF on LECs through the in vivo and in vitro experiments of cells and levels of tissue. The results revealed that PEDF had the effects on inhibiting LECs apoptosis and reducing VEGF expression. According to the literature, these effects of PEDF was stimulated by autocrine and/or paracrine,the effects of cell protection and nutrition, anti-tumor, antioxidant and anti angiogenesis, etc., during the process of lens growth and development. Our research provides a reference for the further investigation of lens growth and development as well as the occurrence and development of cataract.
Conflict of interest statement
We declare that we have no conflict of interest.
[1] Bai Y, Yu W, Han N, Yang F, Sun Y, Zhang L, et al. Effects of semaphorin 3A on retinal pigment epithelial cell activity. Invest Ophthalmol Vis Sci 2013; 54(10): 6628-6638.
[2] Suen WL, Chau Y. Specific uptake of folate-decorated triamcinoloneencapsulating nanoparticles by retinal pigment epithelium cells enhances and prolongs antiangiogenic activity. J Control Release 2013; 167(1): 21-28.
[3] Skiles ML, Sahai S, Rucker L, Blanchette JO. Use of culture geometry to control hypoxia-induced vascular endothelial growth factor secretion from adipose-derived stem cells: optimizing a cell-based approach to drive vascular growth. Tissue Eng Part A 2013; 19(21-22): 2330-2338.
[4] Beckman SA, Chen WC, Tang Y, Proto JD, Mlakar L, Wang B, et al. Beneficial effect of mechanical stimulation on the regenerative potential of muscle-derived stem cells is lost by inhibiting vascular endothelial growth factor. Arterioscler Thromb Vasc Biol 2013; 33(8): 2004-2012.
[5] Gattu AK, Swenson ES, Iwakiri Y, Samuel VT, Troiano N, Berry R, et al. Determination of mesenchymal stem cell fate by pigment epitheliumderived factor (PEDF) results in increased adiposity and reduced bone mineral content. Faseb J 2013; 27(11): 4384-4394.
[6] Sonoda S, Nagineni CN, Kitamura M, Spee C, Kannan R, Hinton DR,et al. Ceramide inhibits connective tissue growth factor expression by human retinal pigment epithelial cells. Cytokine 2014; 68(2): 137-140.
[7] Soejima K, Shinoda K, Kashimura T, Yamaki T, KonoT, Sakurai H, et al. Wound dressing material containing lyophilized allogeneic cultured cells. Cryobiology 2013; 66(3): 210-214.
[8] Szymanska J, Goralczyk K, Klawe JJ, Lukowicz M, Michalska M,Zalewski P, et al. Phototherapy with low-level laser influences the proliferation of endothelial cells and vascular endothelial growth factor and transforming growth factor-beta secretion. J Physiol Pharmacol 2013;64(3): 387-391.
[9] Astern JM, Collier AC, Kendal-Wright CE. Pre-B cell colony enhancing factor (PBEF/NAMPT/Visfatin) and vascular endothelial growth factor(VEGF) cooperate to increase the permeability of the human placental amnion. Placenta 2013; 34(1): 42-49.
[10] Perspicace E, Jouan-Hureaux V, Ragno R, Ballante F, Sartini S, La Motta C, et al. Design, synthesis and biological evaluation of new classes of thieno[3,2-d]pyrimidinone and thieno[1,2,3]triazine as inhibitor of vascular endothelial growth factor receptor-2 (VEGFR-2). Eur J Med Chem 2013; 63(5): 765-781.
[11] Miyashita H, Yokoo S, Yoshida S, Kawakita T, Yamagami S, Tsubota K,et al. Long-term maintenance of limbal epithelial progenitor cells using rho kinase inhibitor and keratinocyte growth factor. Stem Cells Transl Med 2013; 2(10): 758-765.
[12] Yamben IF, Rachel RA, Shatadal S, Copeland NG, Jenkins NA, Warming S, et al. Scrib is required for epithelial cell identity and prevents epithelial to mesenchymal transition in the mouse. Dev Biol 2013; 384(1): 41-52.
[13] Rachitskaya AV, Goldhardt R. Retinal pigment epithelium Tear. Current Ophthal Reports 2015; 3(1): 26-33.
[14] Young M, Chui L, Fallah N, Or C, Merkur AB, Kirker AW, et al. Exacerbation of choroidal and retinal pigment epithelial atrophy after anti-vascular endothelial growth factor. Retina 2014; 34(7): 1308-1315.
[15] Dithmer M, Fuchs S, Shi Y, Schmidt H, Richert E, Roider J, et al. Fucoidanreduces secretion and expression of vascular endothelial growth factor in the retinal pigment epithelium and reduces angiogenesis in vitro. PloS One 2014; 9(2): e89150.
[16] Hazama T, Fukami K, Yamagishi S, Kusumoto T, Sakai K, Adachi T,et al. Dialysate vascular endothelial growth factor is an independent determinant of serum albumin levels and predicts future withdrawal from peritoneal dialysis in uremic patients. Ther Apher Dial 2014; 18(5): 391-397.
