Roots extracts of Adenophora triphylla var. japonica improve obesity in 3T3-L1 adipocytes and high-fat diet-induced obese mice
2015-10-31DongRyungLeeYoungSilLeeBongKeunChoiHaeJinLeeSungBumParkTackManKimHanJinOhSeungHwanYangJooWonSuh
Dong-Ryung Lee, Young-Sil Lee, Bong-Keun Choi, Hae Jin Lee, Sung-Bum Park,Tack-Man Kim, Han Jin Oh, Seung Hwan Yang*, Joo-Won Suh
1NutraPham Tech, Giheung-gu, Yongin, Gyeonggi, Korea
2Center for Nutraceutical and Pharmaceutical Materials, Myongji University, Yongin, Gyeonggi, Korea
3Interdisciplinary Program of Biomodulation, Myongji University, Yongin, Gyeonggi, Korea
4DONG IL Pharmtec, Gangnam-gu, Seoul, Korea
5Department.of Family Medicine, VIEVIS NAMUH Hospital, Seoul, Korea
Roots extracts of Adenophora triphylla var. japonica improve obesity in 3T3-L1 adipocytes and high-fat diet-induced obese mice
Dong-Ryung Lee1#, Young-Sil Lee2#, Bong-Keun Choi1,2, Hae Jin Lee3, Sung-Bum Park3,Tack-Man Kim4, Han Jin Oh5, Seung Hwan Yang2,3*, Joo-Won Suh2
1NutraPham Tech, Giheung-gu, Yongin, Gyeonggi, Korea
2Center for Nutraceutical and Pharmaceutical Materials, Myongji University, Yongin, Gyeonggi, Korea
3Interdisciplinary Program of Biomodulation, Myongji University, Yongin, Gyeonggi, Korea
4DONG IL Pharmtec, Gangnam-gu, Seoul, Korea
5Department.of Family Medicine, VIEVIS NAMUH Hospital, Seoul, Korea
ARTICLE INFO
Article history:
in revised form 20 September 2015
Accepted 15 October 2015
Available online 20 November 2015
Roots of Adenophora triphylla var. japonica extract
High-fat diet-induced obese mice
Adipocytes
Anti-obesity
Lipogenesis
Objective: To investigate the anti-obesity activity and the action mechanism of the roots of Adenophora triphylla var. japonica extract (ATE) in high-fat diet (HFD)—induced obese mice and 3T3-L1 adipocytes. Methods: The roots of Adenophora triphylla were extracted with 70% ethanol. To demonstrate the compounds, linoleic acid was analyzed by using gas chromatography; and the anti-obesity effects and possible mechanisms of ATE were examined in 3T3-L1 adipocytes and HFD-induced obese mice. Results: Treatment with ATE inhibited the lipid accumulation without cytotoxicity in 3T3-L1 adipocytes. Furthermore,200 and 400 mg/kg ATE treatment significantly decreased the body weight gain, white adipose tissues (WATs) weight and plasma triglyceride level, while 100 and 200 mg/kg ATE treatment increased the plasma high-density lipoprotein cholesterol level in the HFD-induced obese mice, as compared with the HFD group. Treatment with 200 and 400 mg/kg ATE also lowered the size of adipocytes in adipose tissue and reduced the lipid accumulation in liver. ATE treatment showed significantly lower expression level of adipogenesis-related proteins,such as peroxisome proliferator-activated receptorγ, fatty acid binding protein (aP2), fatty acid synthase in 3T3-L1 adipocytes; and furthermore, decreased peroxisome proliferatoractivated receptorγ, aP2, fatty acid synthase, sterol regulatory element binding protein-1c, and lipoprotein lipase mRNA expression levels in WAT of the HFD-induced obese mice. Conclusions: These results suggested that the ATE has an anti-obesity effect, which may be elicited by regulating the expression of adipogenesis and lipogenesis-related genes and proteins in adipocytes and WAT of the HFD-induced obese mice.
Document heading doi: 10.1016/j.apjtm.2015.10.011
1. Introduction
Adenophora triphylla (A. triphylla) var. japonica (Korean name: Jandae, Japanese name: lady bell, English name: Three-leaf ladybell)belongs to the Adenophora species (Campanulaceae), which has been used as an oriental medicinal plant in Korea, China and Japan for anti-inflammation, anti-tussive[1] and hepatoprotective effects. Alkaloids, triterpenoids and essential oil components such as pyrrolidine, piperidine, triphyllol, nonacosane, heptacosane,lupenone and saponine have been identified from A. triphylla. These chemicals have anti-inflammatory and anti-oxidant properties, and are used to prevent obesity in Korea.