[17] Dahrouj M, Alsarraf O, Mcmillin JC, Liu Y, Crosson CE, Ablonczy Z. Vascular endothelial growth factor modulates the function of the retinal pigment epithelium in vivo. Invest Ophthalmol Vis Sci 2014; 55(4): 2269-2275.
[18] Hermann MM, Van AstenF, Muether PS, Smailhodzic D, Lichtner P,Hoyng CB, et al. Polymorphisms in vascular endothelial growth factor receptor 2 are associated with better response rates to ranibizumab treatment in age-related macular degeneration. Ophthalmology 2014;121(4): 905-910.
[19] Golan S, Entin-Meer M, Semo Y, Maysel-Auslender S, Mezad-Koursh D, Keren G, et al. Gene profiling of human VEGF signaling pathways in human endothelial and retinal pigment epithelial cells after anti VEGF treatment. Bmc Res Notes 2014; 7(1): 617.
[20] Huang L Y, Zhou X Y. Effect of adenovirus-mediated slit2 shRNA on hypoxia-induced expression of vascular endothelial growth factor in human retinal pigment epithelial cells. Chin JBiol 2014; 27(3): 361-370.
[21] Asao K, Gomi F, Sawa M, Nishida K. Additional anti-vascular endothelial growth factor therapy for eyes with a retinal pigment epithelial tear after the initial therapy. Retina 2014; 34(3): 512-518.
[22] Faby H, Hillenkamp J, Roider J, Klettner A. Hyperthermia-induced upregulation of vascular endothelial growth factor in retinal pigment epithelial cells is regulated by mitogen-activated protein kinases. Graefes Arch Clin Exp Ophthalmol 2014; 252(11): 1737-1745.
[23] Chuderland D, Ben-Ami I, Friedler S, Hashy N, Ninio-Many L,Goldberg K, et al. Hormonal regulation of pigment epithelium-derived factor (PEDF) expression in the endometrium. Mol Cell Endocrinol 2014;390(1-2): 85-92.
[24] Rahimy E, Freund KB, Larsen M, Spaide RF, Costa RA, Hoang Q, et al.Multilayered pigment epithelial detachment in neovascular age-related macular degeneration. Retina 2014; 34(7): 1289-1295.
[25] Doguizi S, Ozdek S. Pigment epithelial tears associated with anti-VEGF therapy: incidence, long-term visual outcome, and relationship with pigment epithelial detachment in age-related macular degeneration. Retina 2014; 34(6): 1156-1162.
[26] Klettner A, Tahmaz N, Dithmer M, Richert E, Roider J.Effects of aflibercept on primary RPE cells: toxicity, wound healing, uptake and phagocytosis. Br J Ophthalmol 2014; 98(10): 1448-1452.
[27] Yi JW, Lee WS, Kim SB, Heo YM, Chae DS. Effect of zoledronate on the expression of vascular endothelial growth factor-a by articular chondrocytes and synovial cells: an in vitro study. J Bone Metab 2014;21(4): 249-255.
[28] Liegl R, Koenig S, Siedlecki J, haritoglou C, Kampik A, Kernt M, et al. Temsirolimus inhibits proliferation and migration in retinal pigment epithelial and endothelial cells via mTOR inhibition and decreases VEGF and PDGF expression. PloS One 2014; 9(2): e88203.
[29] Aldebasi YH, Rahmani AH, Khan AA, Aly SM. The effect of vascular endothelial growth factor in the progression of bladder cancer and diabetic retinopathy. Int J ClinExp Med 2013; 6(4): 239-251.
[30] Kim KJ, Yun JH, Heo JI, Lee EH, Min HS, Choi TH, et al. Role of pigment epithelium-derived factor in the involution of hemangioma:autocrine growth inhibition of hemangioma-derived endothelial cells. BiochemBiophys Res Commun 2014; 454(454): 282-288.
15 August 2015
Lin Zhang, Chief Physician, Professor, Department of Ophthalmology, Renji hospital, School of Medicine, Shanghai Jiao Tong University,Shanghai 200127, China.
Tel:1361179494
E-mail: linlinrj172@hotmail.com
Foundation project: Supported by Scientific Research Projects of Science and Technology Commission of Shanghai Municipality (No. 11DZ1921208).
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Demographic, socioeconomic and environmental changes affecting circulation of neglected tropical diseases in Egypt
- Phenolic profile and biological potential of Endopleura uchi extracts
- Roots extracts of Adenophora triphylla var. japonica improve obesity in 3T3-L1 adipocytes and high-fat diet-induced obese mice
- Anti TB drug resistance in Tanga, Tanzania: a cross sectional facility base prevalence among pulmonary TB patients
- In vitro inhibitory effects of plumbagin, the promising antimalarial candidate, on human cytochrome P450 enzymes
- Vibrio spp. from Macrobrachium amazonicum prawn farming are inhibited by Moringa oleifera extracts