Obesity has become a significant clinical problem in recent decades since it could contribute to life-threatening diseases such as type 2 diabetes, hypertension, hyperlipidemia, and cardiovascular disease[2]. It is characterized by increased fat mass, which isassociated with increased cell number (hyperplasia) and sizes(hypertrophy)[3,4]. Adipocyte hypertrophy is caused by excessive accumulation of lipid such as triglyceride (TG) formed from energy intake[5]. Adipocytes hyperplasia results from a complex interaction between proliferation and differentiation in preadipocytes that is involved in the adipogenic process where undifferentiated preadipocytes are converted to differentiated adipocytes[6,7]. Therefore, adipogenesis and TG accumulation play an important role in fat mass increase. Accordingly, controlling adipogenesis and TG accumulation in adipocytes could help prevent obesity and its associated diseases.
There are many different pharmacological approaches to prevent and treat obesity, but they reportedly have a number of limitations such as undesirable side effects and adverse effects[8,9]. Recently,there is increasing public attention on using natural products to prevent and treat obesity with various plants such as fruits,vegetables, and herbs that have beneficial health effects and minimal side effects[10].
A. triphylla root extracts have anti-obesity and hypolipidemia effects[11,12], however, A. triphylla var. japonica have not yet been studied in adipocytes and high-fat diet (HFD)-induced obese mice related adipogenesis and lipogenesis mechanisms.
In the present study, we examined the effect of A. triphylla var. japonica extracts (ATE) on obesity and the underlying lipid mechanisms, lipid deposition and lipogenesis, in adipocytes and HFD-induced obese mice.
2. Materials and methods
2.1. Chemicals and reagents
5'-diallyl-2, 2'-biphenyldiol, 3-isobutylmethylxanthine insulin, and dexamethasone were purchased from Wako Pure Chemical Industries Ltd. (Osaka, Japan). Oil-Red O, isopropanol, and TRI reagent were purchased from Sigma-Aldrich (MO, USA). Polyclonal antibodies against peroxisome proliferator-activated receptor γ(PPARγ), aP2,and α-tubulin were purchased from Santa Cruz Biotechnology(CA, USA). Horseradish peroxidase-linked anti rabbit IgG and HRP-linked anti-mouse IgG were purchased from Bio-Rad (CA, USA). SYBR Green reaction buffer was purchased from Takara (Shiga,Japan).
2.2. Preparation and component analysis of ATE
A. triphylla var. japonica was provided from DONG IL Pharmtec(Seoul, Korea). The dried A. triphylla var. japonica was extracted with 70% aqueous ethyl alcohol and subjected to vacuum evaporation. Gas chromatography was carried out with an Agilent 7890A GC-FID instrument for the analysis of linoleic acid as a reference compound. GC conditions were equipped on HP-INNOWAX (30 m ×0.25 mm × 0.25 μm) column. The GC oven temperature was maintained at 80 ℃for 3 min and increased to 260 ℃ with helium as a carrier gas at a flow rate of 1.0 mL/min, and . Both injector and detector were set at 260 ℃ and 290 ℃. The total analysis time was 25 min.
2.3. Animal studies
Male C57BL/6J mice were purchased from Samtaco (Gyeonggido, Korea) at 4 wk of age. The mice were housed individually in stainless steel cages and maintained under a temperature of(23 ± 3) ℃ in a humidity-controlled room with a 12 h-light/dark cycle. Mice were given free access to water and food (Certified irradiated global 18% protein diet, 2918C, Harlan Laboratories,Inc., Indianapolis, Indiana, USA). After acclimatization for 1 wk,they were fed with either the normal-fat diet (NFD, n = 8, Rodent diet with 10% kcal fat, D12450B) or HFD, n = 32, Rodent diet with 60% kcal fat, D12492) purchased from Research Diet Inc., (New Brunswick, NJ, USA) for 4 wk to induce obesity. After obesity induction, the mice were divided into 4 subgroups (n = 8/group)that were matched with body weight. The following 4 groups were studied for 6 wk: HFD; HFD with oral administration of ATE at 100 mg/kg of body weight (HFD + ATE 100); HFD with ATE at 200 mg/ kg of body weight (HFD + ATE 200); and HFD with ATE at 400 mg/ kg of body weight (HFD + ATE 400). The body weight of the mice was measured once wk for 6 wk. Food intake was measured every 5 d on a per-cage basis throughout the study. Food intake (g/mouse/ day) was determined by subtracting the remaining food weight from the initial food weight of the previous feeding day, and dividing by the number of mice housed in the cage. At the end of the experiment,the mice were sacrificed, and the liver, kidney, and white adipose tissue (WAT) were excised immediately. The parts were then rinsed,weighed, frozen in liquid N2, and stored at —80 ℃ until analysis. The experimental design was approved by The Animal Experiment Committee of GyeongGi Bio Center, and the mice were maintained in accordance with their guidelines.
2.4. Plasma biochemical analysis
After 6 wk of feeding, 4 h-fasted mice were anesthetized using ether and blood was collected. Plasma was obtained from the blood by centrifugation at 800 g for 10 min at 4 ℃ for biochemical analyses of plasma parameters. The separated plasma was stored at — 80 ℃until analysis. Plasma total cholesterol (T-CHO), TG, high-density lipoprotein (HDL)-cholesterol, low-density lipoprotein (LDL)—cholesterol, and glucose levels were determined enzymatically using commercial assay kits (Asan Chemical, Seoul, Korea). Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured using commercial assay kits (Sigma-Aldrich, MO, USA).
2.5. Histological analysis
Histological examination of liver was conducted, and epididymal adipose tissue were dissected and fixed in 10% neutral formalin solution and embedded in paraffin. The tissues were studied under hematoxylin and eosin staining. The sections were viewed with alight microscope (Olympus, Tokyo, Japan) and photographed at × 200 magnification.
2.6. Cell culture
Mouse 3T3-L1 preadipocytes obtained from the Dr. Rhee, Sang Dal(Korea Research Institute of Chemical Technology, Daejeon, Korea),were cultured in Dulbecco's modified Eagle's medium (DMEM)supplemented with 10% fetal bovine serum (FBS) and antibiotics[100 U/mL penicillin and 100 g/mL streptomycin (Gibco, Carlsbad,CA, USA)] at 37 ℃ under a humidified 5% CO2atmosphere. For differentiation of 3T3-L1 preadipocytes to mature adipocytes,preconfluent cells (defined as day 0) were cultured in differentiation medium containing 0.5 mM 3-isobutylmethylxanthine, 5 μg/mL insulin, and 1 μM dexamethasone in DMEM supplemented with 10% FBS (MDI differentiation medium). After 3 d, the cell culture medium was changed to DMEM containing 5 μg/mL insulin and 10% FBS. The medium was replaced again with DMEM containing 10% FBS after another 2 d. To determine the effect of ATE on adipogenesis, differentiated preadipocytes were treated with presence or absence of ATE for 5 d.
2.7. Oil-red O staining
After differentiation, the cells were fixed with 4% formaldehyde solution in phosphate-buffered saline for 1 h, and were subsequently washed thrice with water. The cells were stained with oil-red O(0.5% in 60% isopropanol) for 1 h and washed thrice with distilled water. Stained cells were photographed under an optical microscope(Olympus, Tokyo, Japan). Stained lipid droplets were extracted with isopropanol and the absorbance was measured at 520 nm with a microplate reader (Tecan, Mannedorf, Switzerland).
2.8. Cell cytotoxicity assay
For cell cytotoxicity, the cell were treated for 24 h with various concentrations (0, 100, 300, and 500 μg/mL) of ATE extracts in DMEM containing 10% FBS. Filtered MTT reagent (5 mg/mL) was added to the cells to reach a final concentration of 0.5 mg MTT/ mL, and the cells were further incubated for 4 h at 37 ℃. The violet formazan crystals were dissolved in dimethyl sulfoxide, and the absorbance was measured at 570 nm by using a microplate reader. The cell viability (%) was calculated by comparing the absorbance of the samples and the control (untreated cell).
2.9. Protein extraction and Western blot analysis
Cold phosphate-buffered saline washed 3T3-L1 cells and epididymal adipose tissue were homogenized in RIPA buffer containing 0.1 mM phenylmethylsulfonyl fluoride and 1% of protease inhibitor cocktail (Sigma-Aldrich, MO, USA). The homogenates were centrifuged at 12 000 rpm for 15 min at 4 ℃, and supernatant was collected. The protein concentrations were calculated by Bio-Rad protein assay reagent (Bio-Rad Laboratories,Hercules, CA, USA). Proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes (Bio-Rad Laboratories,Hercules, CA, USA). The membranes were blocked with 5% of nonfat dried skim milk and then incubated with appropriate antibodies. The membranes were washed with wash buffer and incubated with anti-mouse or anti-rabbit horseradish peroxidaseconjugated secondary antibodies for 1 h. The immunoreactive bands were enhanced by chemiluminescence reagents and detected by chemi-luminometer (CLINX Science Instruments Co. Ltd.,Shanghai, China).
2.10. Isolation of total RNA and quantitative real-time PCR(qRT-PCR)
Total RNA of epididymal adipose tissue was isolated by TRI reagent (Sigma-Aldrich, MO, USA) according to the manufacturer'sprotocols. Total RNA was reverse transcribed to cDNA by using random primers and a reverse transcription system (Promega, Piscataway, NJ) according to manufacturer's recommendations. The mRNA expression levels of adipogenesisrelated genes were analyzed by real-time PCR iQTM5 system (Bio-Rad Laboratories, Hercules, CA, USA) using a SYBR Green Master PCR Kit (Takara, Shiga, Japan) according to the manufacturer's protocols. The real-time PCR cycling parameters were as follows:2 min at 95 ℃; 45 cycles of 20 s at 95 ℃, 20 s at 60 ℃, 40 s at 72 ℃, and 30 s at 72 ℃; and a final extension for 5 min at 72 ℃,followed by a melting curve analysis. All samples were normalized to the expression level of β-actin, and the results were expressed as the fold changes relative to the HFD group. The primers used in the experiments were listed in Table 1.
2.11. Statistical analysis
Statistical evaluation of the data was expressed as the mean ± standard error (SEM). The statistical significance of differences between the mean values for the treatment groups was analyzed by Student's t-tests and one-way analysis of variance (ANOVA) using the software Origin 7 (Microcal Software, USA). Values of P<0.05 were considered as statistical significance.
3. Results
3.1. Linoleic acid content of A. triphylla
The linoleic acid content in A. triphylla was elucidated by comparing the chromatographic profile of standard on gas chromatography analysis. We confirmed that linoleic acid content was approximately 2.0% (Figure 1), and the optimized A. triphylla was used in all subsequent experiments.
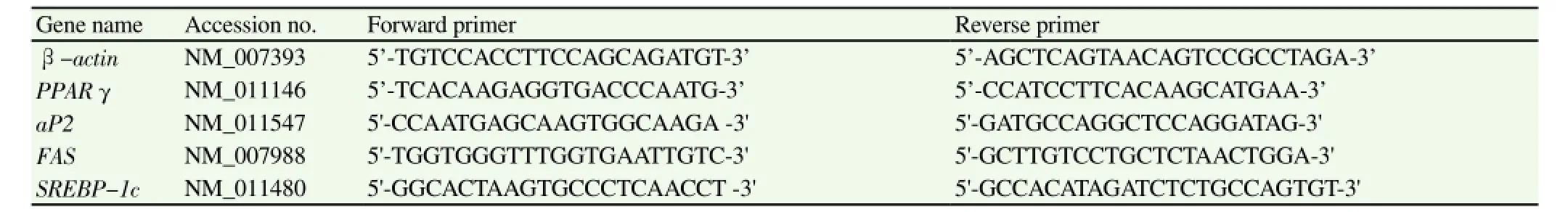
Table 1 Primer sequences used for quantitative real-time PCR.
3.2. Effects of ATE on adipogenesis of 3T3-L1 preadipocytes
To determine the effect of ATE on lipid accumulation during differentiation, 3T3-L1 preadipocytes were induced to differentiate into adipocytes in the presence of ATE for 5 d. Accumulated lipid droplets were measured by Oil-Red O staining. ATE inhibited the lipid accumulation in 3T3-L1 adipocytes (Figure 2A); especially,500 μg/mL ATE treatment significantly reduced the lipid content by 49.6% ,as compared to the control (P<0.05) (Figure 2B). The cytotoxic effect of ATE on 3T3-L1 adipocytes was evaluated by the MTT assay. ATE had no cytotoxic activity at various concentrations ie., 100-500 μg/mL for 24 h (Figure 2C). To investigate how ATE inhibits lipid accumulation, we measured the expression levels of adipogenesis-related proteins using Western blotting. As shown in Figure 2D, PPARγ protein expression level decreased in 300 and 500 μg/mL of ATE treatment, as compared to the control. Target proteins ie.,aP2 and FAS protein expression level also decreased as compared to the control.
3.3. Effects of ATE on body weight and food intake
ATE inhibited lipid accumulation in 3T3-L1 cells. Based on these results, we investigated the effect of ATE on anti-obesity in HFD-induced obese mice. The body weight gain and food intakes were shown in Figure 3. The body weight gain of the HFD group was significantly increased, as compared to the NFD group after 6 wk of feeding; whereas administration of ATE at 100, 200, and 400 mg/kg showed a tendency to decrease body weight gain than the HFD group (Figure 3A). Food intake did not display significant group wise differences (Figure 3B).

Table 2 Effects of ATE on plasma biochemical parameters in HFD-induced obese mice
3.4. Effects of ATE on organs weight
The relative weight of liver, epididymal, perirenal, and mesenteric WAT were compared to those in the HFD group (Figure 4). Supplementation with ATE did not show significant differences in kidney weight in mice fed with HFD (Figure 4A). However, the HFD plus ATE presented a decreased liver weight when compared to the mice fed HFD (Figure 4B). Epididymal, perirenal, mesenteric,and total WAT weights in the HFD group were higher than those of the NFD group (Figure 4C). ATE significantly decreased epididymal,perirenal, mesentric, and total WAT weights in HFD plus ATE 200 and 400 mg/kg fed mice, respectively.
3.5. Effects of ATE on the plasma biochemical levels
The plasma biochemical parameters were compared to the HFD fed group. As shown in Table 2, glucose, T-CHO, and TG levels in the HFD group were significantly increased, as compared to the ND fed mice group. In HFD plus ATE groups, decreased HFD-induced plasma glucose, T-CHO; and TG levels showed a propensity to decrease in a dose-dependent manner, although not significantly, as compare to the HFD group. However, TG level in the HFD plus ATE 200 and 400 groups were significantly decreased, as compared to the HFD group. HDL-cholesterol levels in HFD fed with ATE groups were significantly increased, when compared to the HFD fed group. There were no significant differences in the LDL cholesterol level, among the HFD and ATE-treated groups, but the ATE fed groups presented a tendency towards decreased levels in. ALT level in the HFD fed group was more significantly increased than that of the ND fed group. The HFD + ATE 200 and 400 groups exhibited tendency of decreased ALT, although there were no statistically significant differences, as compared to HFD fed group. AST level in the NFD and HFD + ATE 100 group did not differ, as compared to the HFD group, but showed a significant reduction in HFD fed with 200 and 400 mg/kg of ATE groups.
3.6. Effects of ATE on liver and epididymal adipose tissue morphology
Histological observations of liver and epididymal adipose tissue were conducted through 5 groups as follows: NFD; HFD; HFD + ATE 100, 200, and 400 groups (Figure 5). Sections of liver and epididymal adipose tissue were stained with hematoxylin and eosin to examine the lipid accumulations and cellular morphology. In WAT, adipocytes size of the HFD group showed an increase, as compared to the NFD group; however, adipocytes size of the HFD + ATE 200 and 400 groups were reduced, as compared to the HFD group. In the liver, lipid droplet sizes of the HFD group were larger than that of the NFD group; however, ATE treatment decreased the lipid droplet size, in a dose-dependent manner.
3.7. Effects of ATE on expression levels of adipogenesisrelated genes and proteins
Of the adipogenesis-related protein expression levels in ATE-treated 3T3-L1 adipocytes, we found that PPARγ, aP2, and FAS protein expression levels were decreased in a dose-dependent manner. We determined whether ATE affects the protein expression of adipogenesis-related genes and protein in epididymal adipose tissues of HFD-induced obese mice, using the quantitative real-time PCR and Western blot. As shown in Figure 6A, PPARγ, aP2, FAS,sterol regulatory element binding protein-1c (SREBP-1c), and LPL mRNA expression levels in the HFD fed group were increased than those of the NFD fed group, but the difference was not statistically significant. However, the mRNA expression level was significantly decreased in the HFD plus ATE groups, as compared to the HFD group. SREBP-1c and FAS mRNA expression level in the HFD group was significantly increased, as compared to the NFD group,whereas SREBP-1c mRNA expression level significantly decreased in 3 ATE-treated groups. FAS mRNA expression level showed a tendency of decrease, and was significantly decreased in the HFD plus 400 mg/kg ATE group, as compared to the HFD group. Additionally, ATE treatments increased LPL mRNA level, in a dose dependent manner. Consistently, PPARγ protein expression level in the HFD plus ATE groups was decreased in a dose-dependent manner, and it especially showed statically significant difference in the HFD + ATE 200 and 400 groups (Figure 6B).
4. Discussion
Long-chain polyunsaturated fatty acid, linoleic acid, has less known effects when derived from plants, especially from roots of A. triphylla[14]. Polyunsaturated fatty acid is a potent compound with biological responses that are required for health. In the present study, we demonstrated the 18-carbon polyunsaturated fatty acid,linoleic acid (LA, 18:2n-6, the precursor of arachidonic acid) as a reference compound by gas chromatography analysis with 2 % from ATE. Linoleic acid is an essential fatty acid that is synthesized in the human body as an unsaturated fatty acid for membrane phospholipids. Recent publications have shown that linoleic acid has anti-bacterial[15], anti-oxidative[16], anti-inflammatory[17] activities and attenuates the development of obesity—related pathogenesis,such as insulin resistance[18,19].
Recently, several studies showed that A. triphylla has compounds including saponine, β-sitosterol, cycloartenyl acetate, and taraxenone[11,13]. It has been reported that ATE and isolated compounds show anti-inflammatory, hepatoprotective, and antitumor effects. Based on these results, the present study confirmed an anti-obesity effect including lipid synthesis of ATE and elucidated the mechanisms underlying such effects in 3T3-L1 adipocytes and HFD-induced obese mice.
Our results demonstrated that ATE treatment dose-dependently inhibited the differentiation and lipid accumulation in 3T3-L1 adipocytes. This result was further supported by in vivo experiment,demonstrating that ATE treatment for 6 wk significantly reduced the body weight gain in HFD-induced obese mice. These results are in accordance with previous reports that A. triphylla root ethanol extract has anti-obesity effects[11,12]. Additionally, ATE treatment did not affect the food intake in HFD-induced obese mice. These results suggested that ATE treatment has anti-obesity effects, and reduction in weight gain induced by ATE treatment is related to decreasing food efficiency and not decreasing food intake.
Obesity is characterized by adipose tissue remodeling associated with hyperplasia and hypertrophy[20,21]. Adipocytes hypertrophy is caused by an excessive accumulation of TG from the intakeof excess energy, and it results from an energy imbalance. Since adipocytes hyperplasia results from a complex interaction between proliferation and differentiation in preadipocytes[22]. It is examined by 3T3-L1 adipocytes differentiation system. To differentiate from preadipocytes into adipocytes, proliferation of preadipocytes is a prerequisite[23]. Adipogenic signals MDI differentiation medium,initiating the differentiation after mitotic clonal expansion[24]. It has been suggested that inhibition of proliferation may be the potential mechanism for reducing the adipocytes number[25]. We confirmed that ATE treatment inhibited lipid accumulation during differentiation in 3T3-L1 adipocytes without cytotoxicity. Also,ATE with HFD fed mice exhibited the tendency to decrease the epididymal, perirenal, and total WAT weights in HFD-induced obese mice. Moreover, histological data showed a decrease in the adipocytes size of adipose tissue in ATE-treated mice. These results indicated that ATE treatment may affect both the cell number and sizes of adipocytes in adipose tissue, which is likely to be associated with reduced WATs weight. However, cell proliferation and cell cycle need to be further investigated.
Fatty liver results from increased free fatty acid uptake and endogenous hepatic fatty acid synthesis[26]. Histological data of liver revealed that ATE treatment reduced lipid droplet size and number,indicating that ATE treatment was responsible for the inhibition of lipid accumulation in liver. In our study, ATE treatment lowered plasma TG level, while 100 and 200 mg/kg ATE treatment increased plasma HDL-cholesterol level in HFD-induced obese mice, as shown in previous reports[11,12]. These results collectively suggest that ATE treatment reduces the accumulation in liver, resulting in the improved hyperlipidemia in HFD-induced obese mice.
Excessive fat intake can cause the oxidative stress, which induces dysfunction and fatty degeneration in the liver through abnormal activation of AST and ALT. Previous reports have demonstrated that various extracts of A. triphylla have an antioxidant effect that reduces plasma ALT and AST levels in HFD-induced obese mice[11,12]. In our study, 200 and 400 mg/kg ATE treatment showed a tendency to decrease plasma ALT and AST levels in HFD-induced obese mice,as compared to the HFD group. These results showed that ATE treatment may improve the liver function.
To examine the underlying mechanism, we measured the expression levels of adipogenesis-related genes and proteins in 3T3-L1 adipocytes and WAT in HFD-induced obese mice. Adipogenesis is closely associated with etiologies of obesity, in which the preadipocytes are differentiated into mature adipocytes. Adipogenesis is regulated by sequential expression of adipogenic and lipogenic genes[27]. PPARγ is a transcription factor that regulates adipocytes differentiation and adipogenesis. C/EBP promotes adipocytes differentiation through cooperation with PPAR γ, which increases the expression of adipocytes-specific genes such as aP2[28,29]. SREBP-1c is also a transcription factor that plays key roles in lipid metabolism, by increasing the expressions of several lipogenic genes such as FAS in adipose tissue and liver. It activates PPARγ through regulation of its expression and production of an endogenous PPARγ ligand[30,31]. The lipoprotein lipase is a key regulator of blood TG levels via decomposition of lipoprotein triglyceride. It plays a critical roles in transporting the fats and hydrolysis fat-carried lipoproteins[32]. Combined action of these transcription factors causes expression of target genes and proteins that are responsible for TG synthesis and storage, which leads to accelerated adipogenesis. We found that the high concentration of ATE treatment reduced the PAPRγ, C/EBP , aP2, and FAS protein expression level in 3T3-L1adipocytes. Furthermore, PAPRγ and SREBP-1c mRNA expression level were significantly decreased in epididymal adipose tissue of 100, 200, and 400 mg/kg ATE-treated mice. Additionally, aP2 and FAS mRNA expression level was decreased in 400 mg/kg ATE-treated mice. PPARγ protein expression levels tended to be reduced specifically in adipose tissues of HFD fed with ATE groups, and significant down-regulation was observed in the 200 and 400 mg/kg ATE treatment groups. On the other hand, the reduction in body weight gain and total WATs weight were unfortunately not observed in the 100 mg/kg of ATE treatment group, despite the down-regulation of adipogenesis-related genes. There is a possibility that there are differences in PPARγ protein expression levels in ATE-treated groups. However, more study is needed on the expression levels of other genes related to adipogenesis in adipose and other tissues. Based on our results, ATE treatment affects the expression levels of adipogenesis-related genes and proteins. Furthermore, it is likely that the anti-obesity effects of ATE treatment are caused by suppression of gene expressions and proteins involved in adipogenesis.
In conclusion, our results showed that ATE treatment inhibited the lipid accumulation in 3T3-L1 adipocytes and reduced the body weight gain, WATs weight, adipocytes sizes, and plasma TG levels, without any toxic effects in HFD-induced obese mice. These effects were at least partially accompanied by the regulation of gene expressions and proteins associated with adipogenesis/lipogenesis,in 3T3-L1 adipocytes and HFD-induced obese mice. These findings indicated that ATE prevents the development of obesity and hyperlipidemia in 3T3-L1 adipocytes and HFD-induced obese mice,which suggest the possibility of its use for the prevention of obesity and obesity-related diseases.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
This work was carried out with the support of ‘Cooperative Research Program for Agriculture Science &Technology Development (Project No.PJ01134801)' Rural DevelopmentAdministration, Republic of Korea.
[1] Konno C, Saito T, Oshima H, Hikino C. Kabuto: Structure of methyl adenophorate and triphyllol, triterpenoids of Adenophora triphylla var. japonica roots. Planta Medica 1981; 42(7): 268-274.
[2] World Health Statistics. Geneva: World Health Organization; 2012.
[3] Spiegelman MB, Flier JS. Adipogenesis and obesity: rounding out the big picture. Cell 1996; 87(3): 377-389.
[4] Fujioka K. Management of obesity as a chronic disease:nonpharmacologic, pharmacologic, and surgical options. Obes Res 2002;10: 116S-123S.
[5] Gregoire FM. Adipocytes differentiation: from fibroblast to endocrine cell. Exp Bio Med 2001; 226(11): 997-1002.
[6] Nawrocki AR, Scherer PE. Keynote review: the adipocyte as a drug discovery target. Drug Discov Today 2005; 10(18): 1219-1230.
[7] S. H. Kim SH, Park HS, Lee MS, Cho YJ, Kim YS, Hwang JT, et al. Vitisin A inhibits adipocyte differentiation through cell cycle arrest 3T3-L1 cells. Biochem Biophys Res Commun 2008; 372(1): 108-113.
[8] Derosa G, Maffioli P. Anti-obesity drugs: A review about their effects and their safety. Expert Opin Drug Saf 2012; 11(3): 459-471.
[9] Mayer MA, Hocht CL, Puyo A, Taira CA. Recent advances in obesity pharmacotheraphy. Curr Clin Pharmaco 2009; 4(1): 53-61.
[10] Yun JW. Possible anti-obesity therapeutics from nature-A review. Phytochemistry 2010; 71(14-15): 1625-1641.
[11] Choi HJ, Chung MJ, Ham SS. Antiobese and hypocholesterolaemic effects of an Adenophora triphylla extract in HepG2 cells and high fat diet-induced obese mice. Food Chem 2010; 119(2): 437-444.
[12] Lee SE, Lee HE, Lee TJ, Kim SW, Kim BH. Anti-obesity effects and action mechanism of Adenophora triphylla root ethanol extract in C57BL/6 obese mice fed a high fat diet. Biosci Biotechnol Biochem 2013;77(3): 544-550.
[13] Ahn EK, Oh JS. Lupenone isolated from Adenophora triphylla var. japonica extract inhibits adipogenic differentiation through the downregulation of PPARγ in 3T3-L1 cells. Phytother Res 2013; 27(5):761-766.
[14] Hung PF, Wu BT, Chen HC, Chen CL, Wu MH, Liu HC, et al. Antimitogenic effect of green tea (-) epigallocatechingallate on 3T3-L1 preadipocytes depends on the ERK and Cdk2 pathways. AM J Physiol Cell Physiol 2005; 288(5): C1094-C1108.
[15] Dilika F, Bremner PD, Meyer JJ. Antibacterial activity of linoleic acid and oleic acids isolated from Helichrysum pedunculatum: a plant used during circumcision rites. Fitoterapia 2000; 71(4): 450-452.
[16] Duh PD, Tu YY, Yen GC. Antioxidant activity of water extract of HarngJyur (Chrysantemum morifolium Ramat). LWT Food Sci Tech 1999;32(5): 269-277.
[17] Chen PY, Wang J, Lin YC, Li CC, Tsai CW, Liu TC, et al. 18-carbon polyunsaturated fatty acids ameliorate palmitate-induced inflammation and insulin resistance in mouse C2C12 myotubes. J NutrBiochem 2015. DOI: http://dx.doi.org/10.1016/j.jnutbio.2014.12.007
[18] Matravadia S, Herbst EA, Jain SS, Mutch DM, Holloway GP. Both linoleic acid -linoleica acid prevent insulin resistance but have divergent inpacts on skeletal muscle mitochondrial bioenergetics in obese Zucker rats. Am J Physiol Endocrinol Metab 2014; 307(1): 102-114.
[19] Cranmer-Byng MM, Liddle DM, De Boer AA, Monk JM, Robinson LE. Proinflammatory effects of arachidonic acid in a lipopolysaccharide induceud inflammatory microenvironment in 3T3-L1 adipocytes in vitro. Appl Physiol Nutr Metab 2015; 40(2): 142-154.
[20] Rosenbaum M, Leibel RL, Hirsch J. Obesity. N Engl J Med 1997; 337(6):396-407.
[21] Ducharme NA, Bicke PE. Lipid droplets in lipogenesis and lipolysis. Endocrinology 2008; 149(3): 942-949.
[22] Hsieh YH, Wang SY. Lucidone from Linera erythrocarpa Makino fruits suppresses adipogenesis in 3T3-L1 cells and attenuates obesity and consequent metabolic disorders in high-fat diet C57BL/6 mice. Phytomedicine 2013; 20(5): 394-400.
[23] Zhang JW, Tang QQ, Vinson C, Lane MD. Dominant-negative C/ EBP disrupts mitotic clonal expansion and differentiation of 3T3-L1 adipocytes. Proc Natl Acad Sci USA 2004; 101(1): 43-47.
[24] TangQQ, Otto TC, Lane MD. Mitotic clonal expansion: a synchronous process required for adipogenesis. Proc Natl Acad Sci USA 2003; 100(1):44-49.
[25] Kim HK, Kim NJ, Han SN, Nam JH, Na HN, Ha TJ. Black soybean anthocyanins inhibit adipocytes differentiation in 3T3-L1 cells. Nutr Res 2012; 32(10): 770-777.
[26] Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest 2004; 114(2): 147-152.
[27] Spiegelman BM, Choy L, Hotiamisligil GS, Graves RA, Tontonoz P:Regulation of adipocyte gene expression in differentiation and syndromes of obesity/diabetes. J BiolChem 1993; 268(10): 6823-6826.
[28] Kim JB, Spiegelman BM. ADD/SREBP1 promotes adipocytes differentiation and gene expression linked to fatty acid metabolism. Genes Dev 1996; 10(9): 1096-1107.
[29] Farmer SR. Regulation of PPARγ activity during adipogenesis. Int J Obes 2005; 1: 13-16.
[30] Rosen ED, Puigserver CJP, Spiegelman BM. Transcriptional regulation of adipogenesis. Genes & Dev 2000; 14(11): 1293-1307.
[31] Park J, Rho HK, Kim KH, Choe SS, Lee YS, Kim JB. Overexpression of glucose-6-phosphate dehydrogenase is associated with lipid dysregulation and insulin resistance in obesity. Mol Cell Biol 2005; 25(12): 5146-5157.
[32] Peterson J, Bihain BE, Bengtsson-Olivecrona G, Deckelbaum RJ,Carpentier YA, Olivecrona T. Fatty acid control of lipoprotein lipase: a link between energy metabolism and lipid transport. Proc Natl Acad Sci USA 1990; 87(3): 909-913.
15 August 2015
#These authors contributed equally to this work.
SH Yang. PhD, Professor, JW Suh, PhD, Professor, Center for Nutraceutical and Pharmaceutical Materials, Myongji University, Cheoin-gu,Yongin, Korea.
Tel: +82-31-330-6881 Fax: +82-31-336-0870
E-mail: ymichigan@mju.ac.kr, jwsuh@mju.ac.kr
Foundation project: This work was carried out with the support of ‘Cooperative Research Program for Agriculture Science &Technology Development (Project No.PJ01134801)' Rural Development Administration, Republic of Korea.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Demographic, socioeconomic and environmental changes affecting circulation of neglected tropical diseases in Egypt
- Phenolic profile and biological potential of Endopleura uchi extracts
- Anti TB drug resistance in Tanga, Tanzania: a cross sectional facility base prevalence among pulmonary TB patients
- In vitro inhibitory effects of plumbagin, the promising antimalarial candidate, on human cytochrome P450 enzymes
- Vibrio spp. from Macrobrachium amazonicum prawn farming are inhibited by Moringa oleifera extracts
- Inhibition effect of miR-577 on hepatocellular carcinoma cell growth via targeting β-catenin
